Abstract
Twenty-five amide alkaloids (1–25) from Piper boehmeriifolium and 10 synthetic amide alkaloid derivatives (39–48) were evaluated for antiproliferative activity against eight human tumor cell lines, including chemosensitive and multidrug-resistant (MDR) cell lines. The results suggested tumor type-selectivity. 1-[7-(3,4,5-Trimethoxyphenyl)heptanoyl]piperidine (46) exhibited the best inhibitory activity (IC50 = 4.94 µM) against the P-glycoprotein (P-gp)-overexpressing KBvin MDR sub-line, while it and all other tested compounds, except 9, were inactive (IC50 >40 µM) against MDA-MB-231 and SK-BR-3. Structure-activity relationships (SARs) indicated that (i) 3,4,5-trimethoxy phenyl substitution is critical for selectivity against KBvin, (ii) the 4-methoxy group in this pattern is crucial for antiproliferative activity, (iii) double bonds in the side chain are not needed for activity, and (iv), in arylalkenylacyl amide alkaloids, replacement of an isobutylamino group with pyrrolidin-1-yl or piperidin-1-yl significantly improved activity. Further study on Piper amides is warranted, particularly whether side chain length affects the ability to overcome the MDR cancer phenotype.
Keywords: Piper, Piperaceae, Amide alkaloids, Cytotoxicity, Multidrug resistance
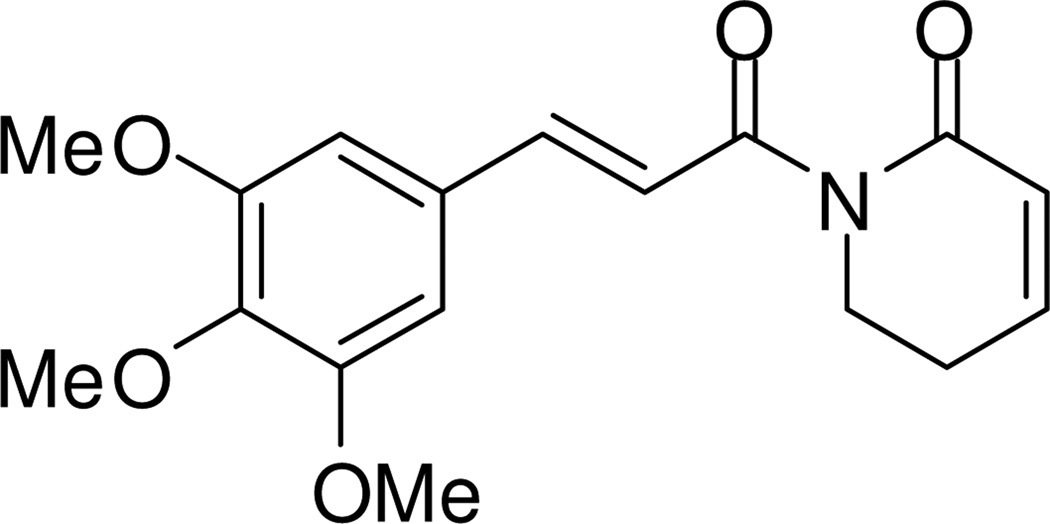
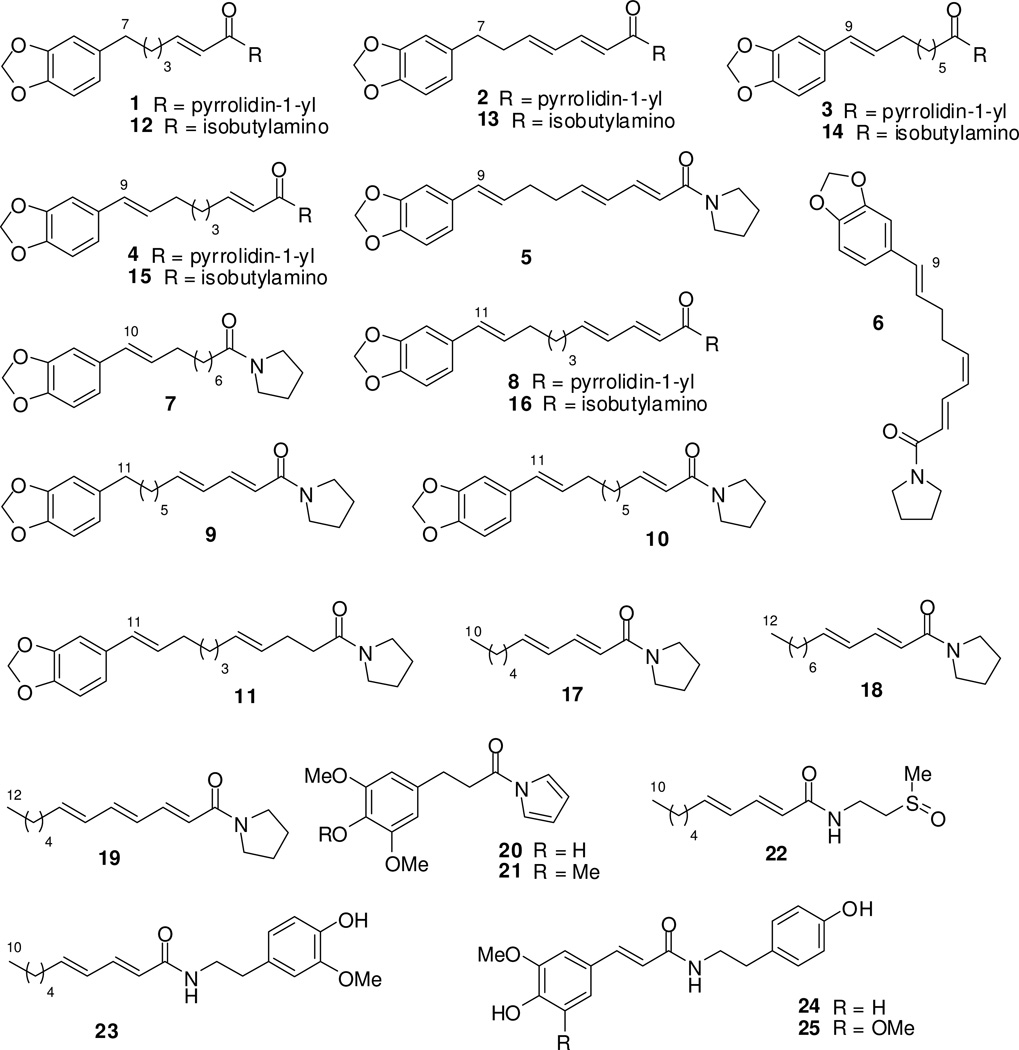
The genus Piper (Piperaceae) contains approximately 2000 plant species distributed mainly in tropical areas.1 Among them, 10 Piper species have been used in traditional medicines to treat cancer or cancer-like symptoms.2 Amide alkaloids, phenylpropanoids, lignans, neolignans, terpenes, steroids, kawapyrones, piperolides, flavonoids, and alkenylphenols have been isolated from Piper plants,3–7 and amide alkaloids are the major cytotoxic constituents.2 Especially, piplartine (piperlongumine, Figure 1) exhibits the most promise showing broad-spectrum cytotoxicity against cancer cell lines in vitro. Furthermore, it also demonstrated excellent anticancer activity in vivo.8,9 The amide alkaloids in Piper plants are usually constructed from acyl and amino moieties. The acyl moiety includes mainly alkylacyl, alkenylacyl, arylalkylacyl, and arylalkenylacyl groups. The amino moiety is generally either isobutylamine, pyrrolidine, piperidine, pyrrole, 5,6-dihydropyridin-2(1H)-one, or arylethylamine. In our previous study, several amides (3–7, and 11, Figure 2) with cytotoxic activity against the human cervical carcinoma HeLa cell line were isolated from P. boehmeriifolium Wall. The active amides belong to the arylalkenylacylpyrrolidine classification and 1-[(9E)-10-(3,4-methylenedioxyphenyl)-9-decenoyl]pyrrolidine (7) exhibited the best inhibitory activity with an IC50 of 7.78 µM.10 However, the structure-activity relationships (SARs) of these amides against cancer cells remain unclear. In the present study, 25 amide alkaloids (1–25, Figure 2) from P. boehmeriifolium and 10 synthetic amide alkaloid analogues (39–48) were evaluated for cytotoxicity against lung adenocarcinoma A549, nasopharyngeal carcinoma KB, P-glycoprotein (P-gp)-overexpressing MDR KB (KBvin), androgen insensitive prostate cancer DU145, and four breast cancer cell lines, including triple-negative [ER−/PgR−/erbB2 (HER2)−] MDA-MB-231, triple-positive ZR-75-1, double-positive expressing multidrug resistance-associated protein (MRP) 1 MCF-7, and HER2-overexpressing SK-BR-3 cell lines. The SARs and selective antiproliferative activity against KBvin are discussed.
Figure 1.
The structure of piplartine (piperlongumine).
Figure 2.
The structures of amide alkaloids (1–25) from Piper boehmeriaefolium.
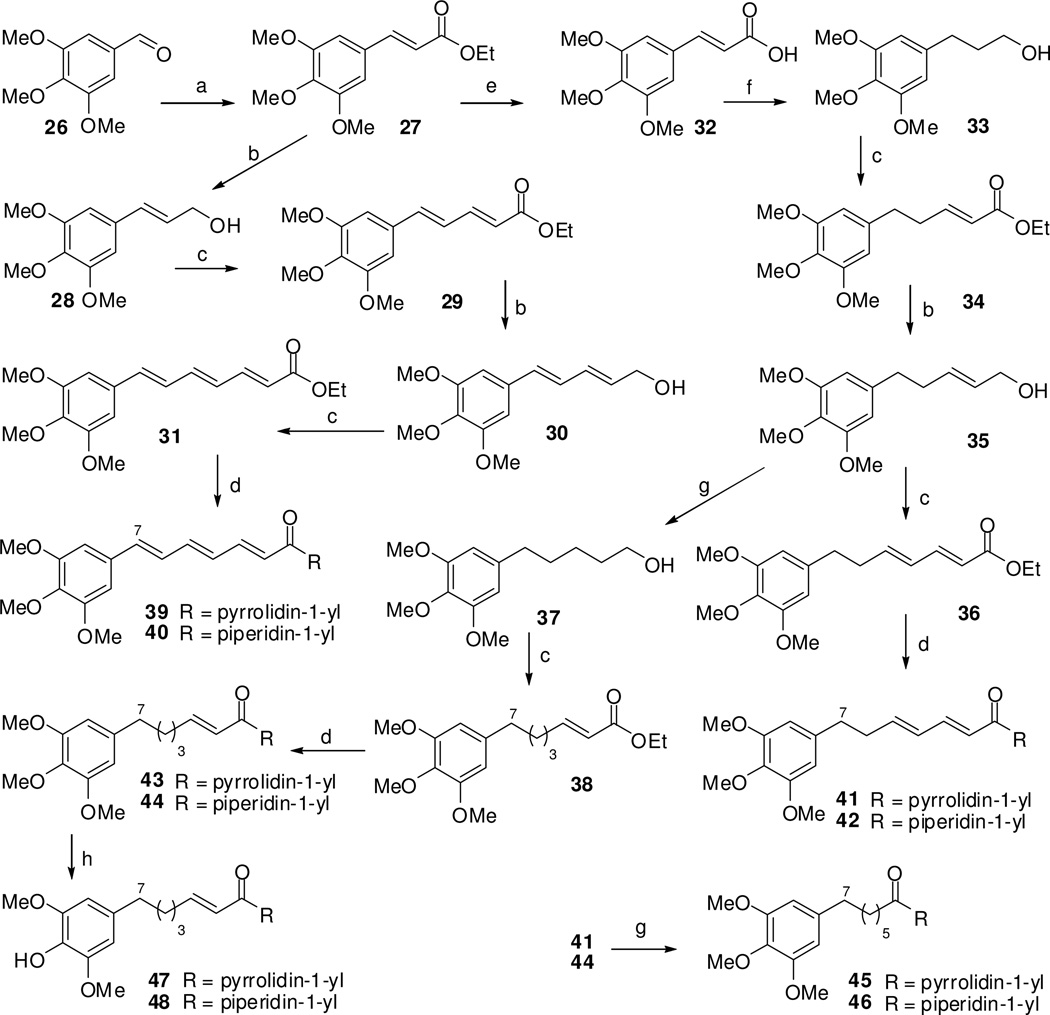
Natural amide alkaloids (1–25) were obtained from the whole plant of P. boehmeriaefolium in our recent study.10 From results of their cytotoxic evaluation (Table 1), both an arylalkenylacyl side chain and pyrrolidin-1-yl moiety were necessary for antiproliferative activity. To determine whether double bonds in the arylalkenylacyl side chain affected activity, ten new amide alkaloids (39–48) were synthesized (Scheme 1). 3,4,5-Trimethoxybenzaldehyde (26) was selected as the starting material to synthesize promising amide piplartine analogues. Ethyl (E)-3-(3,4,5-trimethoxyphenyl)acrylate (27) was obtained from 26 by 2C-Wittig homologation with (carboethoxymethylene)triphenylphosphorane in benzene at reflux.11 The esters 27, 29, and 34 were reduced with diisobutylaluminum hydride to afford alcohols 28, 30, and 35, respectively,12–13 while alcohol 33 was obtained by reduction of acid 32 using lithium aluminium hydride.14 Acid 32 was produced by hydrolysis of ester 27 with lithium hydroxide in a terahydrofuran (THF) and water (2:1) mixture.15 The alcohols 28, 30, 33, 35, and 37 were oxidized using the Parikh-Doering reaction with sulfur trioxide pyridine complex, dimethyl sulphoxide, and triethylamine and then subjected to Wittig reaction to yield esters 29, 31, 34, 36, and 38, respectively.16 Esters 31, 36, and 38 were hydrolyzed with lithium hydroxide in THF:water (2:1) to yield the corresponding acids. Reaction of these acids with pyrrolidine and thionyl chloride gave amides 39, 41, and 43, and with piperidine gave amides 40, 42, and 44, respectively.17 Hydrogenation of 35, 41, and 44 with hydrogen gas at 50 psi yielded 37, 45 and 46, respectively,18 and selective de-4'-O-methylation of 43 and 44 using aluminium trichloride gave 47 and 48, respectively.9
Table 1.
Cytotoxicity of natural (1–25) and synthetic (39–48) amide alkaloidsa
| IC50 (µM) |
SIb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd | A549 | MCF-7 | ZR- 75-1 |
SK- BR-3 |
MDA- MB-231 |
DU145 | KB | KBvin | |
| 1 | 21.20 ± 1.76 | >40 | >40 | >40 | >40 | 36.15 ± 0.03 | 31.08 ± 2.03 | 28.25 ± 4.68 | 1.1 |
| 2 | 24.65 ± 2.37 | >40 | >40 | >40 | >40 | >33 | >33 | 31.00 ± 4.85 | >1.1 |
| 3 | 19.57 ± 2.40 | 30.17 ± 1.64 | 40.99 ± 0.71 | >40 | >40 | 24.90 ± 3.98 | 25.57 ± 2.64 | 21.95 ± 2.80 | 1.2 |
| 7 | 25.74 ± 4.96 | 27.68 ± 1.04 | 40.93 ± 0.61 | >40 | >40 | 23.68 ± 4.78 | 23.74 ± 1.34 | 20.70 ± 3.73 | 1.1 |
| 8 | 18.30 ± 1.30 | 30.13 ± 0.58 | 40.60 ± 0.12 | >40 | >40 | 20.43 ± 1.10 | 21.20 ± 3.82 | 18.93 ± 3.71 | 1.1 |
| 9 | 14.42 ± 1.18 | 22.01 ± 0.31 | 26.71 ± 0.35 | 30.11 ± 0.86 | 31.58 ± 0.59 | 16.02 ± 3.10 | 16.92 ± 2.45 | 15.42 ± 3.07 | 1.1 |
| 10 | 27.55 ± 4.90 | 30.68 ± 0.75 | 36.89 ± 2.24 | >40 | >40 | 23.18 ± 2.54 | 27.32 ± 0.79 | 20.87 ± 3.83 | 1.3 |
| 43 | 9.48 ± 0.43 | 41.27 ± 3.24 | >40 | >40 | >40 | NDc | 35.51 ± 0.83 | 13.03 ± 2.72 | 2.7 |
| 44 | 23.40 ± 1.47 | 29.60 ± 1.20 | 41.01 ± 3.16 | >40 | >40 | ND | 29.79 ± 0.51 | 7.76 ± 0.45 | 3.8 |
| 45 | 20.39 ± 2.07 | 26.85 ± 0.27 | >40 | >40 | >40 | ND | 27.59 ± 1.77 | 8.86 ± 0.74 | 3.1 |
| 46 | 16.28 ± 5.33 | 11.78 ± 2.86 | 33.10 ± 2.39 | >40 | >40 | ND | 13.62 ± 2.48 | 4.94 ± 0.91 | 2.8 |
| PXL (nM)d |
2.40 ± 0.16 | >100 | >100 | >100 | 7.80 ± 0.02 | 4.88 ± 0.21 | 3.70 ± 0.11 | 1580 ± 40 | 0.002 |
Compounds 4–6, 11–25, 39–42, 47 and 48 were inactive against 8 human tumor cell lines used in this study (IC50 > 40 µM).
SI: selective index = (IC50-KB) / (IC50-KBvin).
ND: not determined.
IC50 of paclitaxel (PXL) is represented in nM.
Scheme 1.
Synthesis of amide alkaloids (39–48). Reagents and conditions: (a) Ph3P=CHCO2Et, benzene, refluxed, 4 h; (b) DIBAL-H (1.0 M in THF), CH2Cl2, −50 °C to rt, 2 h; (c) (i) Py·SO3, DMSO, Et3N, CH2Cl2, 0 °C to rt, 2 h; (ii) Ph3P=CHCO2Et, benzene, refluxed, 4 h; (d) (i) LiOH·H2O, THF-H2O (2:1), rt, 36 h; (ii) SOCl2, CH2Cl2, refluxed, 1 h; (iii) pyrrolidine (for 39, 41, and 43) or piperidine (for 40, 42, and 44), CH2Cl2, rt, 30 min; (e) LiOH·H2O, THF-H2O (2:1), rt, 36 h; (f) LiAlH4, THF, 0 °C to rt, overnight; (g) 10% Pd/C, EtOAc, H2, 50 psi, 24 h; (h) AlCl3, CH2Cl2, rt, 4 h.
The cytotoxic activity was determined by the sulforhodamine B (SRB) colorimetric assay as previously described.19 As shown in Table 1, the natural (1–3, and 7–10) and synthetic (43–46) alkaloids showed cytotoxic activity against at least one of the eight human tumor cell lines (Table 1). Within the natural products (1–25), compounds 3 and 7–10 exhibited weak and broad spectrum activity against all tumor cell lines tested except MDA-MB-231 and SK-BR3. Compounds 1 and 2 were inactive (IC50 > 40 µM) against four types of breast cancer cell lines. Interestingly, none of the compounds except 9 were active against MDA-MB-231 and SK-BR-3, suggesting tumor type-selective antiproliferative activity. Compared with the natural products, the synthetic amides 43–46 showed better cytotoxic activity against KBvin cells. 1-[7-(3,4,5-Trimethoxyphenyl)heptanoyl]piperidine (46) was the most active analog against KBvin cells (IC50 = 4.94 µM). P-glycoprotein (P-gp)-overexpressing KBvin was sensitive to the synthetic analogs (43–46), while chemosensitive cell lines, including parental cell line KB, were tolerant. Compound 44 showed the best selective index (3.8-fold selective) against MDR sub-line KBvin compared with parental KB. It is obvious that the 3,4,5-trimethoxy groups in the phenyl ring of the synthetic compounds increase the selectivity. Multidrug resistance-associated protein 1 (MRP1)-expressing MCF-7 was also tolerant to amides 43–46, suggesting that these analogs may be selectively cytotoxic to MDR cells overexpressing P-gp.
Double bonds in the side chain might decrease the cytotoxic activity, because compounds 39–42 were inactive against the tested cell lines. Compounds 47 and 48 were inactive, implying that a 4-methoxy group, rather than 4-hydroxy group, in the 3,4,5-trisubstituted phenyl ring is necessary for activity. Compared with amides 1 and 2, amides 12 and 13 were inactive indicating that pyrrolidin-1-yl derivatives are more potent than isobutylamino derivatives, while both pyrrolidin-1-yl (43 and 45) and piperidin-1-yl (44 and 46) groups are acceptable for activity.
In conclusion, arylalkenylacylpyrrolidines (1–3, 7–10, 43), an arylalkylacylpyrrolidine (45), an arylalkenylacylpiperidine (44), and an arylalkylacylpiperidine (46) showed inhibitory activity against at least one of eight tested human tumor cell lines. Double bonds in the side chain were not needed for activity. 3,4,5-Trimethoxy substitution in the phenyl ring of the amide alkaloids increased selectivity against MDR sub-line KBvin, while the 4-methoxy group in this pattern is required for cytotoxic activity. These results demonstrate that newly synthesized amide alkaloids show tumor type-selective antiproliferative activity, especially against MDR cells. These synthesized analogues may target a unique protein that is highly responsible for the proliferation of KBvin, while dispensable in chemosensitive cell lines, such as SK-BR-3 and MDA-MD-231. Compounds, such as verapamil and desmosdumotin B analogues,19 that show at least a two-fold selective cytotoxicity against MDR cells are called collateral sensitivity (CS) agents.20 Although their mechanisms of action are largely unclear, CS agents are expected to be a new class of chemopreventive adjuvant for cancer chemotherapy to suppress development of MDR phenotype.21 Thus, it is worthwhile to conduct further SAR study of Piper amides, including the effect of the side chain length on the activity of these amide alkaloids against cancer cells showing the MDR phenotype.
Supplementary Material
Acknowledgments
This work was supported by the by the Natural Science Foundation of Yunnan Province, China (No. 2011FZ205). Thanks are also due to partial support from NIH grant CA177584 from the National Cancer Institute awarded to K. H. Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data (experimental details and compound characterization for all synthesized compounds) associated with this article can be found, in the online version, at http:
References and notes
- 1.Tseng YC, Xia NH, Gilbert MG. Flora of China. Vol. 4. Beijing/St. Louis: Science Press/Missouri Botanical Garden Press; 1999. p. 110. [Google Scholar]
- 2.Wang YH, Morris-Natschke SL, Yang J, Niu HM, Long CL, Lee KH. J. Tradit. Complement. Med. 2014;4:8. doi: 10.4103/2225-4110.124811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SX, Sun QY, Yang FM, Hu GW, Luo JF, Wang YH, Long CL. Planta Med. 2013;79:693. doi: 10.1055/s-0032-1328400. [DOI] [PubMed] [Google Scholar]
- 4.Scott IM, Jensen HR, Philogene BJR, Arnason JT. Phytochem. Rev. 2008;7:65. [Google Scholar]
- 5.Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE. Phytochemistry. 1997;46:597. [Google Scholar]
- 6.Kato MJ, Furlan M. Pure Appl. Chem. 2007;79:529. [Google Scholar]
- 7.Liu HX, Chen K, Sun QY, Yang FM, Hu GW, Wang YH, Long CL. J. Nat. Prod. 2013;76:732. doi: 10.1021/np300703u. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra DP, Pessoa C, de Moraes MO, Saker-Neto N, Silveira ER, Costa-Lotufo LV. Eur. J. Pharm. Sci. 2013;48:453. doi: 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li XY, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Nature. 2011;475:231. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Tang GH, Chen DM, Qiu BY, Sheng L, Wang YH, Hu GW, Zhao FW, Ma LJ, Wang HA, Huang QQ, Xu JJ, Long CL, Li J. J. Nat. Prod. 2011;74:45. doi: 10.1021/np100606u. [DOI] [PubMed] [Google Scholar]
- 11.Sabitha G, Srinivas C, Reddy TR, Yadagiri K, Yadav JS. Tetrahedron-Asymmetr. 2011;22:2124. [Google Scholar]
- 12.Purushotham Reddy S, Venkateswarlu Y. Tetrahedron Lett. 2013;54:4617. [Google Scholar]
- 13.Preuss T, Saak W, Doye S. Chem.-Eur. J. 2013;19:3833. doi: 10.1002/chem.201203693. [DOI] [PubMed] [Google Scholar]
- 14.Laurent MY, Stocker V, Temgoua VM, Dujardin G, Dhal R. Tetrahedron Lett. 2011;52:1608. [Google Scholar]
- 15.Rao VR, Muthenna P, Shankaraiah G, Akileshwari C, Babu KH, Suresh G, Babu KS, Kumar RSC, Prasad KR, Yadav PA, Petrash JM, Reddy GB, Rao JM. Eur. J. Med. Chem. 2012;57:344. doi: 10.1016/j.ejmech.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Shi Q, Lai CY, Chen CY, Ohkoshi E, Yang SC, Wang CY, Bastow KF, Wu TS, Pan SL, Teng CM, Yang PC, Lee KH. J. Med. Chem. 2012;55:6751. doi: 10.1021/jm3001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thota N, Koul S, Reddy MV, Sangwan PL, Khan IA, Kumar A, Raja AF, Andotra SS, Qazi GN. Bioorgan. Med. Chem. 2008;16:6535. doi: 10.1016/j.bmc.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Shi Q, Nyarko AK, Bastow KF, Wu CC, Su CY, Shih CCY, Lee KH. J. Med. Chem. 2006;49:3963. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa-Goto K, Chang PC, Lai CY, Hung HY, Chen TH, Wu PC, Zhu H, Sedykh A, Bastow KF, Lee KH. J. Med. Chem. 2010;53:6699. doi: 10.1021/jm100846r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall MD, Handley MD, Gottesman MM. Trends Pharmacol. Sci. 2009;30:546. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pluchino KM, Hall MD, Goldsborough AS, Callaghan R, Gottesman MM. Drug Resist. Update. 2012;15:98. doi: 10.1016/j.drup.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.