Abstract
Alzheimer’s disease (AD) is the most prevalent form of dementia worldwide and is an emerging global epidemic. It is characterized by an imbalance between production and clearance of amyloid β (Aβ) and tau proteins. Oligomeric forms of Aβ and tau are believed to be the most toxic. Dramatic results from AD animal models showed great promise for active and passive immune therapies targeting Aβ. However, there is very limited evidence in human studies of clinical benefits from these approaches. Immunotherapies targeting only tau pathology have had some success but are limited so far to mouse models. The majority of current methods is based on immunological targeting of a self-protein; hence, benefits needed to be balanced against risks of stimulating excessive auto-immune toxic inflammation. For greater efficacy the next generation of vaccines will need to focus more on concurrently targeting all the intermediate toxic conformers of oligomeric Aβ and tau species.
Keywords: amyloid β, tau, vaccination, immunotherapy, immunomodulation, Alzheimer’s disease, transgenic mice
Introduction
Alzheimer’s disease [AD] affects more than 20 million people worldwide currently, with about 135 million people expected to develop it by 2050. The staggering numbers affected by this global health epidemic translates into significant direct and indirect health care expenses, with direct costs for the USA alone estimated to be about $214 billion in 2014. Historically, AD has been characterized as a neurodegenerative disease chiefly defined by its pathological signature including β amyloid deposits in the form of extracellular amyloid β (Aβ) plaques and tau protein aggregates in the form of intracellular neurofibrillary tangles (NFT) (Nelson et al., 2012). A central mechanism underlying the formation of both amyloid plaques and NFTs in AD is pathogenic cerebral protein aggregation. Though both amyloid plaques and aggregated tau have an essential role in AD pathology and are part of the neuropathological definition of the disease, numerous studies suggest that in these precipitated forms they are relatively biologically inert. Hence, the accumulation of aggregated Aβ in plaques correlates poorly with the clinical status of patients (Nelson et al., 2012; Terry, 1996). Soluble oligomeric forms of Aβ and tau, which may replicate via a “prion-like” mechanism, are thought to be the chief mediators of cytotoxicity in AD (Ashe and Aguzzi, 2013). These oligomeric species of Aβ initially accumulate intraneuronally, eventually leading to cell death and the extracellular deposition of amyloid plaques (D’Andrea et al., 2001; Gouras et al., 2000). Both Aβ and tau oligomers have similar but not identical structural and biophysical properties including a high β-sheet content, some resistance to proteolytic degradation and neuronal toxicity. Recent work has also revealed that Aβ and tau related pathology can, in certain scenarios, “seed” or transmit each other (Ashe and Aguzzi, 2013; Jucker and Walker, 2011). Current existing therapies have either no or minimal disease modifying benefit. Hence, a number of novel therapeutic strategies are currently under investigation, many of which involve modulating the immune system.
Preclinical studies in transgenic mouse models have shown great efficacy of immunotherapy in the prevention of both AD and prion diseases (Wisniewski and Goni, 2012; Wisniewski and Goni, 2014). With a central role for Aβ in AD pathogenesis under the “amyloid cascade’ model, several strategies directed towards eradication of Aβ and downstream targets via small molecules or immunotherapies have been and are being explored (Huang and Mucke, 2012; Morgan, 2011; Ozudogru and Lippa, 2012; Wisniewski and Goni, 2014). Though Aβ directed immunization via multiple approaches has shown promising results in AD Tg mouse models, the translation to safe and efficacious therapy for humans still remains a challenge. Insights from these studies have raised further issues that need to be addressed in current and future studies. Questions that have come up from previous work which are critical for the development of successful immunotherapy include identification of the ideal target and the timing of therapy. What is the best design for a vaccine that is both specific and safe? How could we avoid auto-immune toxicity? Would the ideal approach be active or passive immunization? Is stimulation of the innate immune system a viable option? Can a single vaccine be designed to target both amyloid β (Aβ) and tau related pathology simultaneously? This article reviews current preclinical and clinical data for Aβ and phosphorylated tau reduction immunotherapy and discusses how this information may lead to the next generation of more effective vaccines.
Pathogenesis of Alzheimer’s disease
AD is a complex neurodegenerative disease characterized clinically by a progressive deterioration of memory. Pathologically it is defined by deposition of extracellular Aβ as senile neuritic plaques and congophilic angiopathy (CAA), as well as, intracellular hyperphosphorylated fibrillar tau accumulation in the form of neurofibrillary tangles (NFTs) (Figure 1). Genetic studies have shown that AD is a heterogeneous disorder that includes the early-onset (EOAD) form (<5% of all AD patients, with onset at <65yrs) and the much more common sporadic late-onset form (LOAD, with onset >65yrs). EOAD is related to mutations in presenilin 1, presenilin 2 (PS1 and PS2) or the amyloid precursor protein (APP), when associated with autosomal dominant inheritance (Bertram and Tanzi, 2012; Guerreiro and Hardy, 2014; Karch et al., 2014). Epidemiological data suggests that apparent autosomal dominant transmission is found in only ~10% of all EOAD cases (<1% of all AD cases), leaving the genetic association of the majority of EOAD unexplained (Guerreiro and Hardy, 2014; Wingo et al., 2012). LOAD afflicts >95% of patients with AD and is related to both genetic and environmental factors (Bertram and Tanzi, 2012; Guerreiro and Hardy, 2014; Karch et al., 2014; Kim et al., 2014). Some of the known environmental risk factors for LOAD include level of physical activity, educational status, diabetes mellitus, hypertension and head injury (Beydoun et al., 2014; Di Marco et al., 2014). The strongest identified genetic risk factor for LOAD is the inheritance of the apolipoprotein (apo) E4 allele (Kanekiyo et al., 2014; Potter and Wisniewski, 2012). The role of apoE in AD is complex but includes isotype specific effects on the aggregation and clearance of brain Aβ (Kanekiyo et al., 2014). Recently, rare variants of another gene that encodes the triggering receptor expressed on myeloid cells 2 (TREM2; located on 6p21.1) have been reported as a significant risk factor for LOAD, with an odds ratio similar to apoE4 (Boutajangout and Wisniewski, 2013; Hickman and El, 2014). Several hypotheses have been postulated for the development of plaques and tangles, which lead to synaptic and neuronal loss and subsequent decline of memory and cognition in AD. The most favored theory currently in the field that has served as the platform for many therapeutic strategies is the amyloid cascade hypothesis (Hardy and Selkoe, 2002; Holtzman et al., 2012). The central idea proposed in the hypothesis is that Aβ aggregation, especially in its toxic oligomeric form, is the principal insult which produces neuronal toxicity and triggers downstream signaling events that in turn lead to hyperphosphorylation of tau and development of NFTs (Figure 1). A multitude of “chaperone” proteins have been described that stabilize pathological oligomers and mediate a conformational change of soluble Aβ. These include but are not limited to apolipoprotein E (apo E), especially its E4 isoform, ∝1-antichymotrypsin (ACT) or C1q complement factor (Potter and Wisniewski, 2012). Histological and biochemical evidence suggests that “pathological chaperone” proteins co-localize with fibrillar Aβ deposits but not with preamyloid aggregates that are not linked to neuronal loss. From a biochemical perspective, a seminal event in the development of pathologic aggregates is the point at which a critical concentration of soluble Aβ and/or chaperone proteins is achieved. A critical concentration of the precursor proteins would favor a conformational change, drive formation of toxic Aβ oligomers and subsequent activation of downstream signaling cascades. Mechanisms implicated for achieving this in sporadic AD could be a permutation of impaired clearance of Aβ from the brain as a consequence of aging and/or inflow of serum Aβ into the CNS (Holtzman et al., 2012). Several studies in familial Alzheimer’s disease (FAD) patients and in models of FAD have provided evidence in favor of the amyloid cascade hypothesis. The first line of evidence comes from functional analyses of APP gene or in the PS1 or 2 genes that are associated with inherited forms of AD. Mutations in these genes show concomitant changes in APP processing biased towards over production of soluble Aβ (sAβ) or generation of specific species of sAβ such as Aβ1-42 that are more prone to aggregation (Hardy, 2006). In addition, a rare APP mutation first reported in an Icelandic population which protects against AD is supportive of the amyloid cascade (Jonsson et al., 2012). This mutation (A673T) acts by reducing amyloidogenic processing of APP and also mildly decreasing Aβ peptide aggregation (Maloney et al., 2014). The next line of evidence stems from the association of Down’s syndrome with AD related pathology at a very young age. Here, an extra copy of the APP gene secondary to trisomy 21 provides excellent in vivo gain-of-function evidence supporting the amyloid hypothesis (Hartley et al., 2014). Further, animal models where amyloid β and tau are co-expressed reveal that Aβ deposition predates formation of tau aggregates, supporting the concept that NFT formation is downstream from Aβ aggregation (Gotz et al., 2001; Oddo et al., 2003; Wisniewski and Sigurdsson, 2010). Lastly, enhancement of Aβ clearance in transgenic mouse models with over-expression of mutant APP, but with no tau pathology, has been shown to improve cognitive function in mice (Lemere, 2013; Wisniewski and Sigurdsson, 2010). Subsequent work has also revealed that inhibition of Aβ in animal models with overexpression of mutant APP and tau not only prevents development of tau related aggregates but also improves cognitive deficits (Blurton-Jones and LaFerla, 2006; McKee et al., 2008; Oddo et al., 2006). In contrast to the genetic forms of AD where the role of Aβ is well established, definitive evidence regarding Aβ’s central function in late-onset sporadic AD is more limited. A number of autopsy studies have indicated that medial temporal and subcortical tau pathology precedes Aβ pathology in the majority of patients (Jack, Jr. et al., 2013). Genome-wide association studies (GWAS) in LOAD have implicated a number of different genes involved in innate immunity, cholesterol metabolism and endocytosis, suggesting greater etiological heterogeneity (Karch et al., 2014). Supporting a role of Aβ in LOAD the levels of biochemically extracted Aβ peptides from brains of people with sporadic AD, correlate well with cognitive deficits (Naslund et al., 2000). Further, Aβ peptide dimer/oligomer extracts derived from sporadic AD brains have been shown to disrupt synaptic structure, function and plasticity that are critical cellular correlates of memory (Shankar et al., 2008). Interestingly, exogenous injections of Aβ extracts from sporadic AD patients can induce amyloid aggregates in transgenic mice (Ashe and Aguzzi, 2013; Meyer-Luehmann et al., 2006).
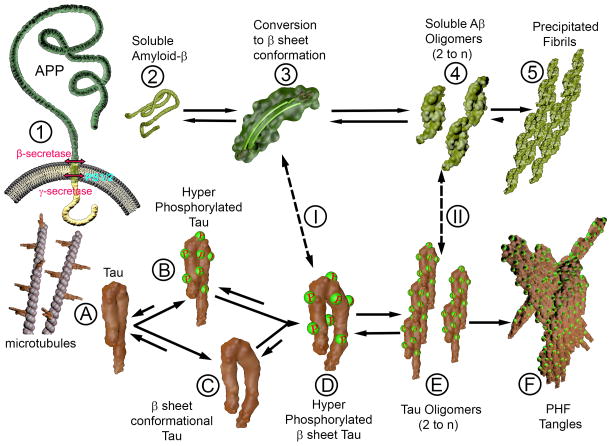
Figure 1. Aβ and tau Conformational Changes in Alzheimer’s disease.
1) Amyloid precursor protein (APP) undergoes normal cleavage by β and γ-secretase (presenilin [PS] is part of the γ-secretase complex) to produce the normal soluble Aβ (2). Soluble Aβ can undergo a conformational change to a β-sheet rich conformer (3) that further aggregates to form soluble, toxic Aβ oligomers. These may also precipitate to form relatively inert fibrils in amyloid plaques and congophilic amyloid angiopathy.
A) Tau is a microtubule binding protein. Tau can undergo hyperphosphorylation (B) or a conformational change to a β-sheet conformer. These species can both further change to hyperphosphorylated tau in a β-sheet rich form (D) that is predisposed to further aggregation into toxic, tau oligomers. These can precipitate to form paired helical filaments (PHF) in the form (F) of neurofibrillary tangles.
The Aβ β-sheet conformers and Aβ oligomers may cross seed, under some circumstances, with intermediate tau species in a β-sheet conformation and with tau oligomers (I and II), to synergistically exacerbate AD pathology.
The most effective immunotherapeutic approaches for AD will need to be able to concurrently reduce levels of the toxic Aβ and tau oligomeric species.
One of the significant concerns with the amyloid cascade hypothesis comes from the post mortem analyses from the active vaccination trials in humans. (Holmes et al., 2008). Individuals from the active immunization or the ‘test’ arm revealed a significant decrease in plaque burden and strikingly reduced Aβ load relative to non-immunized controls. Regardless of these encouraging results, no improvement in long-term survival outcome, time to severe dementia and cognitive function was seen among the immunized groups. Two recent, large phase III trials of passive immunization for AD have also ended with no evidence of clinical benefit, although post-hoc analysis suggested a positive trend in a subpopulation of early AD patients in the Solanezumab trial (Doody et al., 2014; Salloway et al., 2014). One plausible explanation here is that immunization was conducted in the late stage of the disease process, possibly out of the window to translate into a meaningful clinical benefit (Holtzman et al., 2012; Wisniewski and Goni, 2014). One could also theorize that the amyloid hypothesis represents only part of the complete story. The existence of a currently unknown upstream factor(s) or insult that triggers both the Aβ and tau pathways downstream of itself is also possible (Castellani et al., 2008; Small and Duff, 2008). We hypothesize that Aβ and tau abnormal conformers interact; however, the importance of individual abnormal conformers to this interaction and their influence on pathology might differ from patient to patient (Figure 1). Recent data suggests that in many cases a regionally limited tauopathy precedes Aβ pathology; however, for the tau pathology to evolve to AD it requires the concomitant presence of Aβ pathology (Braak and Del, 2011; Crary et al., 2014; Duyckaerts, 2011; Jack, Jr. et al., 2013). Hence tau and Aβ may be independent processes that show pathological synergy in the evolution of AD. Regardless of the heterogeneous pathogenesis of LOAD, immunotherapy remains an attractive and potentially effective strategy, if it is targeted to the common pathways of both Aβ and tau related pathology in early stages, as well as, in clinically symptomatic AD. Here, we will review both active and passive immunotherapeutic approaches along with preclinical and clinical data that has been used to target both Aβ and phosphorylated tau. From past and current experiences we will try to foresee possible pathways to effectively treat AD and related neurodegenerative disorders.
Active Immune Therapy Targeting Aβ in Humans
Preliminary work that suggested a strong role for immunotherapy for AD revealed that anti-Aβ targeting antibodies were capable of preventing Aβ peptide fibrillization, disrupting pre-formed fibrils and thus, thwarting fibril dependent neurotoxicity in in vitro cell culture based assays (Solomon et al., 1997). This initial work motivated further in vivo studies to test the role of Aβ first as an active immunogen and then to assess if it could prevent pathology in mouse models of Aβ related AD pathology. The first in vivo immunization trial, reported in a seminal paper by Schenk et al. demonstrated that full length, aggregated Aβ1-42 in conjunction with Freund’s adjuvant could reduce plaque load in vivo, which at the time was hypothesized to be the main culprit in AD pathology (Schenk et al., 1999). No obvious toxicity was reported in this trial. Results along the same line were confirmed and extended in later studies, where active immunization with Aβ1-42 or Aβ homologous peptides along with Freund’s or alum adjuvants not only prevented Aβ plaque pathology, but also protected against development of cognitive deficits (Asuni et al., 2006; Janus et al., 2000; Lemere, 2013; Morgan et al., 2000; Sigurdsson et al., 2001a;Sigurdsson et al., 2004). Biochemical assays identified the first 15 amino acids of the Aβ peptide as the site of the principal epitope. Further, immunohistochemical assays also revealed that antibodies generated in mice towards Aβ can label amyloid plaques on human AD brain sections, raising the possibility of such immune intervention in humans. Interestingly, peripheral injections of anti-Aβ monoclonal antibodies into the systemic circulation could also reduce Aβ plaque burden and behavior, suggesting that the therapeutic effect of the vaccine was likely mediated by generating a humoral response (Bard et al., 2000; DeMattos et al., 2001; Lemere, 2013; Wisniewski and Goni, 2014). These pilot preclinical trials revealed no evidence of toxicity in the immunized mice. However, there is some debate about the type of immune response involved in mediating the beneficial effects by these peptides. Besides the classical view at the time, that fibrillar Aβ with a strong adjuvant was the appropriate immunogen, another view speculated that the use of non-fibrillogenic, non-toxic Aβ homologous peptides together with adjuvants that activate primarily the humoral, Th-2 response rather than the Th-1 cell mediated response primarily might be more effective and reduce potential toxicity (Lemere et al., 2001; Sigurdsson et al., 2001b;Sigurdsson et al., 2002; Wisniewski and Goni, 2014). The design for the Aβ homologous peptide immunogens, albeit, with a few appropriate amino acid substitutions, is based on the fact that the major B cell epitopes which are within the first 15 amino acids of Aβ form the principal B cell epitopes, while the mid and carboxyl terminus are the chief site for the T cell epitopes (Asuni et al., 2006; Sigurdsson et al., 2001b;Sigurdsson et al., 2004).
The striking results from these preclinical studies served as the launching pad for Elan/Wyeth’s group to launch a randomized, multiple-dose, dose-escalation, double-blind Phase I clinical trial. This trial, started in in April 2000 used the AN1792 vaccine, which was comprised of pre-aggregated Aβ1-42 and QS21 as an adjuvant. The vaccine was designed to generate a strong cell mediated immune response. A strong inducer of Th-1 lymphocytes, QS21 produces an effect similar to that obtained in mice with the use of Freund’s adjuvant (which is not approved for use in humans) (Wisniewski and Frangione, 2005). The pilot study performed in the UK involved 80 patients with mild to moderate AD (Bayer et al., 2005). The primary aim here was to determine the efficacy and safety of full length Aβ1-42 peptide with QS21. Multiple doses were tested and it was demonstrated that 53% of patients could mount an anti-Aβ humoral response. In the later segment of the phase I trial, polysorbate 80, which acts as an emulsifier, was added to increase the solubility of Aβ1-42. The increased emulsifier concentration caused a greater shift from a Th2 humoral response to a proinflammatory Th1 response (Pride et al., 2008). A follow up phase IIatrial was conducted in October 2001 that involved 372 patients. 300 out of 372 enrolled patients were part of the arm that received a higher formulation of QS21 (aggregated Aβ1-42 with QS21 in the polysorbate 80 formulation (AN1792 to placebo ratio of 4:1). As 6% of immunized patients developed symptoms of aseptic meningoencephalitis (18 out of 298 subjects with no placebo patients developing this complication), the trial was concluded early in January11, 2002 (Boche and Nicoll, 2008; Wisniewski, 2005; Wisniewski and Frangione, 2005). The spectrum of onset of symptoms, which included confusion, lethargy and headache ranged from 5 to 168 days after the last immunization the patient had received. Neuroimaging revealed white matter lesions with or without evidence of brain edema, termed amyloid-related imaging abnormalities (ARIA). Consistent with data from animal models, post-mortem analyses in a sub-group of trial patients revealed apparent dramatic clearance of plaques in the brain parenchyma, thus validating the efficacy of this approach for precipitated amyloid fibril clearance in humans (Boche and Nicoll, 2008; Bombois et al., 2007; Ferrer et al., 2004; Masliah et al., 2005a;Nicoll et al., 2005; Nicoll et al., 2006). Histopathology revealed that there were broad stretches of cerebral cortex devoid of plaques, interspersed with areas that had residual plaques. These persistent plaques had a “moth-eaten” appearance or seemed to have a “naked” dense core. Additionally, these plaques were seen along with microglia, that were immunoreactive for Aβ, suggesting that amyloid clearance here was in association with phagocytosis. Other notable features included the presence of unchanged tau reactive NFTs and the persistence of amyloid in cerebral vessels, as well as neuropil threads in apparent regions of plaque clearing, suggesting that this preliminary approach had not targeted vascular amyloid or tau related pathology (Masliah et al., 2005a;Nicoll et al., 2005; Nicoll et al., 2006). In some plaques, a T-cell reaction surrounding some cerebral vessels was observed, reminiscent of an overstimulated deleterious Th-1 immune response. These features suggested that the immune response seemed to be working as a two-edged sword and raised the concern that the potential beneficial effects of the vaccine were being counter balanced by a deleterious auto-toxic T-cell response (Boche and Nicoll, 2008; Sadowski and Wisniewski, 2007). Further support in favor of a toxic response came from in vitro studies, where peripheral blood mononuclear cells from patients in the trial were analyzed after stimulation with the Aβ peptide. Quantification of cytokines by enzyme-linked immunosorbent spot assays revealed that cells of responder patients could generate IL-2 and IFN-γ positive responses suggestive of a Class II (CD4+) Th-1 type response (Pride et al., 2008). Also, follow-up results from the Zurich cohort, a subgroup of the Elan/Wyeth trial (Hock et al., 2002; Hock et al., 2003), indicated that immunization as a strategy might be beneficial for some AD patients. In accordance with the data from the Zurich cohort, the results from a multi- center cohort demonstrated that people with high antibody titers or immune responders performed better in outcome measures that scored memory functions relative to low-and non -responders or to the placebo group of patients (Pride et al., 2008). Though the pathology results with dramatic clearance of plaque burden have been striking, the benefits observed clinically in cognitive function have been very minimal (Gilman et al., 2005). No change in function was noted between the antibody responders and the placebo group when assessed via multiple neuropsychological rating scales. One possibility is that the timing of intervention was incorrect. Thus, there were no effects on NFT pathology with subsequent mild benefits in cognitive deficits. Furthermore, reducing the plaque burden may be inconsequential in the presence of existing widespread neuronal death and dysfunction that is related more directly to toxic oligomeric species of both Aβ and tau. Another explanation is that this approach does not target the complete pathology of AD and other factors driving cognitive dysfunction are not addressed. Thus, the amyloid hypothesis might be an oversimplification of the pathogenesis of sporadic AD.
A number of next generation Aβ vaccination trials in either Phase I or II (www.clinicaltrials.gov) are currently ongoing. Novartis Pharmaceuticals launched a Phase I trial with an active vaccine called CAD106 (Winblad et al., 2012; Wisniewski, 2012). The vaccine CAD106 is designed to target only a B cell epitope, the small amino-terminal Aβ fragment (Aβ1-6) in this case along with an adjuvant carrier that is derived from multiple copies of the coat protein of bacteriophage Qβ. Mild to moderate probable AD subjects with MMSE scores ranging from 16–26 were enrolled in the trial. They were randomized to two cohorts, the first which received 3 injections of either 50 μg CAD106 (24 test and 7 placebo subjects, cohort 1) or a second cohort which received 150 μg CAD106 (22 test and 5 placebo subjects, cohort 2). With a treatment time span of 1 year and a 2 year follow up period, the study revealed that 75% and 100% patients developed anti-Aβ IgM titers in cohorts 1 and 2 respectively, while 67% and 82% developed anti-Aβ IgG titers respectively in cohorts 1 and 2. Nine patients reported serious adverse reactions, but none were thought to be secondary to the immunogen. In support of this idea, no cases of meningitis, meningoencephalitis or vasogenic edema were identified clinically or by imaging during the initial trial or 2 year follow up period. No significant change in CSF biomarkers was noted in the CAD106 subjects. However, some differences were seen in cohort 2 treated subjects compared to controls for plasma Aβ1-40. A limitation of this trial was that it was not sufficiently powered to demonstrate a significant clinical difference between the treatment and control arms. The results from the Phase II CAD106 trial, which was completed in Feb 2013, are yet to be reported.
Another on-going active Phase II immunization trial being conducted by Janssen and Pfizer is ACC-001, that uses the same Aβ(1-6) fragment coupled to a carrier protein, and the surface-active saponin adjuvant QS-21 (Ryan and Grundman, 2009). Further, Affiris AG together with GlaxoSmithKline (GSK) has utilized AFFITOME(R) technology to generate synthetic antigenic peptides called mimotopes to target the unmodified Aβ N-terminus in their AD02 trials (Schneeberger et al., 2009). Affiris AG has also started another Phase I trial with the same technology to target a pyroglutamic-3 modified Aβ N-terminus, a post-translational modified version of Aβ. This post-translational modification of Aβ that renders it more prone to aggregation is believed to happen after its deposition in plaques or vascular amyloid (Frost et al., 2013; Saido et al., 1995). Interestingly, pyroglutamic-3 modified Aβ, which is normally present in plaques and vascular amyloid deposits but is not detectable in the CSF or plasma, is only found in these biological fluids during therapeutic interventions where deposited Aβ has been mobilized (DeMattos et al., 2012). AC Immune has initiated Phase I/IIa trials with their product, ACI-24, which works by generating a humoral immune response to Aβ in a primarily β-sheet conformation. The design is based on previous work by this group in an AD Tg model, where a tetra-palmitoylated amyloid 1–15 peptide that exists chiefly in a β-sheet conformation was used as an immunogen (Hickman et al., 2011; Muhs et al., 2007). The preliminary results from these on-going active immunization trials have yet to be reported. These second generation active immunization strategies were designed to more specifically target pathological conformers of Aβ that hopefully decreased chances of auto-immune toxicity. However, as the immunogens are still derived from the Aβ sequence, some element of cross reactivity to normal Aβ peptides is to be expected, with the plausible risk of inflammatory toxicity. Moreover, none of these approaches directly address tau related pathology.
The Past Passive Immunization Experience for AD
The process of injecting pre-made antibodies to provide host immunity is known as passive immunization. This is in contrast to the process of stimulating the immune system of host by agents like pre-formed antigen, a process that is called as active immunization (Figure 2). One of the easiest ways to provide anti- Aβ antibodies without increasing chances of uncontrolled Th-1 mediated antibody is passive transfer of exogenous monoclonal anti- Aβ antibodies. Importantly, studies have shown that AD Tg model mice treated by this method, developed significantly reduced Aβ level and showed cognitive benefit (Bard et al., 2000; DeMattos et al., 2001). Passive immunization has been associated with problems like: difficulty in selection of appropriate antigen targets, expensive costs, need for repeated injections in chronic diseases, blood –brain barrier penetration, hemorrhagic risk and the triggering of immune response to the antibodies that are injected.
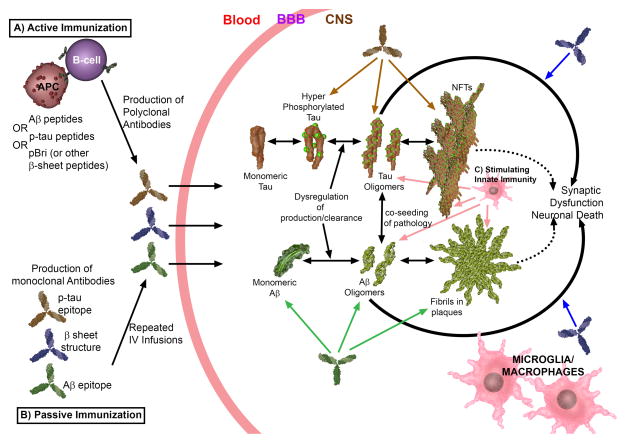
Figure 2. Different Immunotherapeutic Approaches to Ameliorate AD Pathology.
A) Active immunization can be performed using Aβ peptides, phosphorylated tau (ptau) peptides or preparations such as polymerized Bri peptide (pBri) as an immunogen. These immunogens are presented to B-cells by antigen presenting cells (APC). Use of Aβ peptides or ptau peptides will give rise to the production by B-cells of antibodies to Aβ or ptau epitopes, respectively. Use of pBri (or equivalent preparations of an immunogen that is a non-self peptide, in a stabilized, oligomeric β-sheet conformation) will lead to the production of antibodies that recognize both Aβ and tau pathological conformers (but not normal monomeric sAβ or tau proteins).
B) Passive immunization can be performed by the production of monoclonal antibodies (mAb) that bind to Aβ, ptau or β-sheet pathological conformations. These antibodies need to be infused systemically in concentrations sufficient for adequate BBB penetration (typically only ~0.1% of a systemically injected mAb will cross the BBB).
Once antibodies cross the BBB (using either active or passive immunization) they will act to enhance the clearance and degradation of their targets. Additional or alternative mechanisms may include disaggregation or neutralization of their target (i.e. blocking of toxicity). Abs to Aβ will recognize normal sAβ, oligomeric Aβ and/or deposited fibrillar Aβ (with varying preference depending on the type(s) of Abs to Aβ). Similarly, Abs to ptau will recognize monomeric ptau species, oligomeric tau and/or neurofibrillary tangles, with varying preference depending on the specific anti-ptau Ab(s). Abs to β-sheet will simultaneously act to ameliorate both Aβ and tau pathology by specifically binding pathological conformers; without binding to normal sAβ or tau.
C) Stimulation of innate immunity can also be used to ameliorate AD pathology by enhancing microglia/macrophage function via Toll-like receptors (TLRs) or related pathways. Microglia/macrophages are stimulated similarly by the immune complexes produced using active or passive immunization approaches.
Interestingly, studies have shown a number of possible congruent mechanisms of action, which can benefit AD pathology (Farlow and Brosch, 2013; Lemere, 2013; Moreth et al., 2013). Anti- Aβ antibodies, can lead to direct Aβ disassembly by targeting Aβ deposits in the brain. Microglial activation, by antibodies in the brain can also target plaques and clear them. In addition, blockage of Aβ toxicity or sequestration of Aβ monomers by antibodies prevents their aggregation in the CNS. The “peripheral sink effect” has been proposed as an important mechanism via which anti-Aβ antibodies can block Aβ deposition, namely by binding sAβ circulating in the blood stream and reducing free sAβ levels, leading to sAβ being drawn out for the brain. Additional studies are needed in this area for understanding, as to which of these mechanisms is (are) the most important in the transgenic AD mouse model, or in the more limited human trials. At present, several passive immunization trials are under study. Recently reported trials of both Bapineuzumab and Solanezumab are the two most advanced phase III trials in this field, unfortunately both failed to show overall clinical improvement or any clear disease modifying results (Doody et al., 2014; Salloway et al., 2014). Bapineuzumab is a humanized version of the mouse monoclonal antibody 3D6, which has an epitope of residues 1–5 of Aβ. 3D6 is known to cross the BBB, and in Tg mouse studies, it was shown to bind plaques in the brain and elicits Fc receptor mediated, microglial phagocytosis of Aβ plaques (Bard et al., 2000; Bard et al., 2003). The Phase II trials of Bapineuzumab (study 201 with 234 patients and study 202 with 28 patients) involved six infusions that were done every 13 weeks at 4 different doses (0.15mg/kg, 0.5mg/kg, 1mg/kg and 2mg/kg) (Farlow and Brosch, 2013; Salloway et al., 2009). Even though these trials have not showed statistically significant results overall, a post-hoc analysis restricted to subjects who received all infusions showed significant improvement in the pooled treated group compared to controls on the Disability Assessment of Dementia (DAD) and the Alzheimer’s Disease Assessment Scale- Cognitive subscale (ADAS-Cog) (Salloway et al., 2009). In addition, among non-apoE4 carrier patients significant (but small) benefits was documented on the ADAS-cog, NTB, MMSE and CDR scales. The Bapineuzumab treated subjects showed a reduction in cortical fibrillar amyloid deposits compared to both baseline measures and controls over the 78 weeks of the trial as evaluated by 11C-PiB PET imaging in a subset of participants (Rinne et al., 2010). Some of the noteworthy complications in these trials were related to amyloid related imaging abnormalities (ARIA) (Sperling et al., 2012). The abnormalities include the FLAIR MRI signal abnormalities due to parenchymal vasogenic edema and sulcal effusions (ARIA-E) and MRI abnormalities due to microhemorrhages and hemosiderosis as seen on the T2* weighted gradient echo (ARIA-H). Though 36 patients (17% of total patients) developed ARIA-E during treatment, it was symptomatic only in 8 of these 36 patients (22% of patients with ARIA-E). Adverse events included headache, confusion, neuropsychiatric and gastrointestinal symptoms. ARIA-H occurred in 17 patients with ARIA-E and in 7 of 177 patients without ARIA-E (Sperling et al., 2012). An association of these side effects was seen with both the increased dose and presence of apoE4 allele. Out of 8 symptomatic patients 7 were apoE4 carriers and 6 were treated with the two highest doses of Bapineuzumab. A possible mechanism for these adverse events is increase in BBB permeability and microhemorrhages, due to removal of cerebral vessel Aβ (Farlow and Brosch, 2013; Sperling et al., 2012). A higher CAA burden has previously been characterized in apoE4 carriers (Potter and Wisniewski, 2012). On the basis of these Phase II results, the Phase III trials were created, with the aim of giving a lower dose (0.5mg/kg limit) to apoE4 carriers and to restrict the maximum dose in apoE4 non-carriers to 1.0 mg/kg. A total of 1121 patients were involved in this phase III trial, and infusions were given every 13 weeks for total of 6 infusions in one and a half years. No clinical improvement was noted in either the apoE4 carrier or non-carrier groups. In 15% of apoE4 group ARIA occurred, while in apoE4 non-carrier groups, occurrences were 9% and 4%, of the 1.0 mg/kg and 0.5 mg/kg categories, respectively. In light of these observations, the clinical development of Bapineuzumab has been halted. However, AAB-003 a humanized version of Bapineuzumab (3D6), which has mutations in the Fc domain to reduce effector function and reduce ARIA is in two Phase I clinical trials (www.clinicaltrials.gov).
A humanized version of mAb 266, known as Solanezumab, which has an epitope at residues 16–24 of Aβ is also under study. In Tg mouse models, 266 was shown to bind specifically with monomeric soluble Aβ, thereby lowering amyloid pathology, while increasing total sAβ levels in the plasma (Bard et al., 2000; Bard et al., 2003). A reduction of free circulating sAβ and Aβ sequestration in the CNS are proposed as the major mechanisms of action. A total of roughly 800 AD patients with mild to moderate disease, in both control and treated groups have been followed in two Phase III trials. The treatment group was given 400mg of Solanezumab (~5.7 mg/kg) every 4 weeks. Cognition was studied at 80 weeks and no differences were noted compared to controls. Of note when patients with mild AD were studied separately, a small but statistically significant benefit was noted in the cognitive scores (Doody et al., 2014; Farlow and Brosch, 2013). Importantly, even though a high dose of Solanezumab was used (compared to Bapineuzumab), ARIAs were not found as a complication and an increase in plasma Aβ was found (Farlow et al., 2012). Inspired by these results, Solanezumab will be used in two preventive or very early treatment trials. The Dominantly Inherited Alzheimer Network (DIAN) trial will target adult children in families with known mutations and a diagnosis of familial AD; as well as utilizing Gantenerumab, a mAb that selectively binds fibrillar Aβ and is in an on-going Phase III prevention trial, involving ~770 patients that lack clinical AD symptoms but on PET scan have appreciable amyloid disease (Bohrmann et al., 2012). Another significant prevention trial, aiming to test Solanezumab is the A4 or the Anti- Amyloid Treatment for asymptomatic Alzheimer’s disease, which includes ~ 1000 patients that lack symptoms of AD but are positive for amyloid on PET scan.
The Alzheimer’s Prevention Initiative (API) is a further prevention trial, to be performed in ~ 300 people of a Colombian kindred with PS1 mutation (E280A). A very severe AD phenotype is seen in this mutation, characterized by Aβ deposition from ~ 25 years. The study aims to test patients 30 years and older, using Genetech’s Crenezumab mAb. This antibody interacts with multiple species of Aβ (Adolfsson et al., 2012). The effector function of Crenezumab is reduced by using a IgG4 backbone.
Another avenue of active research in passive immunization trials is the role of intravenous immunoglobulin (IVIG) in AD. The basis for its use is that IVIG obtained from a large cohort of donors, contains a small, but significant amount of naturally acting anti-Aβ antibodies. In a number of autoimmune neurological disorders, IVIG is used as an immunosuppressant and even with multiple successive doses, has no major side effects. Remarkably, a decreased risk of developing dementia is seen in patients who receive regular IVIG infusions (Fillit et al., 2009). In a phase I, open label study in 8 mild AD patients IVIg was infused over 6 months, followed by an interruption and then resumed for another 9 months (Relkin et al., 2009). Following each infusion the plasma Aβ levels increased transiently, with CSF Aβ being decreased after 6 months. Moreover the MMSE increased after 6 months by an average of 2.5, returned to baseline level after washout. A total of 23 AD biomarkers were studies in these patients by collecting CSF from spinal taps before initiation of therapy, 6 months afterward and after 3 month wash-out. Out of eight study subjects, significant improvement in biomarkers was seen in six subjects after six month therapy, which gradually returned to baseline levels after IVIg washout (Shayan et al., 2012). Nonetheless, in two recent trials, no significant slowing of AD progression could be documented (Dodel et al., 2013; Lemere, 2013). In the Octapharma, IVIG trial with ~60 mild to moderate AD patients, infusions were done over six months at three different doses. Study methodologies such as MRI volume measurement, FDG-PET or cognitive measures did not show any significant improvement. Of the 43 IVIG treated patients, six patients had new, asymptomatic, microhemorrhages. Studies by Baxter Healthcare Corporation included an eighteen month phase III trial of Gammagrad 10% IVIG in ~ 400 AD patients with mild to moderate disease. The results from this trial have not been fully released but so far no significant improvement in cognitive measures has been detected (Lemere, 2013).
The passive immunization approaches which have been described above might lack an essential element that is the ability to specifically target Aβ oligomers, which are the most deleterious components of Aβ. The prior approaches target either both the normal and pathological conformers of Aβ or only the soluble Aβ (i.e. Solanezumab) (Panza et al., 2012). The lack of specificity to toxic forms is a major setback in these therapeutic approaches as targeting normal sAβ, can interfere with its crucial physiological functions like neuroprotection, modulation of long term potentiation and innate immunity. This may also increase the risk of autoimmune complications (Giuffrida et al., 2009; Puzzo et al., 2008; Soscia et al., 2010; Wisniewski and Goni, 2014). The apparent transient effects of these therapies also implies the need for a large number of administrations with a tremendous burden on health care systems and the increased risk of developing an immune response to the infused immunoglobulins. Another crucial aspect is that these therapies may have to be started very early in AD pathology build up, for them to be therapeutically beneficial. Prior studies have demonstrated that the appearance of the earliest clinical signs of AD corresponds to peak Aβ deposition, along with substantial NFT formation and neuronal loss, which have still not yet reached peak levels (Nelson et al., 2012; Yoshiyama et al., 2013). It is postulated that in order to have a significant effect, amyloid directed therapy targeting soluble Aβ alone, or both the soluble Aβ and deposited Aβ, should be started early, preferably even before cognitive impairment starts. It can be safely said that as of now, these therapeutic approaches have limited utility in symptomatic AD.
Tau Related Pathology As An Immune Target
Neurofibrillary tangles (NFTs), a pathognomonic feature of AD, are intracellular inclusion bodies that consist of deposits of paired helical filaments (PHFs), which are primarily composed of hyperphosphorylated tau. Recently, there is considerable interest on targeting phosphorylated tau for immunomodulation in AD (Boutajangout and Wisniewski, 2014; Kayed and Jackson, 2009; Noble et al., 2009; Sigurdsson, 2008; Yoshiyama et al., 2013). Some recent work has shown that tau pathology precedes formation of amyloid plaques, appearing first in the locus coeruleus and then spreading to other brainstem nuclei and the entorhinal cortex (Braak and Del, 2011; Elobeid et al., 2012; Jack, Jr. et al., 2013). In addition, work by multiple groups has shown that the degree of tau related pathology is better correlated with the degree of dementia when compared to the amyloid plaque burden; hence, making tau as a desirable target in symptomatic AD patients (Arriagada et al., 1992; Bancher et al., 1993; Terry, 1996). Further support for this idea is provided by the results from the human immunization trials (as reviewed above), where the reduction in amyloid plaque load, did not produce cognitive benefits in symptomatic AD subjects.
In animal models, treatment with a phospho-tau peptide (containing the phosphorylated PHF-1 epitopes Ser 396, Ser 404) given prior to the onset of pathology was able to prevent development of tau aggregates in the Tg P301L mouse tau model (Asuni et al., 2007). Phosphorylation at these specific epitopes has been shown to increase the fibrillogenic character of tau and enhances paired helical filaments formation (Eidenmuller et al., 2001; Fath et al., 2002). This model develops neurofibrillary tangles in several brain regions and the spinal cord. Two groups were immunized from 2 to 5 months and from 2 to 8 months. Immunohistochemical analysis using PHF-1 and MC1 antibodies showed a significant reduction in tau related pathology, compared to controls. In addition, an amelioration in the vaccinated groups was seen on a number of sensorimotor tasks. Antibodies generated by this vaccination were found to cross the BBB, bind to phosphorylated tau and reduce pathology without significant adverse effect; thus, providing strong support in favor of the idea that it is possible to also reduce tau related pathology with active immunization (Asuni et al., 2007). These results were also confirmed in a similar study done in an htau/PS1 tau pathology model (Boutajangout et al., 2010). As the transgenic mice used in these studies had severe locomotor deficits, a major limitation of this work was that cognition could not be assessed as a therapeutic endpoint.
How an antibody response to a protein that has intracellular inclusions could have beneficial effects can initially be difficult to understand. However, support for this idea is lent from immunization studies done in a transgenic mouse model of Parkinson’s disease where a reduction in intracellular α-synuclein aggregates was demonstrated (Masliah et al., 2005b). Studies done recently by multiple groups have suggested that anti-tau antibodies can cross the BBB, are translocated inside neurons via low-affinity Fc receptors where they can bind to pathological tau within the endosomal/lysosomal system (Congdon et al., 2013). Additionally, injection of fibrillar tau brain extract into the brains of transgenic wild-type tau expressing mice can push the induction of tau into filaments, along with the spread of pathology from the injection site into adjacent brain regions (Clavaguera et al., 2009). Such an “infectivity” of abnormal protein conformation from outside the cell has also been established for polyglutamine aggregates (Ren et al., 2009) and is well described in prion disease (Colby and Prusiner, 2011). Hence, one can reason that if certain pathological forms of tau can spread and lead to PHF pathology in AD via a “prion like” replicative mechanism, anti-phosphorylated tau antibodies might not necessarily need to enter cells in order to be effective.
With active immunization using tau epitopes, there exists a risk of inducing encephalitis or neuronal apoptosis. This line of thought is backed by an early study, where immunization of female C57BL/6 mice with full length recombinant tau produced neurological deficits, NFT-like changes, gliosis and an inflammatory infiltrate (Rosenmann et al., 2006). Even with phosphorylated tau as an epitope, the possibility of deleterious effects still persists. This risk is evident in a study where E257T/P301S-tau Tg mice and wild type mice were repetitively immunized with a mixture of three phospho-tau peptides producing neuroinflammation, in conjunction with significant neurological disability in the tau Tg mice (Rozenstein-Tsalkovich et al., 2013). Hence, one might speculate that a passive immunization approach with anti-phospho-tau directed mAbs might be safer. Two trials have been conducted where passive immunization was chosen as the targeting strategy, and revealed that tau related pathology and motor deficits were reduced if the timing of the antibody administration was prior to the onset of tau pathology (Boutajangout et al., 2011; Chai et al., 2011). Another study, with serial intracerebroventricular administration of anti-tau antibodies (starting at 6 months of age over a 3 month period), demonstrated a decrease in pathology and contextual fear conditioning deficits in P301S tau Tg mice (Yanamandra et al., 2013). Even though this study demonstrated that administration of anti-tau antibodies at a point when pathology is already present could improve behavior, the intraventricular route used in this case is a major disadvantage. Further, the only study to date to show improvement in pathology after its onset has been unable to show any benefits on animal survival as compared to controls (d’Abramo et al., 2013). In this report, the authors compared DA31 (a pan-tau antibody), PHF1 (detects pSer396/404) and MCI (detects a pathological tau conformation) in P301L Tg tau mice, (which have an onset of pathology at about 3 months of age). Mice injected with MCI revealed a reduction of tau related pathology immunohistochemically and biochemically from 7 to 10 months. However, there was no change in survival between mice injected with either PHF1 or MC1 from 6 to 14 months of age versus control Tg mice (d’Abramo et al., 2013). Previously, it has been shown that PHF1 is able to decrease in tau related pathology when treatment is started prior to the onset of disease (Boutajangout et al., 2011). Together, these results suggest that though immunotherapy directed towards tau holds promise, there is some risk of toxicity. For best results, more work needs to be done to clearly define the tau form to be used and the optimal timing of the directed immunotherapy.
Innate Immunity Stimulation as a Means to Ameliorate AD Pathology
Studies conducted over 20 years ago had suggested the potential critical role of microglia for both the formation and clearance of amyloid lesions in AD (Frackowiak et al., 1992; Wisniewski et al., 1992; Wisniewski and Wegiel, 1994). The importance of inflammatory pathways affecting the function of microglia for the pathogenesis of AD is highlighted by the results of GWAS, where many of the implicated genes have a major role in immunological processes, as well as the recent linkage to AD of a rare variant of TREM2, a gene that regulates phagocytosis and the activation state of microglia/macrophages (Boutajangout and Wisniewski, 2013; Karch et al., 2014). Microglia play a critical role in the innate immune system of the CNS and one of the most potent ways to stimulate this system is via the Toll-like receptors (TLRs). Neuroinflammation can contribute to cognitive impairment and play a significant role in AD progression (Lampron et al., 2013; Lee et al., 2013); however, it is increasingly recognized that tightly regulated stimulation of innate immunity processes and specific microglia activation can be neuroprotective depending on the stimulus and the environment (Schwartz et al., 2013). Microglia lose their Aβ clearing capabilities as AD progresses (Fiala et al., 2005; Lai and McLaurin, 2012; Majumdar et al., 2007). Senescence of microglia function has been suggested to play a fundamental role in both AD and other neurodegenerative diseases (Streit and Xue, 2014). Modulation of innate immunity via TLR2, 4 and 9 signaling pathways has previously been shown to be critical in modulating Aβ deposition. TLR4 deficient mice displayed increases of diffuse Aβ and fibrillar Aβ deposits compared with control mice (Tahara et al., 2006), suggesting that TLR4 signaling is involved in Aβ clearance (Jin et al., 2008). Microglia deficient in TLR2, TLR4, or the co-receptor CD14 are not activated by Aβ and do not show a phagocytic response (Reed-Geaghan et al., 2009). Furthermore, stimulation of microglial cells with TLR2-, TLR4-, or TLR9- specific agonists accelerates Aβ clearance both in vitro and in vivo (Michaud et al., 2013). It has been shown that the administration of the TLR9 agonist CpG oligonucleotides (ODN) containing unmethylated CpG sequences to AD model Tg2576 mice induced a reduction of cortical and vascular Aβ levels without apparent toxicity and improve cognitive function, with a recent study in 3xTg mice showing the same approach can also reduce tau related pathology in associate with cognitive benefits (Scholtzova et al., 2009; Scholtzova et al., 2014). Various CpG DNA drugs that are TLR9 agonists have been shown to be safe for both humans and rodents (Vollmer and Krieg, 2009). Hence, these preclinical studies indicate that modification of microglial function in neurodegeneration is a viable therapeutic target to ameliorate both Aβ and tau pathologies.
Targeting Abnormal Protein Conformation Rather Than Aβ or Tau Related Pathologies Individually
The most pathological conformers of Aβ and aggregated tau have been proposed to be oligomeric. Both of these entities have been demonstrated to be soluble, very mobile forms that spread extracellularly using prion like replicative mechanisms. Notably, recent studies have shown that in the presence of Aβ amyloid pathology, therapeutic interventions that impede Aβ oligomer toxicity can reverse cognitive deficits within a remarkably short treatment duration (Barry et al., 2011; Chung et al., 2010). It can be safely concluded that these molecular targets have great potential even when significant pathology is present. A number of structural and biophysical properties are shared between Aβ and tau oligomers, like a high β-sheet content, neuronal toxicity and imperviousness to proteolytic degradation. A limited number of studies using antibodies that specifically target Aβ oligomers reflect the potentially powerful role of this approach and warrant further attention (Lambert et al., 2007; Lambert et al., 2009; Lee et al., 2006; Mamikonyan et al., 2007; Moretto et al., 2007; Rasool et al., 2013). Another benefit of targeting only the oligomeric form of Aβ or tau is that the normal physiological function of these proteins remains intact. A more recent proposed approach uses conformationally specific antibodies or active immunization which aims to target the shared abnormal β-sheet confirmation of amyloid proteins (Lee et al., 2006; Moretto et al., 2007; Wisniewski et al., 2009). This approach has the benefit of simultaneously targeting both the Aβ and tau related pathologies. Our group is actively engaged in this approach for the last several years (Wisniewski and Goni, 2014). In order to accomplish this goal, we developed a therapeutic immunomodulation, specific for pathological β sheet conformation, shared by Aβ and tau disease associated species. In our studies, we employed a polymerized peptide derived from the carboxyl terminus of the British amyloidosis (ABri) peptide prepared by the use of glutaraldehyde as a cross linker, that results in a stabilized, predominately β sheet oligomeric form that does not form fibrils, which we term pBri (Goni et al., 2010; Goni et al., 2013). One of the rare forms of familial human amyloidosis is ABri, which is associated with a missense mutation in a stop codon that leads to transcription of an intronic sequence, thereby causing production of a highly amyloidogenic protein, with a carboxy terminus that lacks sequence homology to Aβ, tau or any other native human proteins (Rostagno et al., 2005; Vidal et al., 1999). We proposed that via conformational mimicry, the pBri peptide, in its stabilized oligomeric form, can initiate a conformation selective immune response, which is specific to pathological aggregated/oligomeric conformers of phosphorylated tau and Aβ. An immunomodulatory approach of such design will have a decreased risk of causing auto-immune complications, as it is specific to pathological conformers and the immunogen does not have sequence homology to any mammalian peptide. Our past studies have shown that this immunomodulation targeting, of pathological conformation of Aβ is highly effective in reducing amyloid plaques and produces cognitive rescue (Goni et al., 2010). Our recent studies have demonstrated that our approach of targeting abnormal protein confirmation is effective in both the TgSwDI mice, which have a high burden of vascular pathology, and in 3xTg mice which have both Aβ and tau related pathology, decreasing the disease pathology of both deposited conformers, but most importantly the soluble oligomeric levels of Aβ and tau, leading to improvement in cognitive deficits (Goni et al., 2013). With this approach the reductions of deposited Aβ and tau are most likely related to interfering with the intermediate oligomeric forms of Aβ and tau before they fibrillize, rather than directly acting on the plaques and NFTs.
Summary
It is an exciting time, with many different active and passive immunization therapeutic approaches currently either under development or in trials. In addition pre-clinical studies are exploring innate immunity stimulation. Strategies that target Aβ peptides could be effective if used very early in disease onset before the development of any clinical dysfunction and are currently in ongoing prevention trials. Though immunotherapy targeted towards tau pathology has shown some promise, it bears the risk of toxicity. With the current knowledge, it remains undefined if it can be used effectively in symptomatic AD where there is preexisting pathology. Many studies have shown that even at the mild cognitive impairment stage of AD, extensive amyloid and tau pathology is already present (Nelson et al., 2012). In addition, it has been proposed that in sporadic late-onset AD, tau pathology is not simply downstream of Aβ related pathology but that these pathologies could be generated by dual pathways that can interact synergistically (Small and Duff, 2008; Yoshiyama et al., 2013). If this holds true, it would be essential to devise an approach that could simultaneously and effectively target both pathologies.
We postulate that such a strategy that can harness the immune system to clear both Aβ and tau toxic oligomers concurrently might be most efficacious in symptomatic AD. This might be possible by seeking similarities in the tau and Aβ toxic conformers to actively induce a humoral immune response via conformational mimicry. An active immunization approach of the same type can also be used for the development of monoclonal antibodies, to be used alone or as a panel, possibly with other agents in different stages of AD. This “β-sheet buster” approach represents a unifying therapeutic methodology, where pathological protein conformation is being targeted. This offers potential promise for multiple conformational neurodegenerative diseases.
Table 1.
Active and passive trials for immunotherapeutical approaches to treat AD (www.clinicaltrials.gov)
| ACTIVE | ||||
|---|---|---|---|---|
| Pharmaceutical Company | Trial | Stage | Status | |
| Aβ Target | ||||
| ELAN | AN1792 | Phase II | (2000–2002) halted, no improvement, encephalitis 6% | Aggregated Aβ1-42, QS21, Polysorbate80 |
| Novartis | CAD106 | Phase I Phase II |
Aβ titers, no change biomarkers Not reported |
Aβ1-6/Bacteriophage Qβ |
| Janssen/Pfizer | ACC-001 | Phase II | Not reported, finishing | Aβ1-6-QS21 |
| Affinis AG/GSK | AFFITOME AD02 | Phase II | Not reported | Mimotope of unmodified Aβ N-terminus |
| Affinis AG | AFFITOME | Phase I | Ongoing | Mimotope of pyroglutamic-3 modified Aβ N-terminus |
| AC Immune | ACI-24 | Phase I/IIa | Not reported | β-sheet conformation of Aβ peptide |
| Tau Target | ||||
| Axon | AAD vac1 | Phase I | Ongoing continuation, tolerable, safe, antibody titers | Synthetic mutant tau peptide coupled to KLH-ALUM |
| PASSIVE | ||||
| Monoclonal antibody/target | ||||
| Janssen/Pfizer | Bapineuzumab AAB-001 |
Phase II Phase III |
No clinical improvement, ARIA, trend to efficacy No improvement, halted |
Humanized 3D6, anti-Aβ1-5, six infusions, different dosages |
| Eli Lilly | Solaneuzumab | Phase III(2) | No improvement overall, secondary analysis slight improvement in early AD; ongoing extension in early AD | Humanized mAb266 Anti-Aβ16-24 |
| Janssen/Pfizer | Bapineuzumab AAB-003 |
Phase I | Ongoing | Hu IgG4 3D6 to reduce ARIA |
| Hoffman-La Roche | Gantenerumab DIAN |
Phase III | Ongoing, future: in autosomal dominant AD | Antibody to fibrillar form of Aβ Positions 3–12; 18–27 |
| Eli Lilly | Solaneuzumab A4 |
Phase III | Starting: asymptomatic AD with positive PET | Humanized mAb266 Anti-Aβ16-24 |
| AC Immune/Genentech | Crenezumab ABBY BLAZE API |
Phase II/III Phase II Phase II Phase III |
Failed to meet clinical co-primary endpoints No difference in biomarkers/FDG-PET Ongoing in EOAD families with PS1 mut. E280A |
Hu IgG4 anti-multiple epitopes Aβ1-40 |
| Octapharma | IVIG Octapharma | Phase II | Safe, no improvement | Three different doses of naturally occurring anti-Aβ |
| Baxter | IVIG Gammagrad | Phase III | No improvement, data not released | Naturally occurring anti-Aβ |
| Baxter | IVIG newGAM | Phase II | Ongoing | Naturally occurring anti-Aβ |
Acknowledgments
This manuscript is supported by NIH grants NS073502, AG20245 and AG08051. It is also supported by the Alzheimer’s Disease Association (IIRG-13-283707) and the Seix Dow Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurol. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Ashe KH, Aguzzi A. Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion. 2013;7:55–59. doi: 10.4161/pri.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni A, Boutajangout A, Scholtzova H, Knudsen E, Li Y, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Aβ derivative vaccination in alum adjuvant prevents amyloid deposition and does not cause brain microhemorrhages in Alzheimer’s model mice. Eur J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancher C, Braak H, Fischer P, Jellinger KA. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci Lett. 1993;162:179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. Alzheimer’s disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurol. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, LaFerla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- Boche D, Nicoll JA. The role of the immune system in clearance of Aβ from the brain. Brain Pathol. 2008;18:267–278. doi: 10.1111/j.1750-3639.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K, Rauchenberger R, Richter WF, Rothe C, Urban M, Bardroff M, Winter M, Nordstedt C, Loetscher H. Gantenerumab: a novel human anti-Abeta antibody demonstrates sustained cerebral amyloid-beta binding and elicits cell-mediated removal of human amyloid-beta. J Alzheimers Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- Bombois S, Maurage CA, Gompel M, Deramecourt V, kowiak-Cordoliani MA, Black RS, Lavielle R, Delacourte A, Pasquier F. Absence of beta-amyloid deposits after immunization in Alzheimer disease with Lewy body dementia. Arch Neurol. 2007;64:583–587. doi: 10.1001/archneur.64.4.583. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Wisniewski T. The innate immune system in Alzheimer’s Disease. Int J Cell Biol. 2013;2013:e576383. doi: 10.1155/2013/576383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Wisniewski T. Tau-based therapeutic approaches for Alzheimer’s Disease - a mini-review. Gerontology. 2014;60:381–385. doi: 10.1159/000358875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del TK. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Ji Y, Sun Y, Kascsak R, Kascsak RB, Mehta P, Strittmatter SM, Wisniewski T. Anti-PrPC monoclonal antibody infusion as a novel treatment for Aβ oligomer cognitive cognitive deficits. BMC Neuroscience. 2010;11:130. doi: 10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Prusiner SB. Prions. Cold Spring Harb Perspect Biol. 2011;3:a006833. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon EE, Gu J, Sait HB, Sigurdsson EM. Antibody Uptake into Neurons Occurs Primarily via Clathrin Dependent Fcgamma Receptor Endocytosis, and is a Prerequisite for Acute Tau Clearance. J Biol Chem. 2013;288:35452–35465. doi: 10.1074/jbc.M113.491001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Alafuzoff I, Arnold SE, Atterns J, Beach TG, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Kovacs GG, Knopman DS, Kofler J, Masliah E, McKee A, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Abramo C, Acker CM, Jimenez HT, Davies P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS ONE. 2013;8:e62402. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Lu J, Tang Y, Racke MM, DeLong CA, Tzaferis JA, Hole JT, Forster BM, McDonnell PC, Liu F, Kinley RD, Jordan WH, Hutton ML. A plaque-specific antibody clears existing beta-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76:908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Di Marco LY, Marzo A, Munoz-Ruiz M, Ikram MA, Kivipelto M, Ruefenacht D, Venneri A, Soinnen H, Wanke I, Ventikos YA, Frangi AF. Modifiable Lifestyle Factors in Dementia: A Systematic Review of Longitudinal Observational Cohort Studies. J Alzheimers Dis. 2014 doi: 10.3233/JAD-132225. [DOI] [PubMed] [Google Scholar]
- Dodel R, Rominger A, Bartenstein P, Barkhof F, Blennow K, Forster S, Winter Y, Bach JP, Popp J, Alferink J, Wiltfang J, Buerger K, Otto M, Antuono P, Jacoby M, Richter R, Stevens J, Melamed I, Goldstein J, Haag S, Wietek S, Farlow M, Jessen F. Intravenous immunoglobulin for treatment of mild-to-moderate Alzheimer’s disease: a phase 2, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 2013;12:233–243. doi: 10.1016/S1474-4422(13)70014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C. Tau pathology in children and young adults: can you still be unconditionally baptist? Acta Neuropathol. 2011;121:145–147. doi: 10.1007/s00401-010-0794-7. [DOI] [PubMed] [Google Scholar]
- Eidenmuller J, Fath T, Maas T, Pool M, Sontag E, Brandt R. Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem J. 2001;357:759–767. doi: 10.1042/0264-6021:3570759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid A, Soininen H, Alafuzoff I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 2012;123:97–104. doi: 10.1007/s00401-011-0906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M, Arnold SE, Van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, Sethuraman G, DeMattos RB, Mohs R, Paul SM, Siemers ER. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Brosch JR. Immunotherapy for Alzheimer’s disease. Neurol Clin. 2013;31:869–878. doi: 10.1016/j.ncl.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Fath T, Eidenmuller J, Brandt R. Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer’s disease. J Neurosci. 2002;22:9733–9741. doi: 10.1523/JNEUROSCI.22-22-09733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7:221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- Fillit H, Hess G, Hill J, Bonnet P, Toso C. IV immunoglobulin is associated with a reduced risk of Alzheimer disease and related disorders. Neurol. 2009;73:180–185. doi: 10.1212/WNL.0b013e3181ae7aaf. [DOI] [PubMed] [Google Scholar]
- Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84:225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM, Ervin FR, Snigdha S, Cotman CW, Saido TC, Vassar RJ, St George-Hyslop P, Ikezu T, Schilling S, Demuth HU, Lemere CA. Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am J Pathol. 2013;183:369–381. doi: 10.1016/j.ajpath.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Boada Rovira M, Forette F, Orgogozo JM. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interupted trial. Neurol. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De BP, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni F, Herline K, Peyser D, Wong K, Ji Y, Sun Y, Mehta PD, Wisniewski T. Immunomodulation targeting both Aβ and tau pathological conformers ameliorates Alzheimer’s Disease pathology in TgSwDI and 3xTg mouse models. Journal of Neuroinflammation. 2013;10:150. doi: 10.1186/1742-2094-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni F, Prelli F, Ji Y, Scholtzova H, Yang J, Sun Y, Liang FX, Kascsak R, Kascsak R, Mehta P, Wisniewski T. Immunomodulation targeting abnormal protein conformation reduces pathology in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e13391. doi: 10.1371/journal.pone.0013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, van DJ, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Hardy J. Genetics of Alzheimer’s Disease. Neurotherapeutics. 2014 doi: 10.1007/s13311-014-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, Granholm AC, Iqbal K, Krams M, Lemere CA, Lott I, Mobley WC, Ness S, Nixon R, Potter H, Reeves R, Sabbagh M, Silverman W, Tycko B, Whitten M, Wisniewski T. Down syndrome and Alzheimer’s Disease: common pathways, common goals. Alzheimer’s and Dementia. 2014 doi: 10.1016/j.jalz.2014.10.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman DT, Lopez-Deber MP, Ndao DM, Silva AB, Nand D, Pihlgren M, Giriens V, Madani R, St-Pierre A, Karastaneva H, Nagel-Steger L, Willbold D, Riesner D, Nicolau C, Baldus M, Pfeifer A, Muhs A. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem. 2011;286:13966–13976. doi: 10.1074/jbc.M110.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, El KJ. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem Pharmacol. 2014;88:495–498. doi: 10.1016/j.bcp.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Paspassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for β-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8:1270–1276. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Straffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Axel Wollmar M, Umbricht D, de Quervain DJF, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against β–amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JAR. Long term effects of Aβ42 immunization in Alzheimer’s disease: immune response, plaque removal and clinical function. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Mandelkow E, Selkoe DJ. Alzheimer disease in 2020. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Pathogenic protein seeding in alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Jackson GR. Prefilament tau species as potential targets for immunotherapy for Alzheimer disease and related disorders. Curr Opin Immunol. 2009;21:359–363. doi: 10.1016/j.coi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Kim DH, Yeo SH, Park JM, Choi JY, Lee TH, Park SY, Ock MS, Eo J, Kim HS, Cha HJ. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014 doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Lai AY, McLaurin J. Clearance of amyloid-beta peptides by microglia and macrophages: the issue of what, when and where. Future Neurol. 2012;7:165–176. doi: 10.2217/fnl.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL. Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Viola KL, Klein WL. Targeting generation of antibodies specific to conformational epitopes of amyloid beta-derived neurotoxins. CNS Neurol Disord Drug Targets. 2009;8:65–81. doi: 10.2174/187152709787601876. [DOI] [PubMed] [Google Scholar]
- Lampron A, Elali A, Rivest S. Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron. 2013;78:214–232. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lee DC, Rizer J, Hunt JB, Selenica ML, Gordon MN, Morgan D. Review: experimental manipulations of microglia in mouse models of Alzheimer’s pathology: activation reduces amyloid but hastens tau pathology. Neuropathol Appl Neurobiol. 2013;39:69–85. doi: 10.1111/nan.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Lemere CA. Immunotherapy for Alzheimer’s disease: hoops and hurdles. Mol Neurodegener. 2013;8:36. doi: 10.1186/1750-1326-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Maron R, Selkoe DJ, Weiner HL. Nasal vaccination with beta-amyloid peptide for the treatment of Alzheimer’s disease. DNA and Cell Biology. 2001;20:705–711. doi: 10.1089/10445490152717569. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney JA, Bainbridge T, Gustafson A, Zhang S, Kyauk R, Steiner P, van der Brug M, Liu Y, Ernst JA, Watts RJ, Atwal JK. Molecular Mechanisms of Alzheimer Disease Protection by the A673T Allele of Amyloid Precursor Protein. J Biol Chem. 2014;289:30990–31000. doi: 10.1074/jbc.M114.589069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, Mina E, Kiyatkin A, Glabe CG, Cribbs DH, Agadjanyan MG. Anti-A beta 1-11 antibody binds to different beta-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. J Biol Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurol. 2005a;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005b;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- McKee AC, Carreras I, Hossain L, Ryu H, Klein WL, Oddo S, LaFerla FM, Jenkins BG, Kowall NW, Dedeoglu A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, Vigouret JM, Paganetti P, Walsh DM, Mathews PM, Ghiso J, Staufenbiel M, Walker LC, Jucker M. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Michaud JP, Halle M, Lampron A, Theriault P, Prefontaine P, Filali M, Tribout-Jover P, Lanteigne AM, Jodoin R, Cluff C, Brichard V, Palmantier R, Pilorget A, Larocque D, Rivest S. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc Natl Acad Sci U S A. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreth J, Mavoungou C, Schindowski K. Passive anti-amyloid immunotherapy in Alzheimer’s disease: What are the most promising targets? Immun Ageing. 2013;10:18. doi: 10.1186/1742-4933-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto N, Bolchi A, Rivetti C, Imbimbo BP, Villetti G, Pietrini V, Polonelli L, Del SS, Smith KM, Ferrante RJ, Ottonello S. Conformation-sensitive antibodies against alzheimer amyloid-beta by immunization with a thioredoxin-constrained B-cell epitope peptide. J Biol Chem. 2007;282:11436–11445. doi: 10.1074/jbc.M609690200. [DOI] [PubMed] [Google Scholar]
- Morgan D. Immunotherapy for Alzheimer’s Disease. J Intern Med. 2011;269:54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Muhs A, Hickman DT, Pihlgren M, Chuard N, Giriens V, Meerschman C, van dA, Van ILF, Sugawara M, Weingertner MC, Bechinger B, Greferath R, Kolonko N, Nagel-Steger L, Riesner D, Brady RO, Pfeifer A, Nicolau C. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci U S A. 2007;104:9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns N, Davies P, Tredici KD, Duyckaerts C, Frosch MP, Hof PR, Hulette C, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee A, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer’s disease neuropathologic changes with cognitive status: a review of the literature. J Neuropath Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2005;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Noble W, Garwood CJ, Hanger DP. Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion. 2009;3 doi: 10.4161/pri.3.2.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J Biol Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Ozudogru SN, Lippa CF. Disease modifying drugs targeting beta-amyloid. Am J Alzheimers Dis Other Demen. 2012;27:296–300. doi: 10.1177/1533317512452034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, Greco A, Seripa D, Pilotto A. Immunotherapy for Alzheimer’s disease: from anti-beta-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4:213–238. doi: 10.2217/imt.11.170. [DOI] [PubMed] [Google Scholar]
- Potter H, Wisniewski T. Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. Int J Alz Dis. 2012;2012:489428. doi: 10.1155/2012/489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener Dis. 2008;5:194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool S, Martinez-Coria H, Wu JW, LaFerla F, Glabe CG. Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Abeta deposition and tau pathology in 3xTg-AD mice. J Neurochem. 2013;126:473–482. doi: 10.1111/jnc.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, Younkin S, Younkin L, Schiff R, Weksler ME. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, de L, Sr, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. (11)C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, Abramsky O. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006;63:1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- Rostagno A, Tomidokoro Y, Lashley T, Ng D, Plant G, Holton J, Frangione B, Revesz T, Ghiso J. Chromosome 13 dementias. Cell Mol Life Sci. 2005;62:1814–1825. doi: 10.1007/s00018-005-5092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenstein-Tsalkovich L, Grigoriadis N, Lourbopoulos A, Nousiopoulou E, Kassis I, Abramsky O, Karussis D, Rosenmann H. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp Neurol. 2013;248:451–456. doi: 10.1016/j.expneurol.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Grundman M. Anti-amyloid-beta immunotherapy in Alzheimer’s disease: ACC-001 clinical trials are ongoing. J Alzheimers Dis. 2009;17:243. doi: 10.3233/JAD-2009-1118. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Wisniewski T. Disease modifying approaches for Alzheimer’s pathology. Current Pharmaceutic Design. 2007;13:1943–1954. doi: 10.2174/138161207781039788. [DOI] [PubMed] [Google Scholar]
- Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, Aβ N3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR Bapineuzumab 301 and 302 clinical trial investigators (including Wisniewski T. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, Van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurol. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer disease-like pathology in the PDAPP mice. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Mandler M, Otawa O, Zauner W, Mattner F, Schmidt W. Development of AFFITOPE vaccines for Alzheimer’s disease (AD)--from concept to clinical testing. J Nutr Health Aging. 2009;13:264–267. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- Scholtzova H, Chianchiano P, Pan J, Sun Y, Goni F, Mehta PD, Wisniewski T. Toll-like receptor 9 stimulation for reduction of amyloid β and tau Alzheimer’s disease related pathology. Acta Neuropathol Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Induction of Toll-like receptor 9 signaling as a method for ameliorating Alzheimer’s disease related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008 doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayan G, Adamiak B, Relkin NR, Lee KH. Longitudinal analysis of novel Alzheimer’s disease proteomic cerebrospinal fluid biomarkers during intravenous immunoglobulin therapy. Electrophoresis. 2012;33:1975–1979. doi: 10.1002/elps.201100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. J Alzheimers Dis. 2008;15:157–168. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Frangione B, Wisniewski T. Immunization for Alzheimer’s disease. Drug Development Research. 2002;56:135–142. [Google Scholar]
- Sigurdsson EM, Knudsen EL, Asuni A, Sage D, Goni F, Quartermain D, Frangione B, Wisniewski T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-β derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta P, Frangione B, Wisniewski T. Immunization with a non-toxic/non-fibrillar amyloid-β homologous peptide reduces Alzheimer’s disease associated pathology in transgenic mice. Am J Pathol. 2001a;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta P, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-β homologous peptide reduces Alzheimer’s disease associated pathology in transgenic mice. Am J Pathol. 2001b;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer’s Disease-Associated Amyloid beta-Protein Is an Antimicrobial Peptide. PLoS ONE. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Lieberburg I, Arrighi HM, Morris KA, Lu Y, Liu E, Gregg KM, Brashear HR, Kinney GG, Black R, Grundman M. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Human CNS immune senescence and neurodegeneration. Curr Opin Immunol. 2014;29C:93–96. doi: 10.1016/j.coi.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD. The pathogenesis of Alzheimer disease: An alternative to the amyloid hypothesis. J Neuropath Exp Neurol. 1996;55:1023–1025. [PubMed] [Google Scholar]
- Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, Blennow K, Lundmark J, Staufenbiel M, Orgogozo JM, Graf A. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- Wingo TS, Lah JJ, Levey AI, Cutler DJ. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol. 2012;69:59–64. doi: 10.1001/archneurol.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HM, Wegiel J. The role of microglia in amyloid fibril formation. Neuropathol Appl Neurobiol. 1994;20:192–194. [PubMed] [Google Scholar]
- Wisniewski HM, Wegiel J, Wang KC, Lach B. Ultrastructural studies of the cells forming amyloid in the cortical vessel wall in Alzheimer’s disease. Acta Neuropathol. 1992;84:117–127. doi: 10.1007/BF00311383. [DOI] [PubMed] [Google Scholar]
- Wisniewski T. Practice point commentary on “Clinical effects of Aβ immunization (AN1792) in patients with AD in an interupted trial. Nat Clin Prac Neurol. 2005;1:84–85. [Google Scholar]
- Wisniewski T. Active immunotherapy for Alzheimer’s disease. Lancet Neurol. 2012;11:571–572. doi: 10.1016/S1474-4422(12)70136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Frangione B. Immunological and anti-chaperone therapeutic approaches for Alzheimer’s disease. Brain Pathol. 2005;15:72–77. doi: 10.1111/j.1750-3639.2005.tb00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goni F. Could immunomodulation be used to prevent prion diseases? Expert Rev Anti Infect Ther. 2012;10:307–317. doi: 10.1586/eri.11.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goni F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:499–507. doi: 10.1016/j.bcp.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Prelli F, Scholtzova H, Chung E, Mehta PD, Kascsak R, Kascsak R, Goni F. Immunotherapy targeting abnormal protein conformation. Alz Dementia. 2009;5:P113. [Google Scholar]
- Wisniewski T, Sigurdsson EM. Murine models of Alzheimer’s disease and their use in developing immunotherapies. Biochim Biophys Acta Mol Basis Dis. 2010;1802:847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-Tau Antibodies that Block Tau Aggregate Seeding In Vitro Markedly Decrease Pathology and Improve Cognition In Vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Lee VM, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. J Neurol Neurosurg Psychiatry. 2013;84:784–795. doi: 10.1136/jnnp-2012-303144. [DOI] [PMC free article] [PubMed] [Google Scholar]