Abstract
22q11.2 deletion syndrome (22q11DS) is associated with elevated levels of impulsivity, inattention, and distractibility, which may be related to underlying neurobiological dysfunction due to haploinsufficiency for genes involved in dopaminergic neurotransmission (i.e. catechol-O-methyltransferase). The Stop-signal task has been employed to probe the neural circuitry involved in response inhibition (RI); findings in healthy individuals indicate that a fronto-basal ganglia network underlies successful inhibition of a prepotent motor response. However, little is known about the neurobiological substrates of RI difficulties in 22q11DS. Here, we investigated this using functional magnetic resonance imaging while 45 adult participants (15 22q11DS patients, 30 matched controls) performed the Stop-signal task. Healthy controls showed significantly greater activation than 22q11DS patients within frontal cortical and basal ganglia regions during successful RI, whereas 22q11DS patients did not show increased neural activity relative to controls in any regions. Using the Barratt Impulsivity Scale, we also investigated whether neural dysfunction during RI was associated with cognitive impulsivity in 22q11DS patients. RI-related activity within left middle frontal gyrus and basal ganglia was associated with severity of self-reported cognitive impulsivity. These results suggest reduced engagement of RI-related brain regions in 22q11DS patients, which may be relevant to characteristic behavioral manifestations of the disorder.
Keywords: fronto-basal ganglia, impulsivity, response inhibition, velocardiofacial syndrome
Introduction
Chromosome 22q11.2 Deletion Syndrome (22q11DS), also known as DiGeorge/velocardiofacial syndrome, is a recurrent genetic mutation caused by a microdeletion on the long arm of chromosome 22. Associated physical features are variable, but often involve craniofacial, cardiovascular anomalies, immunodeficiency, short stature, and hypocalcemia (Shprintzen et al. 1978; Ryan et al. 1997; Scambler 2000; Guyot et al. 2001). Individuals with 22q11DS also have a characteristic cognitive profile, involving impairment in nonverbal reasoning, working memory, and arithmetic, as well as difficulties with inhibitory control (Swillen et al. 1997; Bearden et al. 2001; Henry et al. 2002). The syndrome is also highly penetrant for Attention Deficit-Hyperactivity Disorder (ADHD), with ∼40% of patients diagnosed with the disorder (Green et al. 2009); of those, the vast majority are diagnosed with the inattentive subtype (Niklasson et al. 2001; Antshel et al. 2007). These symptoms appear to be continuously distributed in 22q11DS patients, as even those that do not meet clinical diagnostic criteria for ADHD show substantially elevated rates of impulsivity, inattention, and distractibility relative to healthy controls (Antshel et al. 2008; Furniss et al. 2011).
The known genetic etiology of this syndrome makes it an ideal model to study the neural basis of inhibitory control deficits. The Go/No-Go and Stop-signal cognitive paradigms are the most commonly used tasks for measuring the neural basis of response inhibition (RI), with some additional evidence that these measures of inhibitory control correlate with self-reported trait impulsivity in healthy individuals (Logan et al. 1997; Enticott et al. 2006). Research on the neural circuitry underlying RI has implicated a fronto-basal ganglia loop, which includes the inferior frontal cortex and subthalamic nucleus (Aron and Poldrack 2006; Aron et al. 2007). Models of basal ganglia function suggest that the subthalamic nucleus is active during the inhibition of already-initiated responses through the “hyperdirect” fronto-subthalamic pathway, as the time to inhibit can be as brief as 120 ms (Alexander and Crutcher 1990; Mink 1996; Nambu et al. 2002; Aron and Poldrack 2006).
To our knowledge, only one prior fMRI study of RI has been conducted in individuals with 22q11DS; this study found that patients exhibited greater activation than healthy controls in left parietal regions during performance of a Go/No-Go task (Gothelf et al. 2007), which was interpreted as reflecting compensatory recruitment for executive dysfunction. It is important to note that adolescent 22q11DS patients were evaluated in this previous cross-sectional study; thus, neural differences captured during this malleable age range may include variance attributable to rapidly changing developmental neural trajectories. It remains unclear whether this pattern of compensatory activity is observed in an adult 22q11DS sample, and further whether this difference generalizes to other RI tasks. The Stop-signal task offers several advantages over other paradigms for investigating RI. An important advantage, given that the task is designed to result in roughly equal proportions of failed and successful inhibition trials, is that it controls for difficulty and allows for different conditions to be directly contrasted, thereby avoiding the confound of the “oddball effect,” or effects related to infrequent trial types (Rubia et al. 2003; Aron and Poldrack 2005).
It is not yet known whether abnormal activation in RI-associated brain circuitry is associated with the symptoms of impulsivity seen in 22q11DS individuals. Early detection of neural vulnerability markers of impulsivity could potentially reduce the clinical severity and functional impairment caused by these symptoms. Using functional magnetic resonance imaging (fMRI), we investigated underlying neural activity in 22q11DS patients compared with healthy controls during RI, as measured using the Stop-signal task. We hypothesized that 22q11DS patients would show abnormal recruitment of brain regions critical for inhibitory control (i.e. prefrontal cortical and basal ganglia regions) during task performance. We also queried whether neural activity within RI-related regions is associated with trait impulsivity in adult 22q11DS patients.
Materials and Methods
Participants
The total sample consisted of 45 (15 22q11DS and 30 healthy) adults (18–38 years old). 22q11DS participants consisted of individuals with a molecularly confirmed diagnosis of 22q11.2 deletion syndrome recruited from an ongoing longitudinal study at the University of California, Los Angeles (UCLA). Healthy controls were recruited from this study and from the Consortium for Neuropsychiatric Phenomics (CNP) at UCLA. Exclusion criteria for all study participants included the following: neurological or medical condition that might affect performance, insufficient fluency in English, substance or alcohol abuse and/or dependence with the past 6 months, any contraindications to scanning, and left-handedness (further details are provided in Supplementary Materials). The Structured Clinical Interview for DSM-IV Axis I Disorders [SCID; (First 1997)] was used to ensure that healthy controls did not meet criteria for any current major mental disorder (see Supplementary Materials and Thakkar et al. 2013 for additional details of inclusion/exclusion criteria). Demographic information for 22q11DS patients and matched controls are presented in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| 22q11.2 Participants (n = 15) | Control participants (n = 30) | P | |
|---|---|---|---|
| Age (years, ± SD) | 22.5 (5.4) | 23.0 (4.4) | 0.72 |
| Participant education (years, ± SD) | 12.1 (1.1) | 14.6 (1.7) | <0.001 |
| Parental education | 16.0 (3.6) | 14.7 (1.7) | 0.30 |
| Gender (N, % female) | 6 (40%) | 12 (40%) | 1.00 |
| Ethnicity (N, % Latino) | 2 (13%) | 2 (37%) | 0.10 |
| Full Scale IQ (mean ± SD)a | 75.7 (14.5) | 123.9 (12.4) | <0.000001 |
| Barratt Impulsivity Scale: Cognitive Impulsivity (mean ± SD)b | 8.00 (2.19) | 6.09 (1.56) | 0.03 |
| Psychotropic medication (N, none/antidepressant/psychostimulant/antianxiety, /antipsychoticc | 7/5/3/2/1 | 29/0/1/0/0 | NA |
based on 2-subtest Wechsler Abbreviated Scale of Intelligence (Vocabulary and Matrix Reasoning).
Cognitive Impulsivity measures were available for 23/30 control participants.
Three 22q11DS participants were prescribed multiple psychotropic medications.
All participants underwent a verbal and written informed consent process. The UCLA Institutional Review Board (IRB) approved all study procedures and informed consent documents.
Procedure
After screening and neuropsychological assessment, participants took part in a behavioral training session immediately prior to a 1-h scan. In the behavioral testing session, participants received training on the Stop-signal task in the form of one initial demo and a trial run before completing the experiment run while inside of the scanner. Stimulus presentation and timing of all stimuli and response events were achieved using Matlab (Mathworks) and the Psychtoolbox (www.psychtoolbox.org, Brainard 1997) on an Apple Powerbook. For the experiment block administered in the scanner, each participant viewed the task through MRI-compatible goggles and responded with his or her right hand on an MR-compatible button box in the scanner.
Measures
Stop-signal Task
Participants completed a tracking version of the Stop-signal task, which enabled isolation of activation associated with the inhibition of an already-initiated motor response, and calculation of an individualized measure of inhibitory control (stop-signal reaction time, SSRT). In this task, participants were instructed to respond quickly when a “go” stimulus was presented on the computer screen (which consisted of leftward or rightward pointing arrows), except on the subset of trials where the “go” stimulus was followed by a “stop” signal (a 500-Hz tone presented through headphones), in which case participants were instructed to withhold their response. The onset of the stop-signal, or stop-signal delay (SSD), was adjusted according to the participant's performance—such that the SSD decreased following a successful inhibition (making the next trial more difficult) and increased following a failed inhibition (making the next trial easier)—which ensured that subjects successfully inhibited on ∼50% of stop trials. A complete description of the task and fMRI acquisition parameters is presented in Supplementary Materials.
Barratt Impulsiveness Scale
The Barratt Impulsiveness Scale (BIS) Version 11 (Patton et al. 1995) is a 30-item questionnaire assessing aspects of impulsivity. The BIS-11 is a versatile and widely used measure of impulsivity that has been applied to various clinical groups, including those with ADHD (Malloy-Diniz et al. 2007; Crunelle et al. 2013). We used the revised, 2-factor scoring method of the BIS, which has been offered as an alternative multidimensional structural representation of impulsivity (Reise et al. 2013). The revised method of scoring the BIS results in 2 correlated factors: 1) Cognitive Impulsivity, reflecting difficulties in attentional control, concentration, careful and deliberate thinking, and planning (e.g. “not a steady thinker,” “no self-control/concentration,” and “not planful”), and 2) Behavioral Impulsivity (with some cognitive elements), which reflects acting impulsively, changing jobs, moving residences relatively often, and a scattered quick-paced cognitive tempo (e.g. “extraneous racing thoughts,” “acts impulsively,” and “changes, moves around”). For both scales, higher scores reflect higher levels of trait impulsivity.
Neurocognitive Measures
Supervised clinical psychology doctoral students or PhD staff administered a comprehensive neurocognitive battery assessing multiple domains of cognitive functioning. IQ data were acquired for all 22q11DS patients and controls using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999) or the Wechsler Adult Intelligence Scale (WAIS-IV, Wechsler 2008).
Behavioral data Analysis
Stop-signal task data were analyzed as has been previously described (Congdon et al. 2010, 2012) and as detailed in the Supplementary Materials. Briefly, SSRT was estimated using the quantile method (Band et al. 2003), with longer SSRT values reflecting poorer inhibitory control. Additional performance measures included mean and standard deviation of reaction time (RT) on Go trials, percent inhibition on Stop trials, and percent correct on Go trials.
To examine differences between the 2 groups (22q11DS vs. controls) with regard to demographics and trait impulsivity, we conducted independent t-tests for continuous variables or χ2 tests for categorical variables. Pearson correlations were conducted to assess the relationship between demographics and Stop-signal task performance. To examine group differences with regard to Stop-signal task performance, we then conducted ANCOVAs for each behavioral measure, with group as a fixed factor, and any demographic measure that showed a significant relationship with Stop-signal performance included as a covariate. All analyses were conducted using SPSS software v. 21 (IBM).
fMRI data Analysis
Analyses were performed using tools from the FMRIB software library (www.fmrib.ox.ac.uk/fsl), version 5.0 (Smith et al. 2004), and preprocessing steps are outlined in Supplementary Materials. For each subject, Stop Successful-Go, Go-Null, and Stop Unsuccessful-Stop Successful contrasts were computed, and the output from the subject-specific analyses was then analyzed using a mixed-effects model with FLAME for between-group comparisons. Group-level statistics images were thresholded with a cluster-forming threshold of z > 2.3 and a cluster probability of P < 0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory.
Follow-up analyses were conducted in order to assess the relationship between neural activity within predefined regions of interest (ROIs) and symptoms of impulsivity within 22q11DS patients. We conducted this analysis within the patient group only as the range of scores was fairly restricted within the subset of controls that completed the measure. We focused on the BIS Cognitive Impulsivity subscore (for details, see Reise et al. 2013) given that 1) the Cognitive Impulsivity subscale captures difficulties in attentional control, and 2) ADHD diagnoses in 22q11DS are primarily of the inattentive subtype (Niklasson et al. 2001; Antshel et al. 2007). Mean percent signal change was extracted from 8 predefined ROIs, selected based on visual inspection of the group contrast and previous literature (Aron and Poldrack 2006; Aron et al. 2007; Congdon et al. 2010; Swick, Ashley and Turken 2011; Bari and Robbins 2013), which included the right inferior frontal gyrus (triangularis and opercularis, separately), right and left middle frontal gyrus, right caudate, right and left thalamus, and right putamen. ROIs were defined using the FSL Harvard-Oxford probabilistic atlas (thresholded at 25%); we then intersected these anatomically defined masks with our group-level Stop Successful-Go contrast in order to isolate voxels within anatomically defined regions that were significantly active during RI. These anatomically defined ROIs were then used to extract average percent signal change values corresponding to a 1-s stimulus convolved with a double-gamma HRF from the Stop Successful-Go contrast in 22q11DS patients alone (following Mumford and Poldrack 2007). Residuals were calculated by regressing percent signal change and Cognitive Impulsivity values on age and gender. Using SPSS, percent signal change residuals were then correlated with Cognitive Impulsivity residuals. Given the exploratory nature of these analyses, we did not correct for multiple comparisons. Finally, we conducted a follow-up analysis to rule out the effect of education on fMRI results given the difference in education between groups (see Supplementary Materials).
RESULTS
Behavioral Results
Demographic and Clinical Characteristics
As shown in Table 1, control and 22q11DS groups were matched on all demographic factors except for participant education and IQ. Among the 22q11DS patients, 33% (5 of 15) had a diagnosis of ADHD.
Demographic Associations with Task Performance
For the 2 groups combined, there was a significant relationship between percent correct on Go trials and age (r = 0.38, P = 0.01), with older participants having higher accuracy than younger participants. There was also a negative relationship between SSRT and years of education (r = −0.42, P = 0.005) and a positive relationship between percent correct on Go trials and years of education (r = 0.62, P < 0.001), such that those with higher levels of education had shorter SSRT values (indicating better inhibitory control) and higher accuracy on Go trials. There were no significant differences between gender and task performance (all P’s ≥ 0.59) or ethnicity and task performance (all P’s ≥ 0.11).
Between-group Comparisons of Stop-Signal Task Performance
As shown in Table 2, there were no significant differences in SSRT or percent total inhibition on Stop trials, after controlling for age and education level, suggesting comparable RI performance between groups. However, there were significant differences in median RT on Go trials and percent correct on Go trials, with controls showing faster RT and higher accuracy on Go trials when compared with 22q11DS patients.
Table 2.
Behavioral Performance on Stop Signal task: 22q11DS participants and healthy controls
| Stop signal fMRI task: Behavioral results | 22q11.2 Participants (N = 15) | Control participants (N = 30) | F | P |
|---|---|---|---|---|
| Median Go RT (ms, ±SD) | 567.0 (169.0) | 440.8 (81.3) | 7.2 | 0.01 |
| Go Trials: % Correct (%, ±SD) | 74.2% (6.3) | 92.5% (11.4) | 15.3 | 0.0003 |
| Stop Trials: % Inhibition (%, ±SD) | 52.1% (12.4) | 49.6% (10.2) | 1.1 | 0.30 |
| SSRT RT (ms, ± SD) | 211.60 (81.6) | 167.9 (71.7) | 0.0 | 0.99 |
Note: As age and level of participant education were associated with task performance, we covaried for these demographic variables in group comparisons of task performance variables.
Between-group Comparisons of Barratt Impulsiveness Scale: Cognitive Impulsivity
As shown in Table 1, there were significant differences in Cognitive Impulsivity between groups, with 22q11DS patients showing significantly elevated Cognitive Impulsivity scores relative to controls, even after controlling for age and years of education.
fMRI Results
Successful Stopping
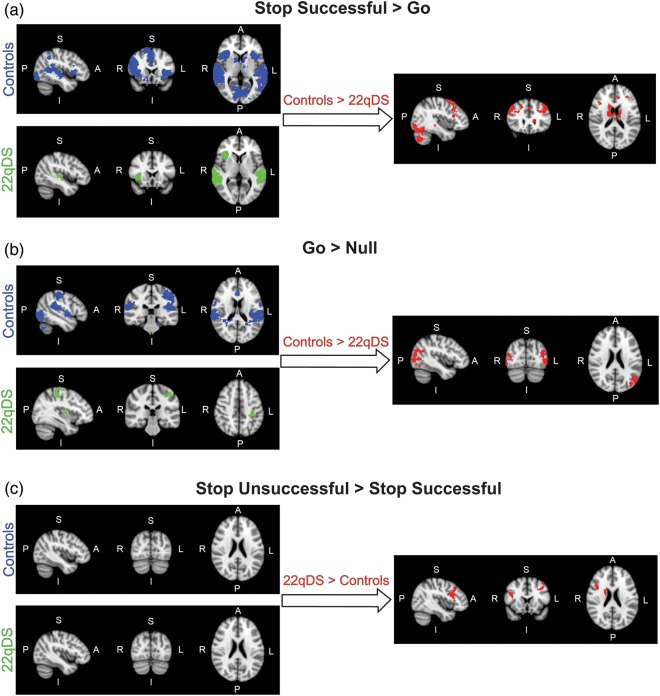
Activation was seen in a broad set of brain regions during successful RI in our sample of healthy controls (Stop Successful-Go contrast), consistent with previous studies (e.g. Aron and Poldrack 2006; Congdon et al. 2010). As illustrated in Table 3 and Figure 1a, activation was seen in bilateral inferior and middle frontal gyri, superior frontal gyrus, middle temporal gyrus, insula, putamen, caudate, thalamus, and occipital cortex. RI-related activation in 22q11DS patients (Stop Successful-Go contrast) was seen in the bilateral middle temporal gyrus and right insula. A direct comparison between controls and 22q11DS patients for the Stop Successful-Go contrast revealed significantly increased activation in controls relative to 22q11DS patients in bilateral inferior and middle frontal gyri, superior frontal gyrus, putamen, caudate, thalamus, occipital cortex, and right middle temporal gyrus. In contrast, there were no regions showing greater activation for 22q11DS patients when compared with controls for successful RI.
Table 3.
Regions of activation for healthy controls and 22q11DS patients during successful stopping, response initiation, and inhibitory failure (Stop Successful-Go, Go-Null, and Stop Unsuccessful-Stop Successful contrasts, respectively)
| Trial type | Contrast | Region | Voxel # | Max Z-Score | Max X (mm) | Max Y (mm) | Max Z (mm) |
|---|---|---|---|---|---|---|---|
| Successful Stopping | |||||||
| Controls | |||||||
| Paracingulate gyrus, Right inferior/middle/superior frontal gyrus, angular gyrus, intraparietal sulcus | 14 827 | 6.45 | 66 | −32 | 2 | ||
| Left insular cortex, orbitofrontal cortex, inferior frontal gyrus, middle/superior temporal gyrus, caudate, pallidum, accumbens, putamen | 6231 | 5.5 | −66 | −26 | 6 | ||
| Bilateral occipital cortex | 4878 | 4.8 | 16 | −96 | −6 | ||
| Right orbitofrontal cortex, pallidum, caudate, accumbens, putamen | 600 | 3.77 | 10 | 6 | 4 | ||
| Posterior cingulate cortex | 426 | 3.66 | 18 | −30 | −4 | ||
| 22q11DS Patients | |||||||
| Right middle/superior temporal gyrus, supramarginal gyrus, angular gyrus | 2372 | 4.58 | 60 | −30 | 0 | ||
| Left middle/superior temporal gyrus, supramarginal gyrus | 1609 | 4.34 | −56 | −36 | 8 | ||
| Right insular cortex, frontal operculum, frontal orbital cortex, inferior frontal gyrus (pars triangularis) | 509 | 4.13 | 36 | 16 | −2 | ||
| Controls > 22q11DS Patients | |||||||
| Right frontal pole, superior/middle/inferior frontal gyrus, anterior cingulate gyrus | 2419 | 5.07 | 16 | 12 | 64 | ||
| Posterior cingulate gyrus, bilateral caudate, putamen, thalamus, pallidum, left accumbens | 1951 | 4.09 | 8 | −2 | 12 | ||
| Left occipital cortex, inferior/middle temporal gyrus, lingual gyrus | 1665 | 3.91 | −8 | −80 | −40 | ||
| Left middle/superior frontal gyrus, frontal pole | 1173 | 4.26 | −40 | 24 | 44 | ||
| Right occipital cortex, inferior temporal gyrus, | 1132 | 4.47 | 40 | −68 | −44 | ||
| Precuneus, cuneus, occipital cortex | 898 | 4.27 | 10 | −76 | 52 | ||
| Right middle/superior temporal gyrus | 683 | 4.44 | 54 | −26 | −8 | ||
| Left occipital cortex, angular gyrus, supramarginal gyrus, superior parietal lobule | 587 | 5.37 | −46 | −50 | 46 | ||
| Go Process | |||||||
| Controls | |||||||
| Left temporal-occipital fusiform gyrus, insular cortex, central opercular cortex | 9441 | 5.46 | 14 | −64 | −48 | ||
| Right superior temporal gyrus, supramarginal gyrus, parietal operculum cortex | 1949 | 5.57 | 68 | −32 | 18 | ||
| Anterior cingulate cortex, supplementary motor cortex | 1212 | 3.99 | 0 | 6 | 38 | ||
| Right middle temporal gyrus, occipital cortex, angular gyrus | 1107 | 5.66 | 56 | −66 | 6 | ||
| Left occipital cortex | 990 | 4.74 | −48 | −70 | −8 | ||
| Left putamen, pallidum, thalamus | 470 | 3.98 | −28 | −4 | −10 | ||
| 22q11DS Patients | |||||||
| Left precentral gyrus, postcentral gyrus, superior frontal gyrus, supramarginal gyrus | 582 | 3.93 | −46 | −20 | 60 | ||
| Left thalamus, putamen, pallidum | 401 | 3.28 | −12 | −18 | 10 | ||
| Controls > 22q11DS Patients | |||||||
| Left occipital cortex, middle temporal gyrus, angular gyrus | 1007 | 4.19 | −40 | −84 | 22 | ||
| Right occipital cortex, middle temporal gyrus | 987 | 3.92 | 56 | −66 | 6 | ||
| Inhibitory Failure | |||||||
| 22q11DS Patients > Controls | |||||||
| Right inferior/middle frontal gyrus | 514 | 3.74 | 36 | 24 | 32 | ||
| Left inferior/middle frontal gyrus | 357 | 3.58 | −38 | 16 | 40 | ||
| Right thalamus, caudate, putamen | 352 | 3.42 | 8 | −4 | 14 | ||
Note: Coordinates for location of maximum activation of significant clusters in MNI space.
Figure 1.
fMRI activity maps during performance of the Stop-signal task. Blue maps represent control activity, green maps represent 22q11DS patient activity, and red maps represent the between-group contrast of Controls > 22q11DS patients. Brain orientations are labeled such that S = superior, I = inferior, P = posterior, and A = anterior; R = right and L = left. (a) Activation maps represent the contrast of Stop Successful-Go, to investigate activity related to successful stopping. (b) Activation maps represent the contrast of Go-Null, to investigate activity related to response initiation. (c) Activation maps represent the contrast of Stop Unsuccessful-Stop Successful, to investigate activity related to inhibition failure.
Response Initiation
When responding to Go stimuli (Go-Null contrast), healthy controls engaged a broad set of regions, consistent with previous studies (Aron and Poldrack 2006; Congdon et al. 2010), with activation seen in the bilateral middle temporal gyrus, temporal-occipital cortex, anterior cingulate cortex, and left primary motor cortex (Fig. 1b, Table 3). In patients, the Go process (Go-Null contrast) was associated with activation in the right cerebellum, left primary motor cortex and right putamen. A direct group comparison for the Go-Null contrast revealed greater activation in the bilateral occipital cortex and left parieto-occipital cortex in controls relative to 22q11DS patients. Again, there were no regions showing greater activation for 22q11DS patients compared with controls for the response initiation contrast.
Inhibitory Failure
There were no regions showing significant activation for the Stop Unsuccessful-Stop Successful contrast (i.e., activation associated with inhibitory failure) in either healthy controls or 22q11DS patients alone. A direct group comparison for the Stop Unsuccessful-Stop Successful contrast revealed greater activation in 22q11DS patients relative to controls in left middle frontal gyrus, right inferior and middle frontal gyrus, and right caudate and thalamus (Fig. 1c, Table 3). There were no regions showing greater activation for controls when compared with 22q11DS patients for inhibitory failure.
Relationship Between Inhibition-Related Activation and Cognitive Impulsivity
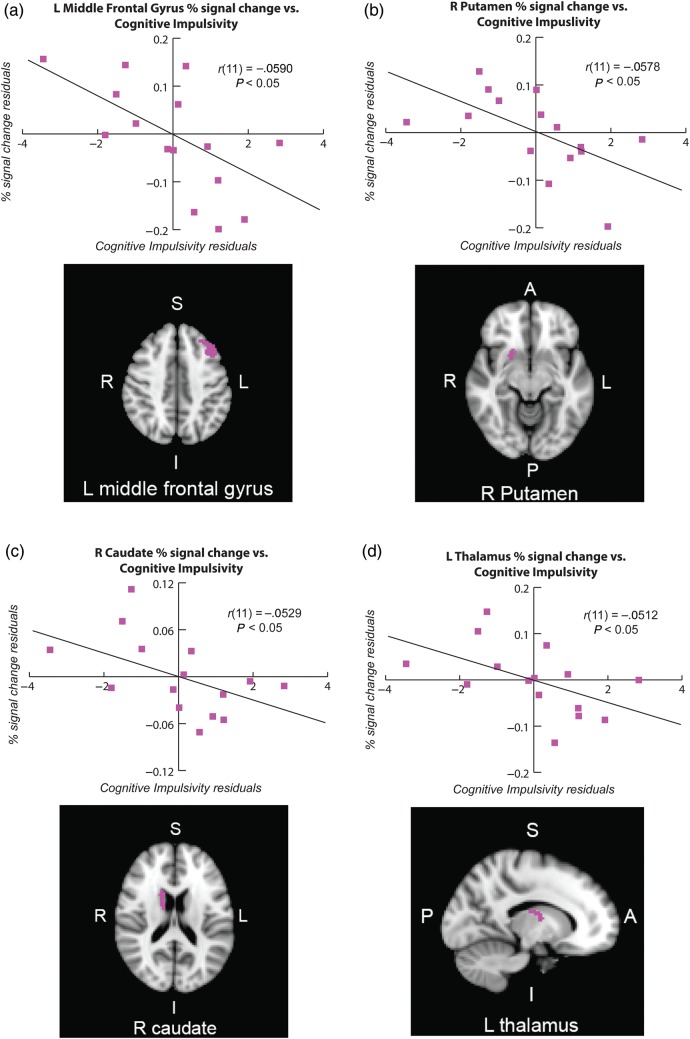
To determine whether Cognitive Impulsivity is associated with RI-related activation, we tested the correlation between the Cognitive Impulsivity subscore from the revised 2-factor scoring of the BIS-11 (Reise et al. 2013) and percent signal change from our set of anatomically defined ROIs, controlling for the effects of age and gender (Fig. 2). Our ROIs included the right inferior frontal gyrus (triangularis), right inferior frontal gyrus (operculum), bilateral middle frontal gyrus, right caudate, bilateral thalamus, and right putamen.
Figure 2.
Relationship between response inhibition-related neural activity and cognitive impulsivity in 22q11DS. The X-axis values represent the residuals of the Cognitive Impulsivity subscore of the Barratt Impulsivity Scale and the Y-axis values represent the residuals for percent signal change during Stop Successful-Go within the following anatomically defined ROIs (after adjusting for age and years of education), which are displayed next to the corresponding plot: (a) left middle frontal gyrus, (b) right putamen, (c) right caudate, and (d) left thalamus. In the partial regression, the residuals (or errors of prediction) represent the parts of our variables of interest that are not predicted by age or gender. Brain orientations are labeled such that S = superior, I = inferior, P = posterior, and A = anterior; R = right, and L = left.
Cognitive impulsivity was significantly associated with activation in 4 of these ROIs. Left middle frontal activation was significantly negatively correlated with Cognitive Impulsivity: r(11) = −0.590, P = 0.017, with a relationship strength, as indexed by η2, of 0.35 (Fig. 2a). Activation in the right putamen was significantly negatively correlated with Cognitive Impulsivity: r(11) = −0.578, P = 0.019, η2 = 0.33 (Fig. 2b). Activation in the right caudate was significantly negatively correlated with Cognitive Impulsivity: r(11) = −0.529, P = 0.032, η2 = 0.28 (Fig. 2c). Activation in the left thalamus was significantly negatively correlated with Cognitive Impulsivity: r(11) = −0.512, P = 0.037, η2 = 0.26 (Fig. 2d). Thus, activity in left middle frontal gyrus, right putamen, right caudate, and left thalamus during RI was significantly inversely correlated with Cognitive Impulsivity in 22q11DS patients.
Discussion
The present study was the first, to our knowledge, to investigate Stop-signal performance in adults with 22q11.2 deletion syndrome, a recurrent genetic mutation associated with substantially elevated rates of ADHD, and high rates of behavioral dysregulation, impulsive behavior, and distractibility (Baker and Vorstman 2012). During RI, patients with 22q11DS failed to show significant activity within brain regions typically associated with successful inhibition on the Stop-signal task, such as inferior frontal and basal ganglia regions. Additionally, exploratory analyses revealed that neural activity in the left middle frontal gyrus, left thalamus, and right striatum was negatively correlated with self-reported Cognitive Impulsivity symptoms in 22q11DS patients, as measured by the BIS. Taken together, these findings suggest a pattern of reduced neural engagement during RI in 22q11DS, and that this dysfunction associated with real-world cognitive impulsivity. These findings provide initial evidence suggesting a neurobiological substrate for inhibitory control deficits in 22q11DS.
Successful Response Inhibition
Consistent with previous reports in healthy adults (Garavan et al. 1999; Braver et al. 2001; Aron and Poldrack 2006; Aron et al. 2007), here we found that RI was associated with activation in bilateral inferior and middle frontal gyri, insula, parietal cortex, and basal ganglia regions. Our sample of healthy controls thus serves as a valid comparison group, to which we can compare our sample of 22q11DS patients in order to examine potential deficits in inhibition-related neural activation.
In contrast to the healthy adults in our sample, when inhibition of a motor response was required, 22q11DS patients only showed significant activation in the bilateral middle temporal gyrus and right insula. Activation in the middle temporal gyrus is expected, given the binaural presentation of the Stop-signal tone (Aron and Poldrack 2006), and was also seen in controls. Similarly, activation in the right insula, a region commonly engaged during RI, was expected and consistent with activation seen in controls. Direct group comparisons confirmed that 22q11DS patients show significantly less engagement of regions typically active during RI (i.e. bilateral inferior and middle frontal gyrus, and subcortical structures including caudate, putamen and thalamus), relative to controls, despite performance being calibrated on these trials such that both groups successfully inhibit responses 50% of the time.
RI-associated neural abnormalities have been reported in multiple clinical populations with impulse control difficulties, particularly those with ADHD. Indeed, a fronto-striatal deficit hypothesis of ADHD has been proposed based on the number of studies consistently showing reduced activation in frontal and basal ganglia regions implicated in inhibitory control processes in children and adolescents with ADHD (Durston et al. 2003; Schulz et al. 2004; Tamm et al. 2004; Dickstein et al. 2006; Epstein et al. 2007). Although fewer studies have investigated RI in adults with ADHD, a recent meta-analysis (Hart et al. 2013) and a comprehensive review by Cubillo et al. (2012) converge on a pattern of reduced activation in the inferior frontal cortex and insula, cingulate, and striatal regions during RI in adults with ADHD when compared with healthy controls. There is, in addition, some evidence for more diffuse-immature activation of the brain in children with ADHD relative to typically developing controls during RI, which is manifested in over-activation of other brain regions (Ma et al. 2012). We did not observe such a pattern in our sample, which is consistent with the interpretation that this is a marker of developmental delay, which eventually normalizes (Brydges et al. 2013). However, we cannot rule out the possibility that we did not observe such over-activation due to limited power. Overall, our findings of reduced RI-related activation in 22q11DS adults during task performance converge remarkably well with findings in children and adults with idiopathic ADHD, suggesting a shared underlying neural substrate for RI deficits. However, future investigations comparing patients diagnosed with idiopathic ADHD directly to 22q11DS patients with and without a diagnosis of ADHD are needed to directly test this.
To our knowledge, there has been only one prior fMRI investigation of RI in 22q11DS patients. Specifically, Gothelf et al. (2007) examined neural activation during performance of a Go/No-Go task in an adolescent sample of 22q11DS patients, when compared with typically developing controls and controls with developmental disability. Using a block design, these authors examined activation during Go/NoGo when compared with Go conditions, revealing greater left parietal activation in the 22q11DS group when compared with both typically developing and developmentally disabled controls. The authors interpreted these findings as consistent with a pattern of compensatory activation, in which 22q11DS patients compensate for executive dysfunction via increased recruitment of parietal regions. In contrast, using an event-related fMRI design of the Stop-signal task, we report here less activation in stopping-related brain regions in a sample of 22q11DS adults when compared with controls. In addition, while we found decreased activation in RI-related regions in 22q11DS patients relative to controls for the inhibition of a motor response, we found increased activation in 22q11DS patients compared with controls in several regions when failing to inhibit a motor response. We do not consider these findings inconsistent with those of Gothelf et al. (2007), since the Stop-signal design allows for the isolation of the each of these processes, as opposed to combining activity across these conditions in the Go/No-Go task. Other methodological issues, including differences in task demands and the age of the study participants, may also account for differences in our findings.
While the broader literature of RI-related neural activation in other neurogenetic syndromes is limited, one fMRI investigation of RI found reduced activity in cortical and subcortical structures in Williams Syndrome individuals compared with age and gender-matched typically developing control subjects, supporting the pattern of reduced neural engagement found in our 22q11DS sample (Mobbs et al. 2007). Similarly, adolescent males with Fragile X Syndrome showed reduced activation in prefrontal and basal ganglia regions compared with controls during RI using a Go/No-Go task (Hoeft et al. 2007). In conclusion, the pattern of activation differences in 22q11DS patients relative to controls depends on a number of factors, and a complete understanding of neural deficits that contribute to inhibitory control deficits associated with these disorders of distinct genetic origin require additional studies in larger samples. In particular, translational studies in which RI deficits are compared across transgenic mouse models of these disorders could provide novel insights into the underlying molecular mechanisms.
Response Initiation
Consistent with previous research (Mink 1996; Liddle et al. 2001; Mostofsk et al. 2003; Aron and Poldrack 2006), the “Go” network identified in our sample of healthy adults implicates the fronto-striatal-pallidal pathway in response initiation. When compared with controls, 22q11DS patients showed less activity in the bilateral occipital cortex and left parieto-occipital cortex when initiating a motor response. This difference may reflect decreased neural activity during visual processing of the arrow stimulus and the arrow direction for 22q11DS patients when compared with controls (Haxby et al. 1991; Malach et al. 1995), an interpretation that is supported by behavioral performance data on the Stop-signal task, with patients showing poorer performance on Go trials compared with controls.
Inhibitory Failure
In contrast to the pattern observed for RI and initiation processes, 22q11DS patients showed greater activation within bilateral frontal gyri, the right caudate and thalamus, when failing to inhibit a motor response, when compared with controls. This contrast indexes activation during trials where the inhibition process was initiated, but unsuccessful, and therefore encompasses activation associated with the RI process, as well as activation associated with error processing, conflict detection, and response initiation. As activation in 22q11DS patients was seen in regions that are part of a RI-related network, as opposed to a medial frontal error processing network (e.g., Ridderinkhof et al. 2004), our results suggest a delayed or ineffectual inhibition process in the patients, which is reflected by increased activation during failed, when compared with successful, trials—when compared directly to the pattern of activation observed in healthy controls.
Summary of fMRI Findings
Our findings of altered neural activity in 22q11DS patients compared with healthy individuals during RI, response initiation, and when failing to inhibit a motor response provides evidence for an overall impairment in executive function processes, which may be related to common neurobiological mechanisms. An initiated response is suggested to involve the fronto-striatal-pallidal “direct pathway,” whereas when inhibiting a response this pathway is blocked via discharges through the “hyperdirect” fronto-subthalamic pathway, or through the “indirect” fronto-striatal-palladal-subthalamic pathway (Alexander and Crutcher 1990; Mink 1996; Nambu et al. 2002). The Stop-signal task allows for the isolation of activity related to these cognitive components of RI.
The absence of behavioral differences in our primary measure of RI (SSRT) led us to conclude that stopping performance was comparable between 22q11DS patients and controls, suggesting that the reported difference in fMRI activation between groups was due specifically to engagement of stopping-related brain regions, as opposed to potential confounds of task-associated differences (including differences in difficulty experienced or number of trials). In addition, a follow-up analysis showed that the fMRI findings were not accounted for by group differences in education. Furthermore, to confirm that differences in brain activation during performance of Go trials were not driving the observed differences in successful inhibition, we examined group differences for the Go-Null contrast, and found that there were no differences between groups for this contrast in any stopping-related region. Overall, our results suggest that at least 2 pathways in this common fronto-basal ganglia loop are compromised during different executive function processes, response initiation and RI, in 22q11DS patients.
RI-Related Activity and Self-Reported Cognitive Impulsivity
The BIS-11 was originally designed as a measure of total trait impulsivity (Patton et al. 1995), with impulsivity defined as the predisposition to respond to internal and external events without regard to the potential consequences (Moeller et al. 2001). The original questionnaire was constructed to assess a global score, in addition to 3 subscales of impulsivity: cognitive, motor, and nonplanning. However, following exploratory and confirmatory factor analyses in a large community sample, a revised factor structure has been recently proposed, in which this multidimensional trait is represented as 2 factors, Cognitive Impulsivity and Behavioral Impulsivity (Reise et al. 2013). In our present investigation, we chose to focus on the revised Cognitive Impulsivity subscore, given previous literature on the phenotypic expression of ADHD in 22q11DS, which suggests that ADHD symptomatology in 22q11DS patients is characterized by inattention, rather than hyperactive or impulsive symptoms (Niklasson et al. 2001; Antshel et al. 2007).
Our preliminary findings of an association between RI-related activation and trait impulsivity, as measured with the Cognitive Impulsivity sub-scale of the BIS-11, suggest that reduced engagement of neural circuitry relevant to RI has potential clinical significance for 22q11DS patients. While the BIS is a self-report measure that reflects the subjective view that an individual possesses of his/her own behavior, and is therefore not equivalent to a clinical diagnosis of ADHD, elevated scores on this measure correlate strongly with clinical diagnoses of ADHD (Malloy-Diniz et al. 2007).
Genetics and Impulsivity in 22q11DS
Elevated levels of impulsivity, inattention, and distractibility in 22q11DS may be related to underlying neurotransmitter dysfunction in these patients (Fallgatter and Lesch 2007). Notably, the deleted region includes the catechol-O-methyltransferase (COMT) gene, which codes for an enzyme important for prefrontal cortical dopamine metabolism (Lachman et al. 1996; Egan et al. 2001). Previous research suggests that 22q11DS patients exhibit abnormal neural recruitment of attention- and inhibition-related brain regions depending on COMT genotype (Gothelf et al. 2007), and dopaminergic function has been implicated in trait impulsivity in the general population (Cools et al. 2007; Cole et al. 2012). A recent study in healthy adults found that a functional polymorphism of the COMT gene (i.e. Met homozygosity at the Val158Met locus) was associated with impulsivity related to non-planning (Soeiro-De-Souza et al. 2013). Taken together, these findings suggest that dopaminergic dysfunction resulting from COMT haploinsufficiency may be related to behavioral manifestations of inattention and impulsivity in patients with 22q11DS (Shashi et al. 2006). Given the sample size, the current study was under-powered for genetic analyses, but this is an important area warranting further study in larger, consortium-based samples. Given its known genetic etiology this syndrome provides an ideal model for the investigation of genes related to cognitive impulsivity.
Implications
Impulsivity is a multidimensional construct that characterizes multiple psychiatric disorders and has serious functional consequences (Swann et al. 2005). The findings reported here are broadly relevant for the study of disorders involving impulsive behavior, including ADHD, addiction, and personality disorders. First, we utilized a dimensional approach to study trait impulsivity, rather than a categorical ADHD diagnosis, which allows us to capitalize on the full range of variation as opposed to focusing on a subset of impaired patients. This approach is in agreement with the RDoC initiative, which aims to promote innovative methods for characterizing psychopathology based on dimensions of observable behaviors or neurobiological measures rather than traditional diagnostic systems. Indeed, previous research using maternal ratings of attention problems and neurobiological data from children with symptoms of hyperactivity and impulsivity suggests that ADHD diagnoses are better characterized from a dimensional view (Shaw et al. 2011; Lubke et al. 2009). Second, given that 22q11DS patients exhibit deficits primarily related to the domain of Cognitive Impulsivity, we focused our analysis on this particular dimension, as opposed to the behavioral/motor impulsivity features. A similar approach can be used for studying other populations exhibiting impulsive behaviors, with a more targeted investigation of the specific symptom dimension of interest. Finally, this study sought to characterize the neurobiology of inhibition, with the goal of investigating RI-related neural activity as a potential endophenotype for disorders involving impulsivity. The identification of inhibition as an endophenotype for impulsivity can also be applied to investigations in animal models of 22q11DS, in order to better understand the contribution of genetics, neurotransmitter function, and neural function on impulsivity across species.
Limitations
The primary limitation of our study is the small sample size. Although 22q11DS is the most common microdeletion syndrome, it is estimated to occur in 1 in 4000 to 6000 live births, rendering a substantial challenge for subject ascertainment at a single site. As such, although our sample size is large relative to the existing literature examining neural circuitry in 22q11DS, our results should be considered preliminary until replicated. Second, given the lack of prior evidence regarding relationships between trait impulsivity measures and RI-related activation in this population, we tested our hypothesis in a selection of 8 anatomically defined ROIs, known to play a role in RI, and did not correct for multiple comparisons. Although the relationship across activation in ROIs associated with trait impulsivity is consistent across ROIs, and in line with our group difference results, caution is warranted in drawing conclusions based on the uncorrected nature of these results, which require replication. Finally, 22q11DS patients differed in overall cognitive abilities from healthy controls; however, Stop-signal task difficulty level was equated across subjects, making it ideal for use in a clinical population (Aron and Poldrack 2005).
Conclusions
We examined the neural correlates of RI in a sample of 22q11DS adults in order to avoid age-related confounds associated with the development of inhibitory control and associated neural activation (Hart et al. 2013). Relative to healthy adults, we found that during RI patients with 22q11DS showed reduced engagement of fronto-striatal regions typically involved in stopping a behavioral response. Furthermore, in exploratory analyses, we report the novel association between reduced activation in key RI-related regions and increased trait Cognitive Impulsivity in 22q11DS. Our preliminary findings provide a characterization of neural abnormalities in 22q11DS during RI, and suggest RI as a potentially valuable endophenotype for identifying impulse-related dysfunction in 22q11DS.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This manuscript was supported by grants from the NIH: RO1 MH085953 (C.E.B.), grant P50-HD-055784 (Pilot Project Grant to C.E.B.), 5T32MH073526-05 (Postdoctoral Training Program Fellowship given to C.A.M.), 5T32MH073526-05 (Neurogenetics Training Grant given to M.J.), and UL1-DE019580 and PL1MH083271 (NIH Roadmap grants to R.M.B.). These funding sources had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Supplementary Material
Notes
We thank the participants and their families for being a part of our research. We thank Therese K. Vesagas for assistance with figure illustrations. We also thank Ms Chelsea Gilbert, Dr Sarah Marvin, and Dr Laurie Brenner, who assisted in conducting clinical assessments and administering neuropsychological measures to our participants; Wen-Ching Tran, who assisted in data management and scoring; and Angelica Bato who assisted with fMRI data collection. Conflict of Interest: None declared.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Kates WR. The neurocognitive phenotype in velo-cardio-facial syndrome: a developmental perspective. Dev Disabil Res Rev. 2008;14:43–51. doi: 10.1002/ddrr.7. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Stephen VF, Fremont W, Monuteaux MC, Kates WR, Doyle A, Mick E, Biederman J. Comparing ADHD in velocardiofacial syndrome to idiopathic ADHD: a preliminary study. J Atten Disord. 2007;11(1):64–73. doi: 10.1177/1087054707299397. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Vorstman JA. Is there a core neuropsychiatric phenotype in 22q11. 2 deletion syndrome? Curr Opin Neurol. 2012;25(2):131–137. doi: 10.1097/WCO.0b013e328352dd58. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop signal procedure. Acta Psychol (Amst) 2003;112(2):105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Mos E, McDonald-McGinn D, Zackai E, Cannon TD. The neurocognitive phenotype of the 22q11. 2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brydges CR, Anderson M, Reid CL, Fox AM. Maturation of cognitive control: delineating response inhibition and interference suppression. PLoS One. 2013;8(7):e69826. doi: 10.1371/journal.pone.0069826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, Beckmann CF. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2012;23(7):1509–1516. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53(2):653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Front Psychol. 2012;3(37):1–10. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27(20):5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Veltman DJ, van Emmerik-van Oortmerssen K, Booij J, van den Brink W. Impulsivity in adult ADHD patients with and without cocaine dependence. Drug Alcohol Depend. 2013;129:18–24. doi: 10.1016/j.drugalcdep.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48(2):194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Watts R, Ulug AM, Casey BJ. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20(4):2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Ogloff JRP, Bradshaw JL. Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Pers Indiv Differ. 2006;41(2):285–294. [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, et al. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry. 2007;48(9):899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Lesch KP. 22q11. 2 deletion syndrome as a natural model for COMT haploinsufficiency-related dopaminergic dysfunction in ADHD. Int J Neuropsychopharmacol. 2007;10(3):295. doi: 10.1017/S1461145706006985. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American. Psychiatric Publishing, Incorporated. 1997 [Google Scholar]
- Furniss F, Biswas AB, Gumber R, Singh N. Cognitive phenotype of velocardiofacial syndrome: a review. Res Dev Disabil. 2011;32:2206–2213. doi: 10.1016/j.ridd.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Garavan HH, Ross TJT, Stein EAE. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Hinard C, Hallmayer JF, Van Dover Stoecker J, Antonarakis SE, Morris MA, Reiss AL. Abnormal cortical activation during response inhibition in 22q11.2 deletion syndrome. Hum Brain Mapp. 2007;28(6):533–542. doi: 10.1002/hbm.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Weizman A, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11. 2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Guyot L, Dubuc M, Pujol J, Dutour O, Philip N. Craniofacial anthropometric analysis in patients with 22q11 microdeletion. Am J Med Genet. 2001;100(1):1–8. doi: 10.1002/1096-8628(20010415)100:1<1::aid-ajmg1206>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA. 1991;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JC, Van Amelsvoort T, Morris RG, Owen MJ, Murphy DGM, Murphy KC. An investigation of the neuropsychological profile in adults with velo-cardio-facial syndrome (VCFS) Neuropsychologia. 2002;40(5):471–478. doi: 10.1016/s0028-3932(01)00136-1. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp. 2007;28(6):543–554. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, Goldberg R, Kucherlapati R, Papolos DF. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67(5):468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychol Sci. 1997;8:60–64. [Google Scholar]
- Lubke GH, Hudziak JJ, Derks EM, van Bijsterveldt TC, Boomsma DI. Maternal ratings of attention problems in ADHD: evidence for the existence of a continuum. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1085–1093. doi: 10.1097/CHI.0b013e3181ba3dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Lei D, Jin X, Du X, Jiang F, Li F, Zhang Y, Shen X. Compensatory brain activation in children with attention deficit/hyperactivity disorder during a simplified Go/No-Go task. J Neural Transm. 2012;119(5):613–619. doi: 10.1007/s00702-011-0744-0. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92(18):8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13(04):693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, Galaburda AM, Reiss AL. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biol Psychiatry. 2007;62(3):256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Mostofsk SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Cogn Brain Res. 2003;17(2):419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Poldrack RA. Modeling group fMRI data. Soc Cogn Affect Neurosci. 2007;2:251–257. doi: 10.1093/scan/nsm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Niklasson LL, Rasmussen PP, Oskarsdóttir SS, Gillberg CC. Neuropsychiatric disorders in the 22q11 deletion syndrome. Genet Med. 2001;3:79–84. doi: 10.1097/00125817-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale-11: reassessment of Its Structure in a Community Sample. Psychol Assess. 2013;25(2):631–642. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Sci Signal. 2004;306(5695):443. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. Am J Med Genet. 1997;34(10):798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum Mol Gen. 2000;9(16):2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. Am J Psychiatry. 2004;161(9):1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Shashi V, Keshavan MS, Howard TD, Berry MN, Basehore MJ, Lewandowski E, Kwapil TR. Cognitive correlates of a functional COMT polymorphism in children with 22q11. 2 deletion syndrome. Clin Genet. 2006;69(3):234–238. doi: 10.1111/j.1399-0004.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168(2):143. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Levin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Soeiro-De-Souza MGM, Stanford MSM, Bio DSD, Machado-Vieira RR, Moreno RAR. Association of the COMT Met158 allele with trait impulsivity in healthy young adults. Mol Med Report. 2013;7:1067–1072. doi: 10.3892/mmr.2013.1336. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005;162(9):1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. Am J Med Genet. 1997;34(6):453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(11):1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Congdon E, Poldrack RA, Sabb FW, London ED, Cannon TD, Bilder RM. Women are more sensitive than men to prior trial events on the Stop-signal task. Br J Psychol. 2013 doi: 10.1111/bjop.12034. doi:10.1111/bjop.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WASI manual. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Wechsler . Wechsler Adult Intelligence Scale (WAIS-IV) Pearson Assessment. San Antonio: Pearson; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.