Abstract
Background and purpose
We reviewed the current state of research on microRNAs in age-related diseases in cartilage and bone.
Methods
PubMed searches were conducted using separate terms to retrieve articles on (1) the role of microRNAs on aging and tissue degeneration, (2) specific microRNAs that influence cellular and organism senescence, (3) microRNAs in age-related musculoskeletal conditions, and (4) the diagnostic and therapeutic potential of microRNAs in age-related musculoskeletal conditions.
Results
An increasing number of studies have identified microRNAs associated with cellular aging and tissue degeneration. Specifically in regard to frailty, microRNAs have been found to influence the onset and course of age-related musculoskeletal conditions such as osteoporosis, osteoarthritis, and posttraumatic arthritis. Both intracellular and extracellular microRNAs may be suitable to function as diagnostic biomarkers. In particular
Interpretation
The research data currently available suggest that microRNAs play an important role in orchestrating age-related processes and conditions of the musculoskeletal system. Further research may help to improve our understanding of the complexity of these processes at the cellular and extracellular level. The option to develop microRNA biomarkers and novel therapeutic agents for the degenerating diseases of bone and cartilage appears to be promising.
Musculoskeletal tissue degeneration is generally thought to be an age-related process that may be aggravated or accelerated by injury or disease (Loeser 2010). It has been well established that the cellular and molecular processes during degeneration can be directly associated with those happening during aging. During the last 3 decades, research has shown that apart from the many environmental stimuli, genetic factors play an important role in the regulation of aging (Smith-Vikos et al. 2012). More recently, the role of micro RNAs (miRNAs) as regulators of aging of cells and organisms has been increasingly studied (Jung et al. 2012, Ukai et al. 2012, Yu et al. 2013). These short and non-coding RNAs regulate the translation of mRNAs at the posttranscriptional level, and therefore widely influence the biological function of cells. Currently, around 1,400 different human miRNAs have been identified and reported in miRBase (http://www.mirbase.org). In terms of aging, a number of miRNAs have been shown to be up- or downregulated; thus, they are believed to play a key role in regulation of aging processes (Grillari et al. 2010, Weilner et al. 2012).
In this review article, we first describe the present state of research on miRNAs regarding their function in the aging and tissue degeneration processes. We then consider studies that have focused on specific miRNAs that influence aging of cells and organisms. Then we discuss age-related diseases in cartilage and bone that are already being associated with miRNAs. We end by exploring the future role of miRNAs in the field of orthopedic research.
MiRNAs and cellular aging
The phenomenon of irreversible cellular growth arrest of normal human cells after serial passage in vitro is called cellular senescence, and was first described by Hayflick and Moorhead (1961). This cell cycle block can be caused by critically short telomeres, DNA damage, oncogenic signaling, or cellular stress. Senescent cells are not only characterized by a changed morphology and function but also by altered intracellular protein expression and an altered secretion profile compared to early-passage cells (Campisi et al. 2007). It was long believed that cellular senescence is just an in vitro phenomenon, but in recent years an increasing number of papers have been published showing not only that senescent cells accumulate with age in vivo—for instance, in the skin (Herbig et al. 2006, Jeyapalan et al. 2007), at sites of arteriosclerosis (Erusalimsky 2009), or in the kidney (Melk et al. 2004, Koppelstaetter et al. 2008)—but that they also contribute to overall aging and age-related diseases in an organism. For example, Baker et al. (2011) demonstrated that the onset of age-related diseases could be delayed by removing p16Ink4a-positive cells, a typical marker for cellular senescence, in mice. Furthermore, the influence of senescent cells in aging of an organism was also shown by reactivating telomerase in mouse tissues, which subsequently “became rejuvenated” (Jaskelioff et al. 2011, Bernardes de Jesus et al. 2012). Today, 2 pathways are known whose activation can lead to cellular senescence. Firstly, replicative senescence is triggered by exhausted replicative potential caused by critically short telomeres (Campisi and d’Adda di Fagagna 2007). Secondly, stress can induce premature senescence, triggered by cellular stressors such as DNA damage or oncogenic signaling (Chen et al. 2008). Whatever the trigger for cellular senescence, so far the 2 executors of irreversible cell cycle arrest appear to be the p21- and the p16-dependent pathways. Both pathways result in activation of the retinoblastoma cell cycle inhibitory pathways, leading to cell cycle arrest, possibly followed by permanent growth arrest and cellular senescence (Campisi and d’Adda di Fagagna 2007).
Interestingly, even in musculoskeletal tissue such as bone and cartilage, accumulation of senescent cells with age has been observed. Mesenchymal stem cells (MSCs) are the source of bone matrix-forming osteoblasts and of cartilage matrix-forming chondroblasts. Since bone is continuously remodeled, a functionally adequate number of proliferating and differentiating stem cells for tissue regeneration is required. A number of studies (reviewed by Kassem and Marie 2011) examining differences in the number of colonies formed by MSCs isolated from young and aged donors, led to rather contradictory results. In summary, a strong decline in the number of MSCs can be observed in early adulthood as soon as skeletal growth is completed, but this reduction does not contribute to the frequently observed reduction in bone matrix density in old age (Kassem and Marie 2011). Although no conclusive reduction in the number of MSCs after the completion of skeletal growth has been found so far, a higher percentage of MSCs, exhibiting typical markers of senescence—such as β-galactosidase—was observed in cultures established from elderly donors, indicating that senescent MSCs accumulate in vivo with age, and that they may thereby contribute to age-related bone loss (Stenderup et al. 2003, Zhou et al. 2008). In support of this, a reduced replicative potential of human MSCs (Stolzing et al. 2008, Laschober et al. 2009) and osteoblasts (Kassem et al. 1997) isolated from elderly donors compared to young individuals has been reported.
Furthermore, increased levels of the senescence-inducing proteins p53 and p21 have been observed in aged MSCs (Zhou et al. 2008). Interestingly, p53 has been shown to have an impact on osteogenesis of MSCs, since p53 knock-down resulted in the initiation of osteogenic differentiation (Kim et al. 2012b. Taken together, these studies show that the proportion of senescent MSCs increases with population doubling in vitro as well as with donor age, and these observations are associated with reduced differentiation capacity.
It is not only MSCs and osteoblasts that have been shown to enter cellular senescence. Chondrocytes of aged donors have been found to show typical markers of senescence, such as reduced telomere length and enhanced β-galactosidase expression compared to young donors (Martin and Buckwalter 2003). In addition, transfection of chondrocytes isolated from an elderly donor with the gene for telomerase reverse transcriptase hTERT not only resulted in elongated telomeres, but also enabled the transduced cells to undergo 275 more population doublings compared to normal cells, indicating that chondrocytes also are affected by replicative senescence (Martin and Buckwalter 2003).
Another example of senescent cells and their contribution to the age-associated degeneration of cartilage was provided by Hoyland’s group. They hypothesized that the reason for degenerated intervertebral discs may be senescent cells. Comparing cells isolated from non-degenerated and degenerated human tissue, reduced telomere length, reduced replicative potential, increased p16INK4A levels, and a higher proportion of senescence-associated β-galactosidase-positive cells were detected in cell cultures isolated from degraded tissues. Even when cells isolated from 2 discs of the same individual were compared, differences in their mean telomere length could be observed, depending on the grade of tissue degradation (Le Maitre et al. 2007). Summarizing the data, it is clear that cells known to be relevant to musculoskeletal tissue are also affected by cellular senescence, and that they accumulate with age or when there is musculoskeletal disease.
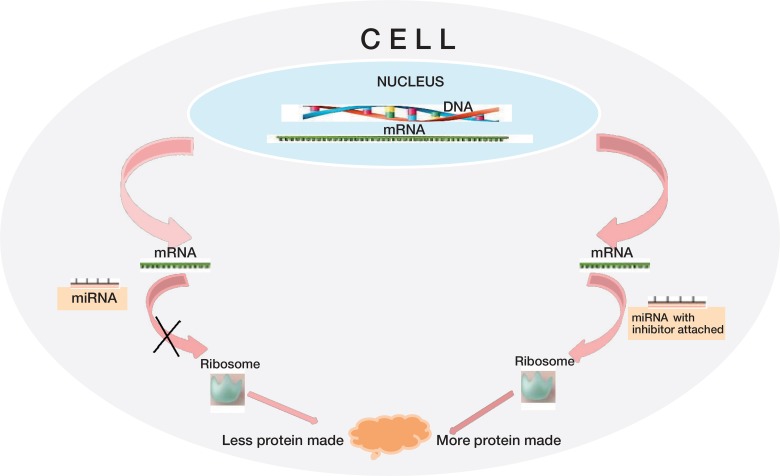
MiRNAs are single-stranded 22- to 24-nucleotide non-protein coding RNAs that have the ability to inhibit protein translation by recognizing and binding to the 3´-untranslated region of specific target mRNAs (Figure). In 1993, the first miRNA, lin-4, was discovered in Caenorhabditis elegans (C. elegans) (Lee et al. 1993), and more than 10 years later, elevated levels of lin-4 were found to have a positive effect on the lifespan of the nematode (Boehm and Slack 2005). MiRNAs not only target 1 but up to several hundred mRNAs, resulting in the capability to repress the expression of components within one pathway, and even in related pathways. Thus, they can rapidly control cellular processes such as differentiation, proliferation, migration, autophagy, apoptosis, and senescence (Bartel 2004, Zhao and Srivastava 2007, Thum et al. 2008), but they also affect processes of the entire organism—such as aging (Boehm and Slack 2005). The importance of miRNAs in bone formation in adult organisms is highlighted by the results of several studies showing that deletion of Dicer, a protein necessary for biogenesis of miRNA in mature osteoblasts and in osteoclasts leads to a high-bone-mass phenotype in mice (Sugatani and Hruska 2009, Gaur et al. 2010, Mizoguchi et al. 2010).
MicroRNAs (miRNAs) control the translation of messenger RNA (mRNA) into protein. Binding of miRNA to a target mRNA inhibits protein expression. Inhibitors of miRNA block the function of miRNA and therefore enhance translation.
How do miRNAs and senescence interact?
Originally, different miRNA expression profiles in replicative senescence were shown for various cell types, such as arterial and umbilical vein-derived endothelial cells, replicated CD8(+) T cells, renal proximal tubular epithelial cells, and skin fibroblasts (Maes et al. 2008b, Hackl et al. 2010, Rippe et al. 2012), indicating that miRNAs might play an important role in orchestrating replicative senescence. Also, in the case of human bone marrow-derived MSCs, an altered miRNA expression profile due to cellular senescence was observed. In particular, upregulation of miR-369-5p, miR-29c, and let-7f was demonstrated in replicative senescence (Wagner et al. 2008). However, whether the altered miRNA profile is a consequence of senescence or whether the altered miRNA profile triggers replicative senescence is still a matter of debate. Another prominent example of miRNAs being differentially regulated during senescence is miR-34a. This miRNA is highly expressed in senescent fibroblasts (Tazawa et al. 2007), endothelial cells, and in the heart and spleen of aged mice, suggesting a possible role of miR-34 in cardiovascular diseases (Ito et al. 2010). MiR-34a is known to target the class-III histone deacetylase silent information regulator 1 (SIRT1) (Yamakuchi et al. 2008), and expression of miR-34a—which leads to reduced SIRT1 levels—contributes to a positive p53-activating feedback loop leading to increased p21 levels, thereby triggering senescence (Yamakuchi and Lowenstein 2009). Regarding bone formation and tumorigenesis in bone, expression of miR-34a has been found to decrease in osteosarcoma cell lines (Yan et al. 2012, Li et al. 2013). Furthermore Yan et al. (2012) demonstrated that overexpression of miR-34a inhibits the tumor growth and metastasis of osteosarcoma. This observation was confirmed by Li et al. (2011), who demonstrated that tumor cells overexpressing miR-34a have reduced replicative potential, indicating that this miRNA does indeed have an effect on cell cycle progression. However, whether the observed downregulation of miR-34a in osteosarcoma directly impairs bone formation, or whether the enhanced formation is due to a prevention of senescence, which would otherwise inhibit tumor formation, is not clear (He et al. 2009).
MiRNAs are not only differentially regulated during replicative senescence, but also in stress-induced premature senescence (Li et al. 2009a, Cufi et al. 2012, Li et al. 2012). Regarding bone formation, data on miRNA expression profiles during senescence have shown changes in irradiation-induced prematurely senescent osteoblasts compared to early-passage cells (Li et al. 2012). Other studies detected the miR-17-92 cluster, a group of miRNAs that were found to be downregulated in low-level irradiation-induced senescent cells (Maes et al. 2008a, b) but upregulated in osteosarcoma cell lines (Baumhoer et al. 2012), indicating that this family of miRNAs might also play a critical role in controlling cell cycle progression during bone formation.
Oxidative stress has been shown to induce premature cellular senescence in various cell types (Sohal and Orr 1998, Feng et al. 2001, Yudoh et al. 2005, Brandl et al. 2011, Estrada et al. 2013). Several studies have demonstrated that miRNAs play an important role in reactive oxygen species-induced senescence (Menghini et al. 2009, Ito et al. 2010, Bai et al. 2011, Magenta et al. 2011, Cufi et al. 2012). Magenta et al. (2011) reported that miR-200c is upregulated upon H2O2 treatment of human endothelial cells, and that overexpression of miR-200c alone causes growth arrest, apoptosis, and cellular senescence. Interestingly, Li et al. (2013) reported that miR-200b and miR-200c are usually downregulated in osteosarcomas and that overexpression of those 2 miRNAs leads to a reduced proliferation potential, indicating that these miRNAs do indeed affect cell cycle progression in bone-relevant cells (Li et al. 2013). However, whether the altered miRNA profile is a consequence of stress-induced senescence or of the DNA damage afflicted remains unclear.
What is the functional consequence of miRNAs in senescence?
Differential miRNA expression of various cellular senescenct cell types strongly suggests functional consequences of the differentially expressed miRNAs in the context of cellular aging, for which we present only some examples, predominantly of studies of bone-derived tumor cells.
It has been shown that miR-21 levels are upregulated in osteosarcoma cell lines, and that its overexpression correlates with enhanced cell invasion and migration of tumor cells by targeting and inhibiting the expression of reversion-inducing cysteine-rich protein with kazal motifs (RECK), a protein that negatively regulates matrix metalloproteinase-9 (MMP-9) (Ziyan et al. 2011). It has also been shown that miR-21 expression is downregulated in bone marrow-derived MSCs of postmenopausal patients suffering from osteoporosis, as well as in ovariectomized mice (Yang et al. 2013). Recombinant expression of miR-21 in murine MSCs resulted not only in enhanced osteoblastogenesis in vitro, but also in increased bone formation in vivo (Yang et al. 2013).
Another prominent example of miRNAs playing an important role in the process of cellular senescence is miR-24. MiR-24 targets p16 mRNA and is downregulated in replicative senescent diploid fibroblasts (Lal et al. 2008), thereby promoting the p16-dependent senescence pathway. Interestingly, miR-24 has also been shown to have a key role in facilitating osteogenesis of MSCs (Goff et al. 2008). It is therefore tempting to speculate that reduced miR-24 levels contribute to the phenomenon of reduced proliferation potential, by facilitating p16-mediated senescence, as well as to decreased osteogenic differentiation potential of MSCs with age.
Not only p16, but also p21—as an ultimate effector of senescence—has been shown to be regulated by miRNAs. Apart from miRNAs such as miR-663, the miR-17-92 cluster is a prominent example of the posttranscriptional repression of p21 through miRNAs (Yi et al. 2012). This cluster has not only been reported in the context of cell cycle regulation, but also to be differentially expressed in various cancer cell lines including osteosarcomas (Thayanithy et al. 2012).
MiRNAs and age-related diseases in cartilage and bone (Table)
Some miRNAs of importance for bone and joint
| miRNA 34a | decreased bone formation | Yan et al. 2012, Li et al. 2013 |
| miRNAs 200b/c | decreased bone formation | Li et al. 2013 |
| miRNA 21 | osteoporotic patients, ovx rats | Yang et al. 2013 |
| miRNA 21 | increased bone formation | Yang et al. 2013 |
| miRNA 24 | decreased osteogenic differentiation of MSCs | Goff et al. 2008 |
| miRNA 34a | osteoarthritis | Abouheifet et al. 2010 |
| miRNA 146a | cartilage preservation | Li et al. 2011 |
| miRNA 133a | osteoporotic patients | Li et al. 2008, Wang et al. 2012 |
| miRNA 138 | osteoporotic mice | Eskildsen 2011 |
| miRNA 148a | increased bone mass | Cheng et al. 2013 |
| miRNA 2861 | reduced osteogenesis | Li et al. 2009 |
Several studies have revealed that miRNAs play a central role in age-related diseases such as cardiovascular diseases, Alzheimer’s disease (AD), arthritis, dementia, cataract, osteoporosis, diabetes, and cancer (as reviewed by Schraml et al. 2012). Here are a few examples of deregulated miRNAs in patients suffering from age-related diseases (involving bone or cartilage).
Several studies have demonstrated the importance of miRNAs in cartilage development, maintenance, and destruction (Sumiyoshi et al. 2010, Ukai et al. 2012, Song et al. 2013a, b, Karlsen et al. 2014, Vonk et al. 2014). miRNA expression profiles were established using chondrocytes isolated from patients with osteoarthritis and compared to those from healthy age matched controls. 7 differentially regulated miRNAs were found, among them hsa-miR-483-5p, which was more highly expressed in cells of osteoarthritic donors (Diaz-Prado et al. 2012). In contrast, hsa-miR-149*, hsa-miR-582-3p, hsa-miR-1227, hsa-miR-634, hsa-miR-576-5p, and hsa-miR-641 were downregulated in osteoarthritis. Interestingly, these miRNAs have been shown to influence pathways associated with cartilage function (Diaz-Prado et al. 2012). In this regard, it was also shown that SIRT1 levels are deregulated in osteoarthritis chondrocytes. It is known that SIRT1 expression levels are tightly regulated by miRNAs, and it has already been shown that SIRT1 levels change during the development of osteoarthritis (Diaz-Prado et al. 2012). One example of miRNA that has an influence on SIRT1 expression levels is miR-34a. It has been shown that miR-34a levels are upregulated in response to activated p53, and in turn inhibit the expression of SIRT1 (Zhao et al. 2010). Interestingly, elevated levels of miR-34a contribute to osteoarthritis, since silencing of miR-34a in an osteoarthritis rat model resulted in reduced cartilage destruction (Abouheif et al. 2010). A positive effect on the preservation of cartilage in an osteoarthritis mouse model was observed on overexpression of miR-146a (Li et al. 2011). miRNAs have also been shown to have a high impact in other age-related diseases such as osteoporosis or osteopenia. Several miRNAs are known to influence the function of bone-forming osteoblasts. For example, miR-133a was not only shown to inhibit osteoblastogenesis by targeting Runx-2 but it also turned out to be a potential biomarker, since miR-133a levels are higher in patients suffering from osteoporosis than in healthy postmenopausal women (Li et al. 2008, Wang et al. 2012).
Another promising candidate for a marker of osteoporosis is miR-214, the expression of which negatively correlates with alkaline phosphatase and osteocalcin levels—2 established markers of bone formation (Wang et al. 2013). Furthermore, overexpression of miR-138, which has been shown to inhibit osteogenic differentiation of MSCs in vitro, was also found to cause an osteoporotic phenotype in mice (Eskildsen et al. 2011). MiR-182 suppresses osteogenesis of bone-forming osteoblasts by knocking down its target, Forkhead box O1 (FOXO1) (Kim et al. 2012a). On the other hand, some miRNAs are known to enhance the activity of bone-resorbing osteoclasts. MiR-148a was recently shown to increase osteoclastogenesis in vitro by knocking down the expression of V-maf musculoaponeurotic fibrosarcoma oncogene homolog B (MAFB) (Cheng et al. 2013). In support of this, the group of Luo could demonstrate that inhibiting miR-148a expression in vivo led to impaired osteoclast development and increased bone mass in ovariectomized (OVX) and sham-operated control mice (Cheng et al. 2013).
The influence of miRNAs on the development of osteoporosis was demonstrated by a study by Li et al. (2009a). MiR-2861 was shown to target histone deacetylase 5 (HDAC5), a protein facilitating Runx2 degradation (Li et al. 2009b). Silencing of miR-2861 resulted in reduced osteogenesis in mice (Li et al. 2009b). Furthermore, these authors also reported on 2 young, related individuals who were suffering from primary osteoporosis due to mutations in miR-2861.
The future role of miRNAs in musculoskeletal research
The high potential of miRNAs as diagnostic markers or biomarkers has been mentioned on several occasions (Mitchell et al. 2008, Kosaka et al. 2010, Wittmann and Jack 2010, Pauley and Cha 2011, Miyaki and Asahara 2012). As the majority of studies have focused on the intracellular role of miRNAs, it was only recently that they were also detected in blood and other body fluids (Miyaki and Asahara 2012). Mitchell et al. (2008) were the first to describe stable circulating miRNAs in cancer patients, which was subsequently supported by findings in other studies (Kosaka et al. 2010, Wittmann and Jack 2010). In terms of arthritis, Pauley and Cha (2011) showed an increased expression of miR-146a in peripheral blood mononuclear cells from patients with rheumatoid arthritis. Murata et al. (2010) described miRNAs in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. MiR-16, miR-146a, miR-155, and miR-223 were all found to be at higher levels in synovial fluid in rheumatoid arthritis than in osteoarthritis. MiRNAs as biomarkers in synovial fluids appear to be a very exciting field for further research; the development of disease-specific profiling using synovial fluid and peripheral blood could be especially promising. Regarding osteoarthritis and posttraumatic arthritis, the role of extracellular miRNAs in signaling that directly activates the arthritic process has been mentioned (Wittmann and Jack 2011). We have recently reviewed that extracellular miRNAs are packed in secretory microparticles, such as exosomes, thus being transferable from tissue to tissue (Weilner et al. 2013). Miyaki and Asahara (2012) mentioned that extracellular miRNAs may play a role similar to cytokines, and could be considered to be paracrine regulatory molecules for cell-to-cell or tissue-to-tissue communication. They also pointed out that the mechanisms involving transport and functions of exosomes are not yet well understood and need to be explored further. This may not only lead to new biomarkers, but may also introduce novel therapeutic targets in osteoarthritis.
Wang et al. (2012) described miR-133a in human peripheral blood monocytes as a potential biomarker of postmenopausal osteoporosis. MiR-133a was more highly expressed in the low bone mineral density group than in high bone mineral density group (Wang et al. 2012). They found 3 potential osteoclast-related target genes—CXC11, CXCR3, and SLC39A1—which are known to play an important role in osteogenesis (Wang et al. 2012).
The use of miRNAs as therapeutic agents in joint diseases was demonstrated by Nagata et al. (2009). They injected double-stranded miR-15a into arthritic joints of mice. It was subsequently found in synovial cells but not in chondrocytes. MiR-15a led to increased cell apoptosis by inhibiting the expression of B-cell CLL/lymphoma 2 (BCL-2), which is known to act as an inhibitor of apoptosis and [authors. OK?] is overexpressed in rheumatoid arthritis synovial fibroblasts. Another possible therapeutic target might be miR-210. Shoji et al. (2012) reported on the effect of intraarticular injection of miR-210 on partially transected anterior cruciate ligaments in rats. They observed healing of the ligament following injection of miR-210 through enhancement of angiogenesis by upregulation of VEGF and FGF2 expression.
Conclusion
MiRNAs play an important role in the aging process in general, and in age-related diseases (Schraml and Grillari 2012). Due to the complexity of the spectrum of intracellular and extracellular miRNAs, our current understanding in this area is far from complete. However, the number of reports on specific miRNAs and their role in tissue degeneration is constantly increasing. With regard to the age-related diseases of the musculoskeletal system, such as osteoporosis and osteoarthritis, promising studies have been undertaken that will contribute to a better understanding of the underlying mechanisms of these conditions. The role of miRNAs as diagnostic biomarkers—tissue-specific as well as in body fluids—has gained enormous research interest recently. Furthermore, it has been shown that miRNAs, both locally and systemically, may be able to be used as therapeutic agents in future.
Acknowledgments
Work in the laboratory of JG is supported by EU-FP7 “Frailomics”, the Austrian Science Fund (FWF-P24498-B20), CERIES, and the Christian Doppler Research Association. The laboratory of RGV is supported by EU FP7 “Sybil”, Herzfelder’sche Familienstiftung, and the Austrian Science Fund (FWF I-510-B19).
SW: conception, design, and drafting of the article. RGV: revision for important intellectual content. HR and JG: revision for important intellectual content, and final approval. TN: conception, design, drafting, revision, and final approval. As the corresponding authors, TN and JG also take responsibility for the integrity of the work as a whole.
Competing interests: JG and RGV are co-founders of Evercyte GmbH.
References
- Abouheif MM, Nakasa T, Shibuya H, Niimoto T, Kongcharoensombat W, Ochi M. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro . Rheumatology. 2010;49:2054–60. doi: 10.1093/rheumatology/keq247. [DOI] [PubMed] [Google Scholar]
- Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM. MiR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes . J Am Soc Nephrol. 2011;22:1252–60. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders . Nature. 2011;479:232–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function . Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Baumhoer D, Zillmer S, Unger K, et al. MicroRNA profiling with correlation to gene expression revealed the oncogenic miR-17-92 cluster to be up-regulated in osteosarcoma . Cancer Genet. 2012;205:212–9. doi: 10.1016/j.cancergen.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer . EMBO Mol Med. 2012;4:691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans . Science. 2005;310:1954–7. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P, Oxidative stress induces senescence in chondrocytes J. Orthop Res . 2011;29:1114–20. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells . Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chen J, Patschan S, Goligorsky MS. Stress-induced premature senescence of endothelial cells . J Nephrol. 2008;21:337–44. [PubMed] [Google Scholar]
- Cheng P, Chen C, He HB, et al. MiR-148a regulates osteoclastogenesis via targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog BMAFB . J Bone Miner Res. 2013;28:1598–606. doi: 10.1002/jbmr.1845. [DOI] [PubMed] [Google Scholar]
- Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Quirantes R, et al. Metformin lowers the threshold for stress-induced senescence: a role for the microRNA-200 family and miR-205 . Cell Cycle. 2012;11:1235–46. doi: 10.4161/cc.11.6.19665. [DOI] [PubMed] [Google Scholar]
- Diaz-Prado S, Cicione C, Muinos-Lopez E, et al. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes . BMC Musculoskelet Disord. 2012;13:144. doi: 10.1186/1471-2474-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology . J Appl Physiol. 2009;106:326–32. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S, Kassem M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo . Proc Natl Acad Sci USA. 2011;108:6139–44. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada JC, Torres Y, Benguria A, et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy . Cell Death Dis. 2013;4:e691. doi: 10.1038/cddis.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, He W, Ochi H. A new murine oxidative stress model associated with senescence . Mech Ageing Dev. 2001;122:547–59. doi: 10.1016/s0047-6374(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Gaur T, Hussain S, Mudhasani R, et al. Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse . Dev Biol. 2010;340:10–21. doi: 10.1016/j.ydbio.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff LA, Boucher S, Ricupero CL, et al. Differentiating human multipotent mesenchymal stromal cells regulate microRNAs: prediction of microRNA regulation by PDGF during osteogenesis . Exp Hematol. 2008;36:1354–69. doi: 10.1016/j.exphem.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillari J, Grillari-Voglauer R. Novel modulators of senescence, aging, and longevity: Small non-coding RNAs enter the stage . Exp Gerontol. 2010;45:302–11. doi: 10.1016/j.exger.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Hackl M, Brunner S, Fortschegger K, et al. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging . Aging Cell. 2010;9:291–6. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains . Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- He C, Xiong J, Xu X, Lu W, Liu L, Xiao D, Wang D. Functional elucidation of MiR-34 in osteosarcoma cells and primary tumor samples . Biochem Biophys Res Commun. 2009;388:35–40. doi: 10.1016/j.bbrc.2009.07.101. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates . Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence . Biochem Biophys Res Commun. 2010;398:735–40. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice . Nature. 2011;469:102–6. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates . Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Suh Y. MicroRNA in Aging: From Discovery to Biology . Curr Genomics. 2012;13:548–57. doi: 10.2174/138920212803251436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen T A Jakobsen RB, Mikkelsen TS, Brinchmann JE. microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN . Stem Cells Dev. 2014;23:290–304. doi: 10.1089/scd.2013.0209. [DOI] [PubMed] [Google Scholar]
- Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions . Aging Cell. 2011;10:191–7. doi: 10.1111/j.1474-9726.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- Kassem M, Ankersen L, Eriksen EF, Clark BF, Rattan SI. Demonstration of cellular aging and senescence in serially passaged long-term cultures of human trabecular osteoblasts . Osteoporos Int. 1997;7:514–24. doi: 10.1007/BF02652556. [DOI] [PubMed] [Google Scholar]
- Kim KM, Park SJ, Jung SH, et al. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1 . J Bone Miner Res. 2012a;27:1669–79. doi: 10.1002/jbmr.1604. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim C, Choi YS, Kim M, Park C, Suh Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects . Mech Ageing Dev. 2012b;133:215–25. doi: 10.1016/j.mad.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Koppelstaetter C, Schratzberger G, Perco P, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation . Aging Cell. 2008;7:491–7. doi: 10.1111/j.1474-9726.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis . Cancer Sci. 2010;101:2087–92. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Kim HH, Abdelmohsen K, et al. p16(INK4a) translation suppressed by miR-24 . PLoS one. 2008;3:e1864. doi: 10.1371/journal.pone.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschober GT, Brunauer R, Jamnig A, Fehrer C, Greiderer B, Lepperdinger G. Leptin receptor/CD295 is upregulated on primary human mesenchymal stem cells of advancing biological age and distinctly marks the subpopulation of dying cells . Exp Gerontol. 2009;44:57–62. doi: 10.1016/j.exger.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration . Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14 . Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence . Mech Ageing Dev. 2009a;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xie H, Liu W, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans . J Clin Invest. 2009b;119:3666–77. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gibson G, Kim JS, et al. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis . Gene. 2011;480:34–41. doi: 10.1016/j.gene.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Ha CT, Fu D, Xiao M. Micro-RNA30c negatively regulates REDD1 expression in human hematopoietic and osteoblast cells after gamma-irradiation . PloS one. 2012;7:e48700. doi: 10.1371/journal.pone.0048700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Ha CT, Fu D, Xiao M. REDD1 protects osteoblast cells from gamma radiation-induced premature senescence . PloS one. 2012;7:e36604. doi: 10.1371/journal.pone.0036604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang J, Zhang L, Si M, Yin H, Li J. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling . Carcinogenesis. 2013;34:1601–1610. doi: 10.1093/carcin/bgt065. [DOI] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Volinia S, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program . Proc Natl Acad Sci USA. 2008;105:13906–11. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis . Clin Geriatr Med. 2010;26:371–86. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes OC, An J, Sarojini H, Wang E. Murine microRNAs implicated in liver functions and aging process . Mech Ageing Dev. 2008a;129:534–41. doi: 10.1016/j.mad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Maes OC, An J, Sarojini H, Wu H, Wang E. Changes in MicroRNA expression patterns in human fibroblasts after low-LET radiation . J Cell Biochem. 2008b;105:824–34. doi: 10.1002/jcb.21878. [DOI] [PubMed] [Google Scholar]
- Magenta A, Cencioni C, Fasanaro P, et al. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition . Cell Death Differ. 2011;18:1628–39. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair . J Bone Joint Surg (Am) (Suppl 2) 2003;85:106–10. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney . Kidney Int. 2004;65:510–20. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1 . Circulation. 2009;120:1524–32. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection . Proc Natl Acad Sci USA. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis . Nat Rev Rheumatol. 2012;8:543–52. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi F, Izu Y, Hayata T, et al. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption . J Cell Bioch. 2010;109:866–75. doi: 10.1002/jcb.22228. [DOI] [PubMed] [Google Scholar]
- Murata K, Yoshitomi H, Tanida S, et al. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis . Arthritis Res Ther. 2010;12:R86. doi: 10.1186/ar3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Nakasa T, Mochizuki Y, et al. Induction of apoptosis in the synovium of mice with autoantibody-mediated arthritis by the intraarticular injection of double-stranded MicroRNA-15a . Arthritis Rheum. 2009;60:2677–83. doi: 10.1002/art.24762. [DOI] [PubMed] [Google Scholar]
- Pauley KM, Cha S. miRNA-146a in rheumatoid arthritis: a new therapeutic strategy . Immunotherapy. 2011;3:829–31. doi: 10.2217/imt.11.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Blimline M, Magerko KA, et al. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation . Exp Gerontol. 2012;47:45–51. doi: 10.1016/j.exger.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml E, Grillari J. From cellular senescence to age-associated diseases: the miRNA connection . Longevity and healthspan. 2012;1 doi: 10.1186/2046-2395-1-10. DOI 10.1186/2046-2395-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Nakasa T, Yamasaki K, et al. The effect of intra-articular injection of microRNA-210 on ligament healing in a rat model . Am J Sports Med. 2012;40:2470–8. doi: 10.1177/0363546512458894. [DOI] [PubMed] [Google Scholar]
- Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging . J Cell Sci. 2012;125:7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. Role of oxidative stress in senescence. Aging. 1998;10:149–51. [PubMed] [Google Scholar]
- Song J, Kim D, Chun CH, Jin EJ. MicroRNA-9 regulates survival of chondroblasts and cartilage integrity by targeting protogenin . Cell Commun Signal. 2013a;11:66. doi: 10.1186/1478-811X-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lee M, Kim D, Han J, Chun CH, Jin EJ. MicroRNA-181b regulates articular chondrocytes differentiation and cartilage integrity . Biochem Biophys Res Commun. 2013b;431:210–4. doi: 10.1016/j.bbrc.2012.12.133. [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells . Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies . Mech Ageing Dev. 2008;129:163–73. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function . J Biol Chem. 2009;284:4667–4678. doi: 10.1074/jbc.M805777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi K, Kubota S, Ohgawara T, et al. Identification of miR-1 as a micro RNA that supports late-stage differentiation of growth cartilage cells . Biochem Biophys Res Commun. 2010;402:286–90. doi: 10.1016/j.bbrc.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells . Proc Natl Acad Sci USA. 2007;104:15472–7. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayanithy V, Sarver AL, et al. Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma . Bone. 2012;50:171–81. doi: 10.1016/j.bone.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease . Cardiovasc Res. 2008;79:562–570. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- Ukai T, Sato M, Akutsu H, Umezawa A, Mochida J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism . J Orthop Res. 2012;30:1915–22. doi: 10.1002/jor.22157. [DOI] [PubMed] [Google Scholar]
- Vonk LA, Kragten AH, Dhert WJ, Saris DB, Creemers LB. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes . Osteoarthritis Cartilage. 2014;22:145–53. doi: 10.1016/j.joca.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process . PloS one. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guo B, Li Q, et al. miR-214 targets ATF4 to inhibit bone formation . Nat Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Moore BT, et al. MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis . PloS one. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilner S, Schraml E, Redl H, Grillari-Voglauer R, Grillari J. Secretion of microvesicular miRNAs in cellular and organismal aging . Exp Gerontol. 2013;48:626–33. doi: 10.1016/j.exger.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann J, Jack HM. Serum microRNAs as powerful cancer biomarkers . Biochim Biophys Acta. 2010;1806:200–7. doi: 10.1016/j.bbcan.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Wittmann J, Jack HM. microRNAs in rheumatoid arthritis: midget RNAs with a giant impact . Ann Rheum Dis. 2011;70(i92)(96) doi: 10.1136/ard.2010.140152. Suppl. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis . Proc Natl Acad Sci USA. 2008;105:13421–6. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop . Cell Cycle. 2009;8:712–5. doi: 10.4161/cc.8.5.7753. [DOI] [PubMed] [Google Scholar]
- Yan K, Gao J, Yang T, et al. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo . PloS one. 2012;7:e33778. doi: 10.1371/journal.pone.0033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Wang G, Hu C, et al. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis . J Bone Miner Res. 2013;28:559–73. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- Yi C, Wang Q, Wang L, et al. MiR-663, a microRNA targeting p21(WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma . Oncogene. 2012;31:4421–33. doi: 10.1038/onc.2011.629. [DOI] [PubMed] [Google Scholar]
- Yu KR, Lee S, Jung JW, et al. MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24 . J Cell Sci. 2013;126:5422–31. doi: 10.1242/jcs.133314. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Nguyen v T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function . Arthritis Res Ther. 2005;7:R380–91. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1 . Am J Physiol Endocrinol Metab. 2010;299:E110–6. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Srivastava D. A developmental view of microRNA function . Trends Biochem Sci. 2007;32:189–97. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts . Aging Cell. 2008;7:335–43. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyan W, Shuhua Y, Xiufang W, Xiaoyun L. MicroRNA-21 is involved in osteosarcoma cell invasion and migration . Med Oncol. 2011;28:1469–74. doi: 10.1007/s12032-010-9563-7. [DOI] [PubMed] [Google Scholar]