Abstract
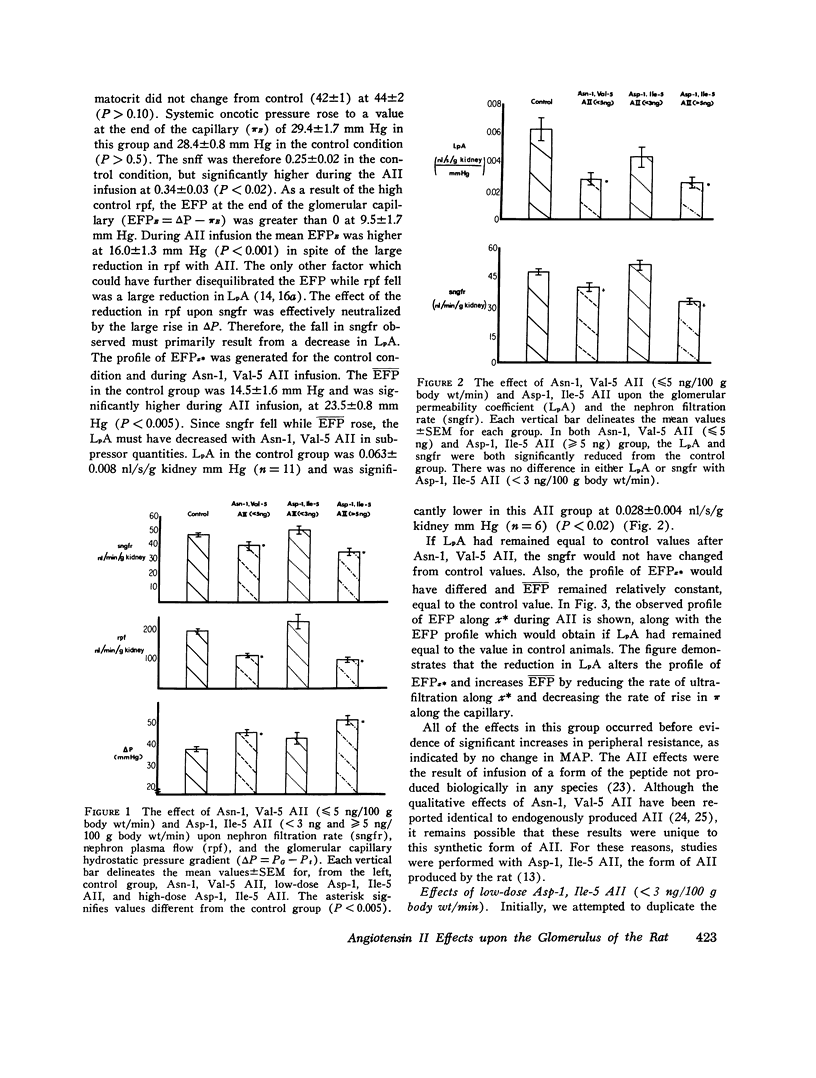
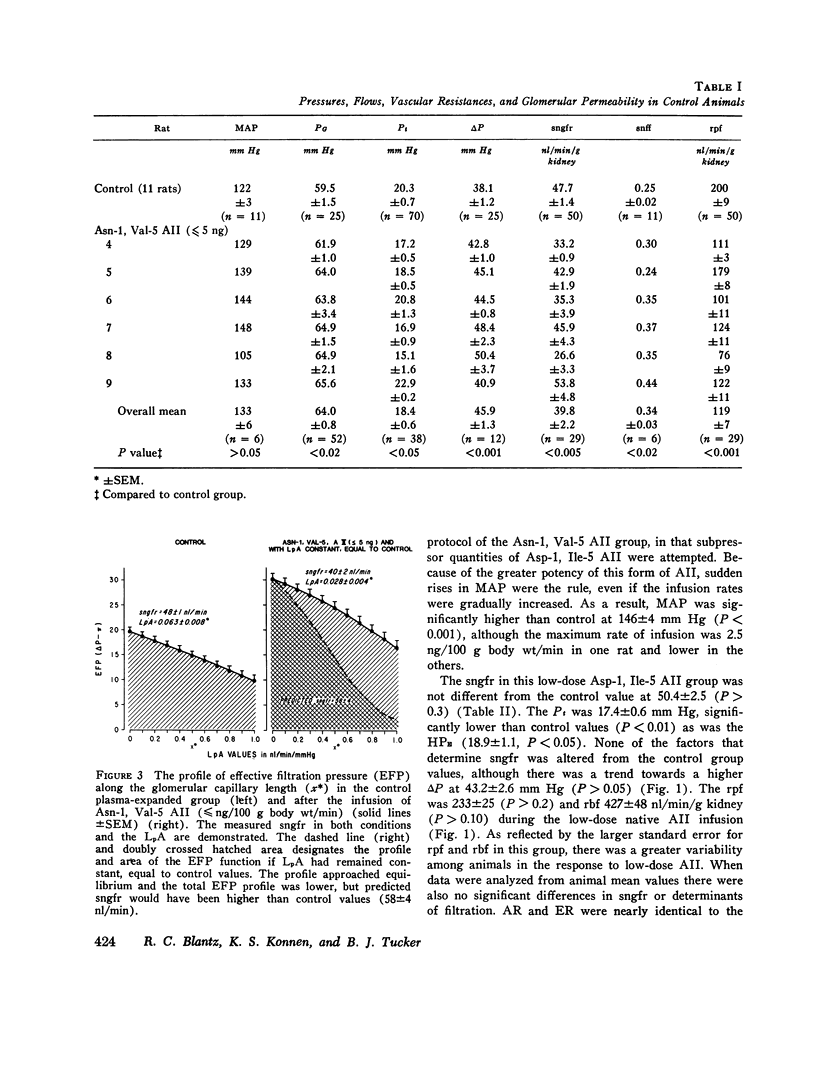
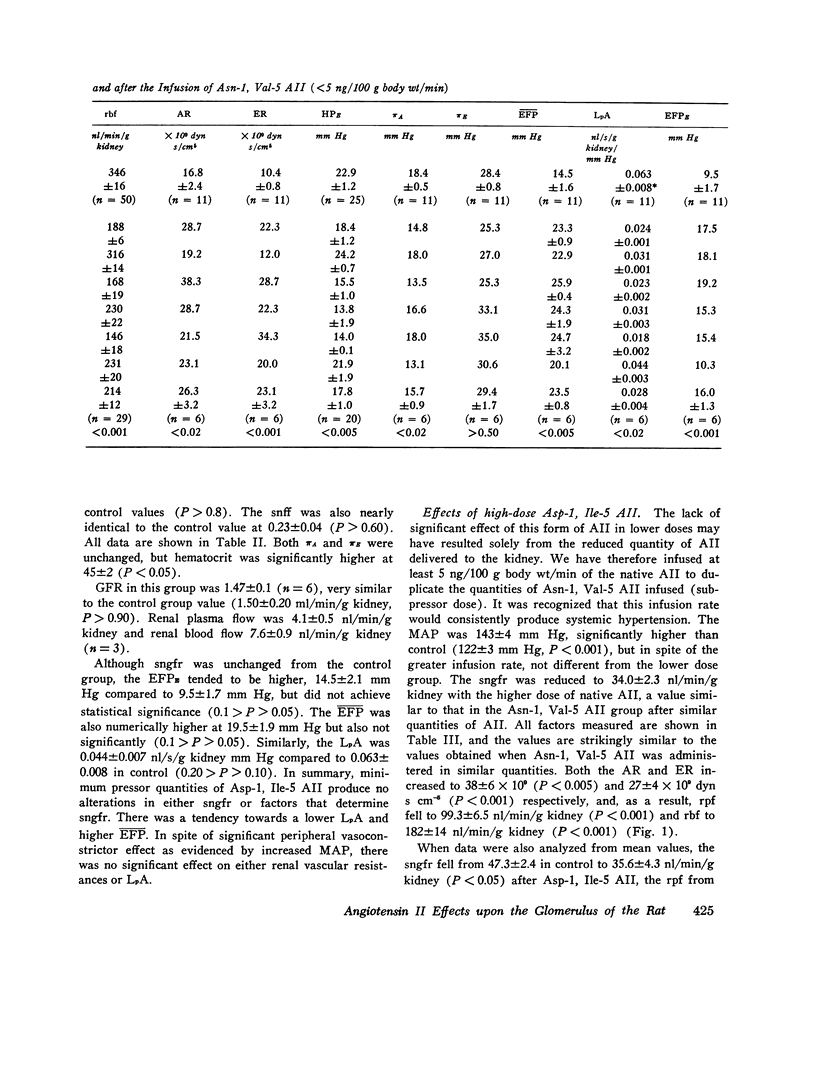
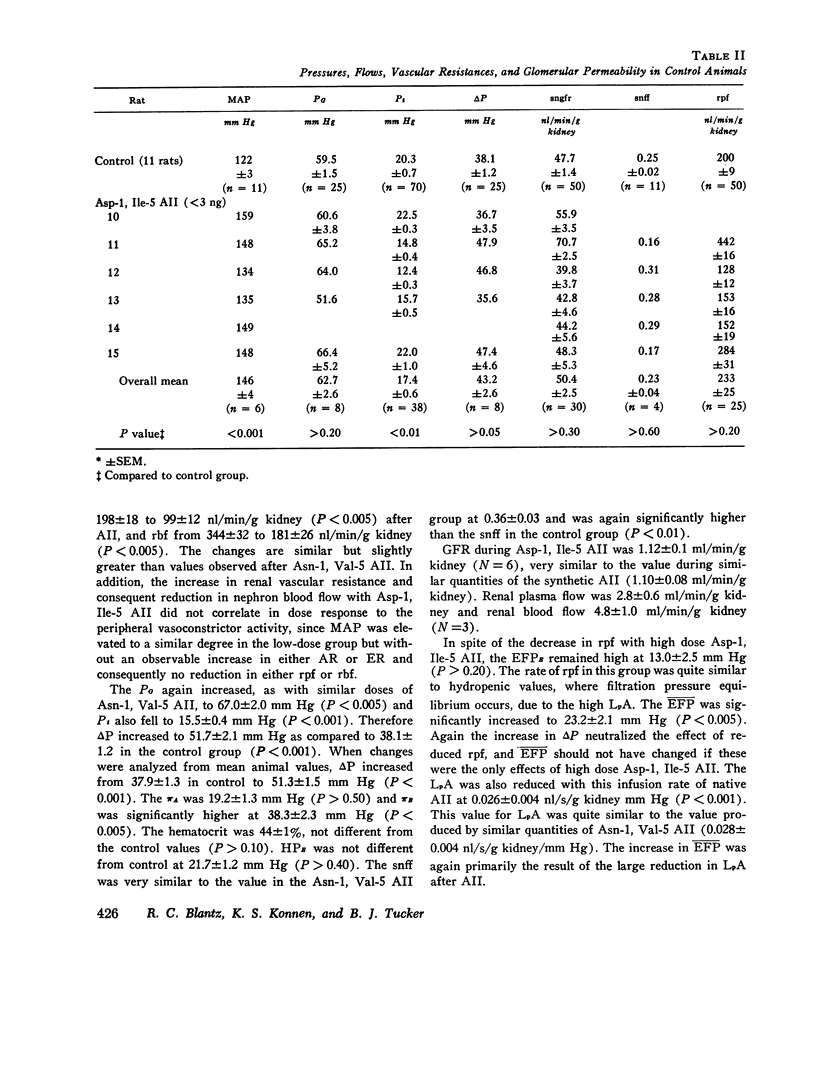
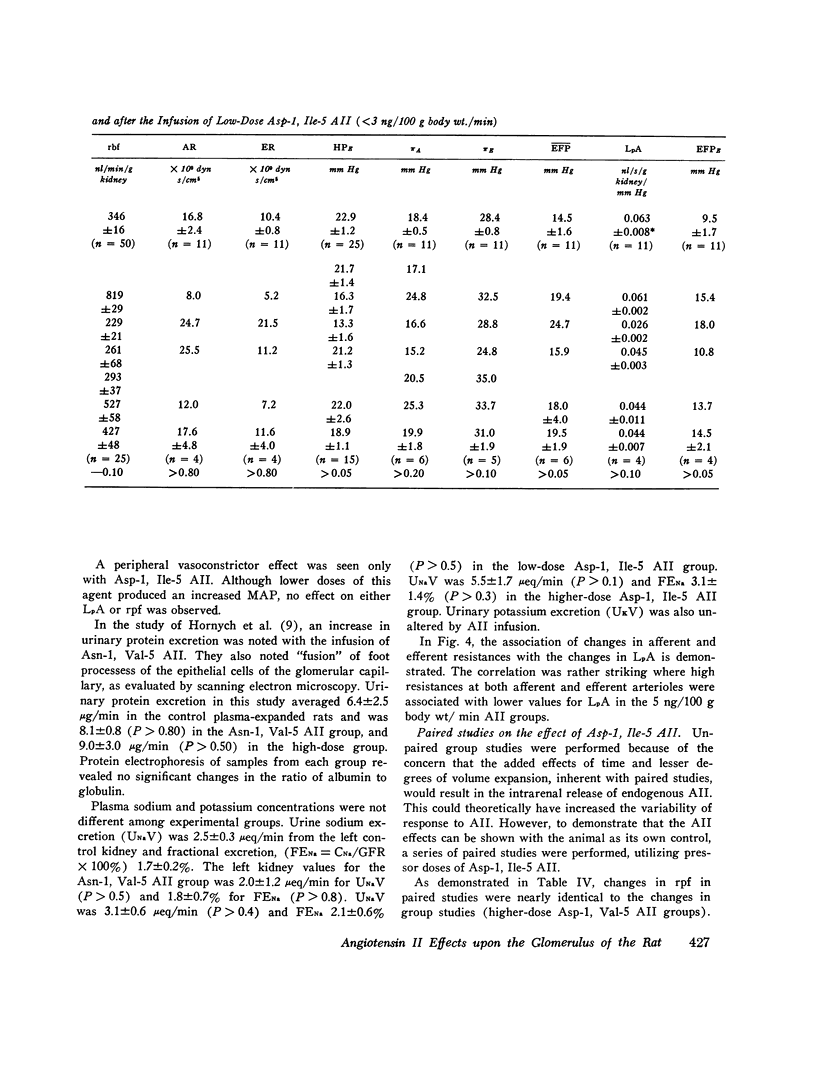
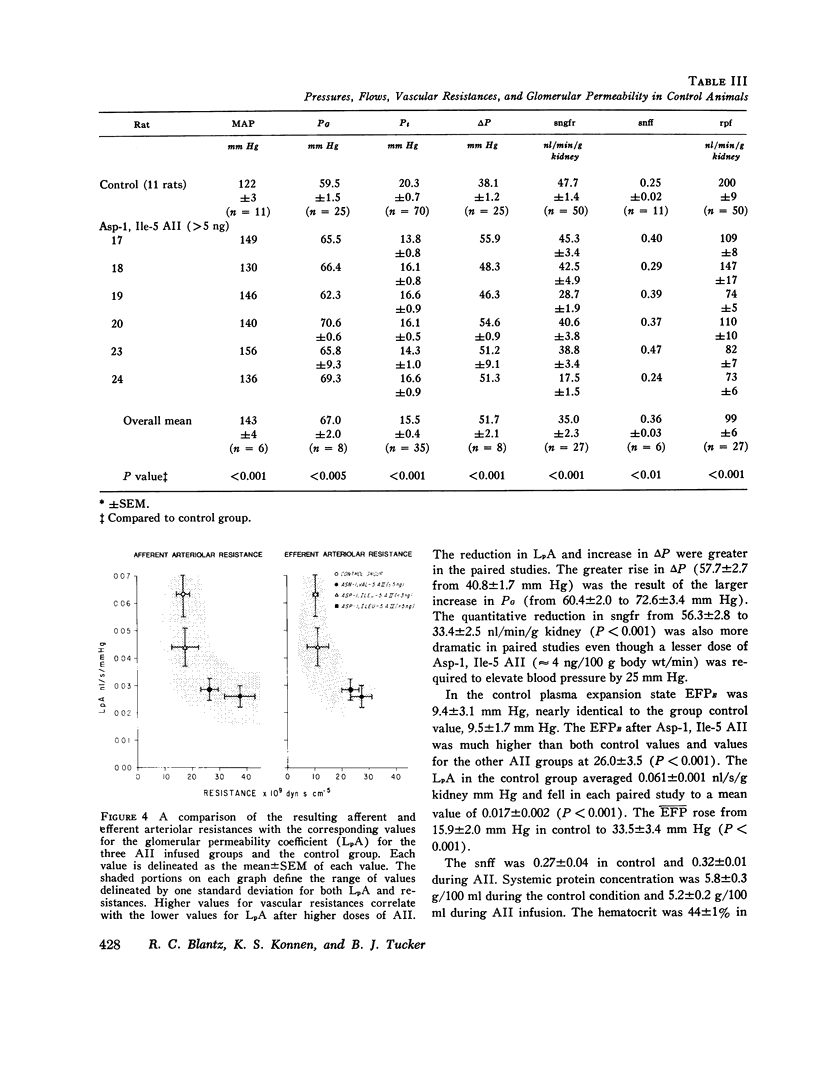
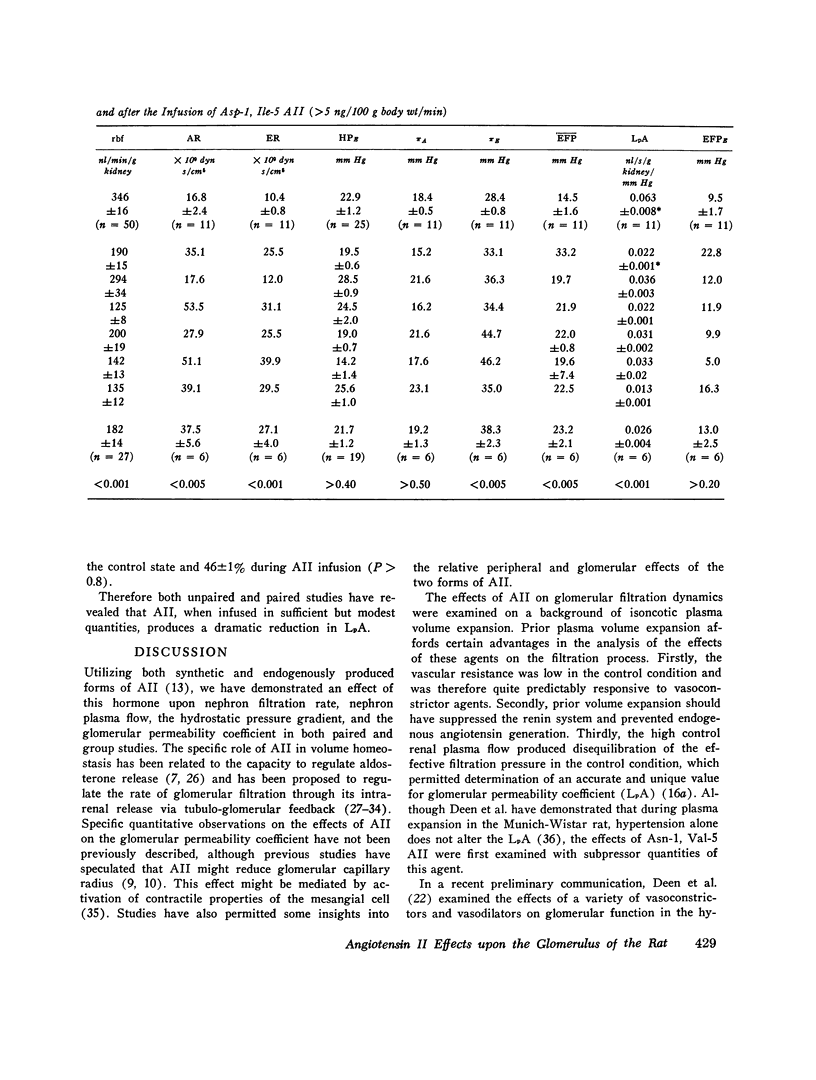
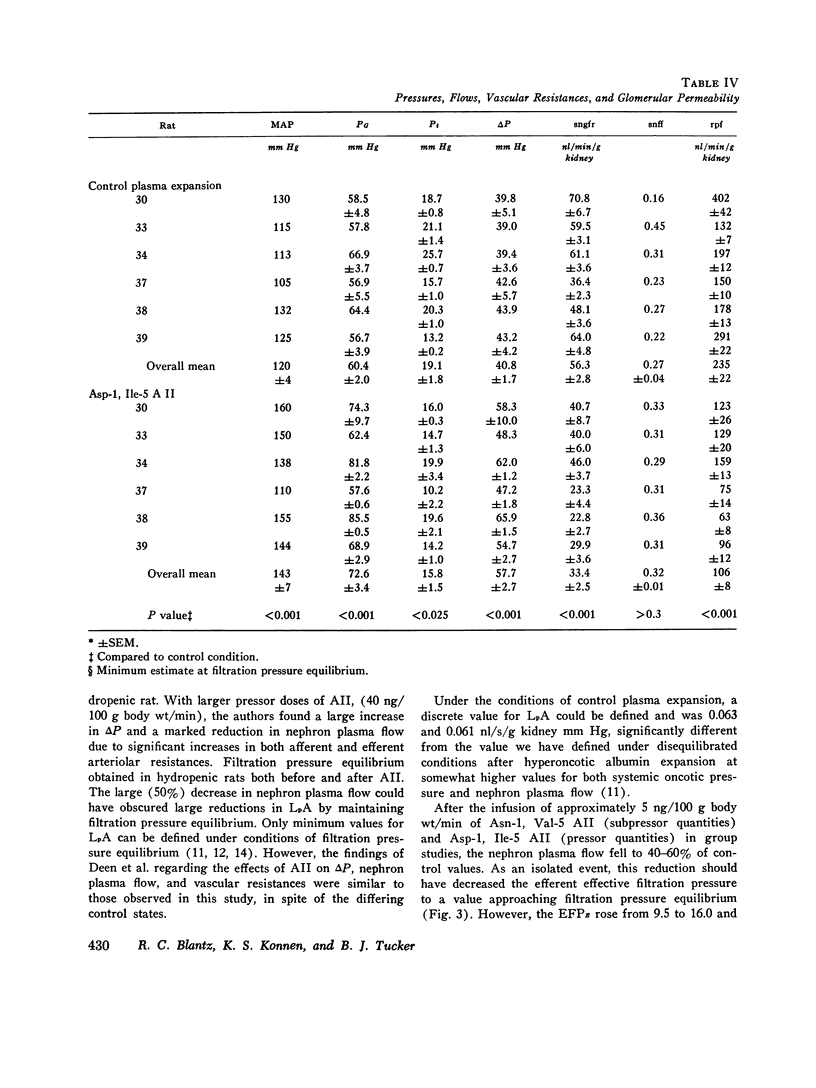
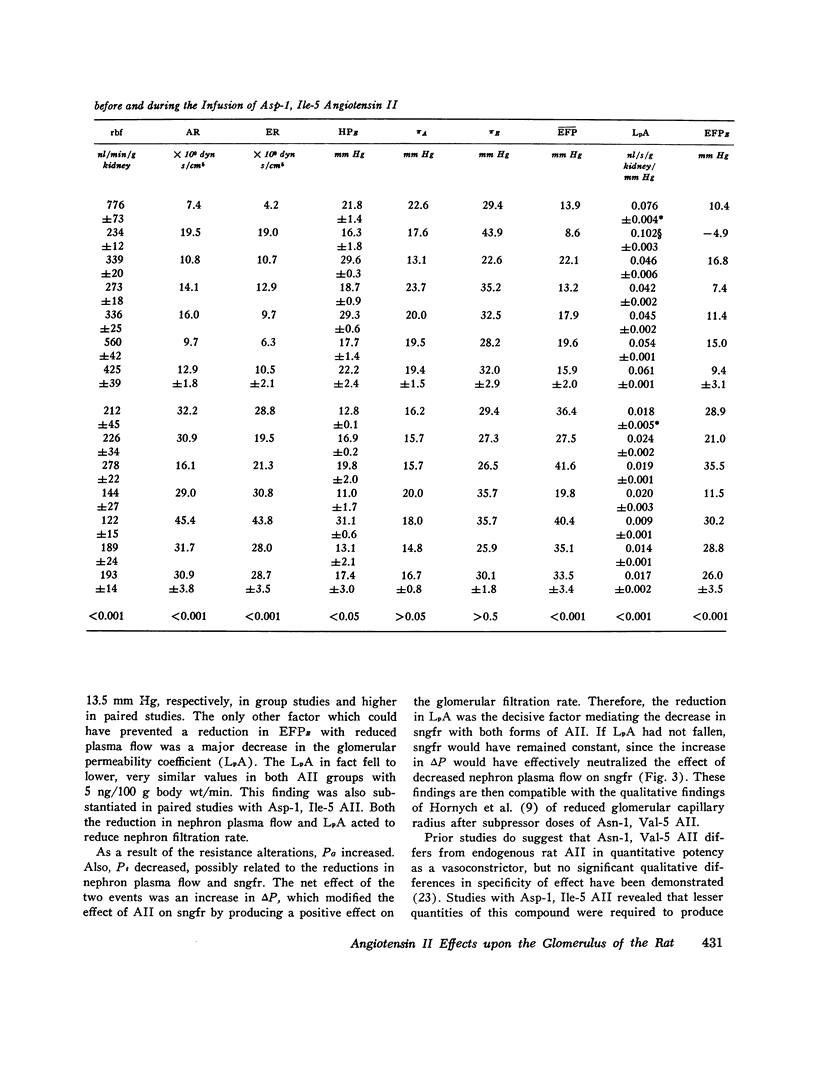
The effects of both synthetic and biologically produced angiotensin II (AII) upon the process of glolerular filtration were examined in the plasma-expanded (2.5% body wt) Munich-Wistar rat, by micropuncture evaluation of pressures, nephron plasma flow (rpf) and filtration rate (sngfr). Plasma expansion was chosen as a control condition because (a) response to AII was uniform and predictable, (b) endogenous generation of AII was presumably suppressed, and (c) the high control values for rpf permitted accurate determination of values for the glomerular permeability coefficient (LpA) before and during AII infusion. With subpressor quantities of synthetic Asn-1, Val-5 AII (less than 5 ng/100 g body wt/min), sngfr fell from 47.7 in the control group to 39.8 nl/min/g kidney (P less than 0.005). The rpf fell to 60% of control values (P less than 0.001). Measurement of glomerular capillary (PG) and Bowman's space (Pt) hydrostatic pressures in surface glomeruli with a servo-nulling device permitted evaluation of the hydrostatic pressure gradient (deltaP = PG - Pi). DeltaP increased from 38.1 +/- 1.2 in control to 45.9 +/- 1.3 mm Hg after Asn-1, Val-5 AII and essentially neutralized the effect of decreased rpf in sngfr. The sngfr then fell as a result of a decreased in LpA from 0.063 +/- 0.008 in control to 0.028 +/- 0.004 nl/s/g kidney/mm Hg after Asn-1, Val-5 AII (P less than 0.02). Lower doses of Asp-1, Ile-5 AII (less than 3 ng/100 g body wt/min) had no effect on sngfr, rpf, deltaP, and afferent and efferent vascular resistance, but significantly elevated systemic blood pressure, suggesting peripheral effects on smooth muscle at this low dose. LpA was 0.044 +/- 0.007 nl/s/g kidney/mm Hg after low-dose Asp-1, Ile-5 AII, and 0.063 +/- 0.008 in the control group (0.02 greater than P greater than 0.1). Higher, equally pressor doses of native AII (5 ng/100 g body wt/min) produced effects almost identical to similar quantites of synthetic Asn-1, Val-5 AII upon rpf, deltaP, sngfr, and renal vascular resistance. LpA again fell to 0.026 +/- 0.004 nl/s/g kidney/mn Hg, a value almost identical to that after the synthetic AII. Paired studies with Asp-1, Ile-5 AII also demonstrated a consistent reduction in LpA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARAJAS L., LATTA H. THE JUXTAGLOMERULAR APPARATUS IN ADRENALECTOMIZED RATS. LIGHT AND ELECTRON MICROSCOPIC OBSERVATIONS. Lab Invest. 1963 Nov;12:1046–1059. [PubMed] [Google Scholar]
- Bailie M. D., Rector F. C., Jr, Seldin D. W. Angiotensin II in arterial and renal venous plasma and renal lymph in the dog. J Clin Invest. 1971 Jan;50(1):119–126. doi: 10.1172/JCI106465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C. Effect of mannitol on glomerular ultrafiltration in the hydropenic rat. J Clin Invest. 1974 Nov;54(5):1135–1143. doi: 10.1172/JCI107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Israelit A. H., Rector F. C., Jr, Seldin D. W., Green J. M. Relation of distal tubular NaCl delivery and glomerular hydrostatic pressure. Kidney Int. 1972 Jul;2(1):22–32. doi: 10.1038/ki.1972.66. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Glomerular filtration response to elevated ureteral pressure in both the hydropenic and the plasma-expanded rat. Circ Res. 1975 Dec;37(6):819–829. doi: 10.1161/01.res.37.6.819. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Rector F. C., Jr, Seldin D. W. Effect of hyperoncotic albumin expansion upon glomerular ultrafiltration in the rat. Kidney Int. 1974 Oct;6(4):209–221. doi: 10.1038/ki.1974.102. [DOI] [PubMed] [Google Scholar]
- Blantz R. C. The mechanism of acute renal failure after uranyl nitrate. J Clin Invest. 1975 Mar;55(3):621–635. doi: 10.1172/JCI107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Britton K. E. Renin and renal autoregulation. Lancet. 1968 Aug 10;2(7563):329–333. doi: 10.1016/s0140-6736(68)90534-5. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMER O. M. ACTION OF NATURAL ANGIOTENSIN II AND SYNTHETIC ANALOGUES ON STRIPS OF RABBIT AORTA. Am J Physiol. 1964 Aug;207:368–370. doi: 10.1152/ajplegacy.1964.207.2.368. [DOI] [PubMed] [Google Scholar]
- HUHN D., STEINER J. W., MOVAT H. Z. [The fine structure of the mesangium in the kidney glomerulus of the dog and mouse]. Z Zellforsch Mikrosk Anat. 1962;56:213–230. [PubMed] [Google Scholar]
- Hatt P. Y., Berjal G. Le mésangium glomérulairedans l'hypertension artérielle expérimentale du rat. Etude au microscope électronique. J Urol Nephrol (Paris) 1965 Apr-May;71(4):286–294. [PubMed] [Google Scholar]
- Hornych H., Beaufils M., Richet G. The effect of exogenous angiotensin on superficial and deep glomeruli in the rat kidney. Kidney Int. 1972 Dec;2(6):336–343. doi: 10.1038/ki.1972.117. [DOI] [PubMed] [Google Scholar]
- Krahé P., Hofbauer K. G., Gross F. Effects of angiotensin infusion on the isolated rabbit kidney. Proc Soc Exp Biol Med. 1971 Sep;137(4):1324–1327. doi: 10.3181/00379727-137-35781. [DOI] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. Relations of the centrolobular region of the glomerulus to the juxtaglomerular apparatus. J Ultrastruct Res. 1962 Jun;6:562–578. doi: 10.1016/s0022-5320(62)80010-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGiff J. C. Tissue hormones: angiotensin, bradykinin and the regulation of regional blood flows. Med Clin North Am. 1968 Mar;52(2):263–281. [PubMed] [Google Scholar]
- Michielsen P. La fonction du mésangium. J Urol Nephrol (Paris) 1965 Apr-May;71(4):283–285. [PubMed] [Google Scholar]
- Nakajima T., Sakakibara S., Sakuma A., Sokabe H. Specific pressor activity of angiotensins I and II. Jpn J Pharmacol. 1973 Aug;23(4):591–593. doi: 10.1254/jjp.23.591. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Nakajima T., Sokabe H. Comparative studies on angiotensins. II. Structure of rat angiotensin and its identification by DNS-method. Chem Pharm Bull (Tokyo) 1972 Jul;20(7):1579–1581. doi: 10.1248/cpb.20.1579. [DOI] [PubMed] [Google Scholar]
- Navar L. G., Burke T. J., Robinson R. R., Clapp J. R. Distal tubular feedback in the autoregulation of single nephron glomerular filtration rate. J Clin Invest. 1974 Feb;53(2):516–525. doi: 10.1172/JCI107585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peytremann A., Nicholson W. E., Brown R. D., Liddle G. W., Hardman J. G. Comparative effects of angiotensin and ACTH on cyclic AMP and steroidogenesis in isolated bovine adrenal cells. J Clin Invest. 1973 Apr;52(4):835–842. doi: 10.1172/JCI107247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D., Gauthier R. Site of action of angiotensin and other vasoconstrictors on the kidney. Can J Physiol Pharmacol. 1971 Jun;49(6):608–612. doi: 10.1139/y71-078. [DOI] [PubMed] [Google Scholar]
- Regoli D., Park W. K., Rioux F. Pharmacology of angiotensin. Pharmacol Rev. 1974 Jun;26(2):69–123. [PubMed] [Google Scholar]
- SCHMID H. E. Renin, a physiologic regulator of renal hemodynamics? Circ Res. 1962 Jul;11:185–193. doi: 10.1161/01.res.11.1.185. [DOI] [PubMed] [Google Scholar]
- SCHWYZER R., TURRIAN H. The chemistry and pharmacology of angiotensin. Vitam Horm. 1960;18:237–288. doi: 10.1016/s0083-6729(08)60864-x. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Persson A. E., Agerup B. Tubuloglomerular feedback. Nonlinear relation between glomerular hydrostatic pressure and loop of henle perfusion rate. J Clin Invest. 1973 Apr;52(4):862–869. doi: 10.1172/JCI107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J., Wright F. S., Davis J. M., von Stackelberg W., Grill G. Regulation of superficial nephron filtration rate by tubulo-glomerular feedback. Pflugers Arch. 1970;318(2):147–175. doi: 10.1007/BF00586493. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Effect of angiotensin and beta-adrenergic stimulation on venous smooth muscle. Am Heart J. 1966 Apr;71(4):568–570. doi: 10.1016/0002-8703(66)90227-4. [DOI] [PubMed] [Google Scholar]
- Sraer J. D., Sraer J., Ardaillou R., Mimoune O. Evidence for renal glomerular receptors for angiotensin II. Kidney Int. 1974 Oct;6(4):241–246. doi: 10.1038/ki.1974.105. [DOI] [PubMed] [Google Scholar]
- Steele J. M., Jr, Lowenstein J. Differential effects of an angiotensin II analogue on pressor and adrenal receptors in the rabbit. Circ Res. 1974 Oct;35(4):592–600. doi: 10.1161/01.res.35.4.592. [DOI] [PubMed] [Google Scholar]
- THURAU K. RENAL HEMODYNAMICS. Am J Med. 1964 May;36:698–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- THURAU K., SCHNERMANN J. DIE NATRIUMKONZENTRATION AN DEN MACULA DENSA-ZELLEN ALS REGULIERENDER FAKTOR FUER DAS GLOMERULUMFILTRAT (MIKROPUNKTIONSVERSUCHE) Klin Wochenschr. 1965 Apr 15;43:410–413. doi: 10.1007/BF01483845. [DOI] [PubMed] [Google Scholar]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. H., McDonnell L. M., Raux M. C., Hollenberg N. K. Evidence for different angiotensin II receptors in rat adrenal glomerulosa and rabbit vascular smooth muscle cells. Studies with competitive antagonists. Circ Res. 1974 Mar;34(3):384–390. doi: 10.1161/01.res.34.3.384. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974 Jun;53(6):1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA E. The fine structure of the renal glomerulus of the mouse. J Biophys Biochem Cytol. 1955 Nov 25;1(6):551–566. doi: 10.1083/jcb.1.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]




