Abstract
Background and purpose
The optimal hip replacement for young patients remains unknown. We compared patient-reported outcome measures (PROMs), revision risk, and implant costs over a range of hip replacements.
Methods
We included hip replacements for osteoarthritis in patients under 60 years of age performed between 2003 and 2010 using the commonest brand of cemented, cementless, hybrid, or resurfacing prosthesis (11,622 women and 13,087 men). The reference implant comprised a cemented stem with a conventional polyethylene cemented cup and a standard-sized head (28- or 32-mm). Differences in implant survival were assessed using competing-risks models, adjusted for known prognostic influences. Analysis of covariance was used to assess improvement in PROMs (Oxford hip score (OHS) and EQ5D index) in 2014 linked procedures.
Results
In males, PROMs and implant survival were similar across all types of implants. In females, revision was statistically significantly higher in hard-bearing and/or small-stem cementless implants (hazard ratio (HR) = 4) and resurfacings (small head sizes (< 48 mm): HR = 6; large head sizes (≥ 48 mm): HR = 5) when compared to the reference cemented implant. In component combinations with equivalent survival, women reported significantly greater improvements in OHS with hybrid implants (22, p = 0.006) and cementless implants (21, p = 0.03) (reference, 18), but similar EQ5D index. For men and women, National Health Service (NHS) costs were lowest with the reference implant and highest with a hard-bearing cementless replacement.
Interpretation
In young women, hybrids offer a balance of good early functional improvement and low revision risk. Fully cementless and resurfacing components are more costly and do not provide any additional benefit for younger patients.
Implants in young patients must perform to a higher level while lasting longer. Literature from the 1980s described high rates of early loosening and implant failure following cemented hip replacement in younger patients (Chandler et al. 1981, Dorr et al. 1983, Collis 1984, Ranawat et al. 1984, Sharp and Porter 1985). These problems drove the development of cementless implants; larger, more anatomical head sizes; and hard bearings (Lord et al. 1979, Sedel et al. 1990, Cuckler et al. 2004, Delaunay et al. 2008). It was hoped that these advances would reduce rates of aseptic revision by addressing the main causes of failure (polyethylene (PE) debris and polymethylmethacrylate-bone interface loosening) and reduce early dislocation rates. Resurfacing devices were also introduced to provide an anatomical solution, providing a lower risk of dislocation, a perceived greater function, and “easier” revision if required (Spencer 2011). Implant failures remain, however, but the mode of failure has changed: high dislocation rates with ceramic-on-ceramic (CoC) bearings and metal wear-related failures with large-head metal-on-metal (MoM) bearings (Sexton et al. 2009, Haddad et al. 2011, Smith et al. 2012b).
Findings from worldwide registry data show that cemented implants outperform all others, in terms of implant survival (Finnish National Arthoplasty Registry 2006, New Zealand National Joint Registry 2008, Norwegian Arthroplasty Registry 2008, Australian National Joint Registry 2010, Swedish Hip Registry 2010, England and Wales National Joint Registry 2012). Following their analysis of the literature, Sedrakyan et al. (2011) found that there were no benefits of using hard bearings instead of PE bearings.
Of the primary hip replacements performed in 2011 in England and Wales with patient data available, 20% (13,871 of 68,331; 7,249 women and 6,622 men) were implanted in patients under 60 years of age. The majority of these replacements used cementless fixation (either fully cementless (60%, 8,372 of 13,871) or hybrid (15%, 2,064)), or used a resurfacing device (8.4%, 1,159). The evidence for this practice therefore remains elusive. It may be that some combinations improve implant survival or function, but the subtleties of brand differences may be lost when implants are analyzed within groups, as in joint registry analyses.
Implant survival data and patient functional outcome can now be assessed by using linked data from the Patient-Reported Outcomes Measures (PROMs) project and the National Joint Registry (NJR) in England and Wales. Hypothesizing that no implants offer superior functional outcome and survival, we compared different types of replacements to identify optimal combinations for young patients, employing the most commonly used standard cemented hip replacement as the reference case.
Methods
Design
We conducted a cohort study using prospectively collected patient-level NJR and PROMs data to compare implant survival and patient-reported outcomes in different primary hip replacements. Material costs were analyzed using National Health Service (NHS) procurement data.
Data
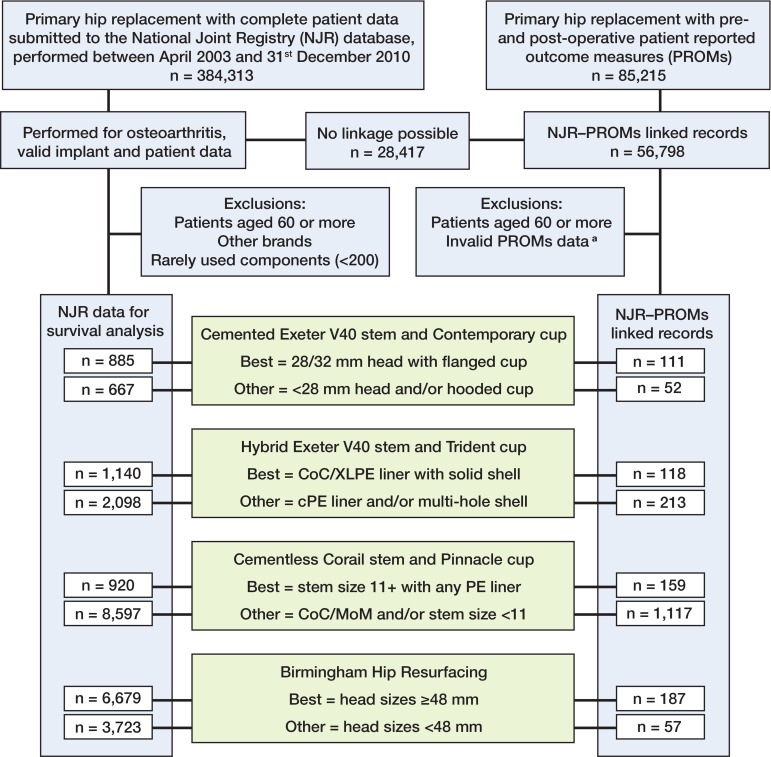
We chose the single most commonly used brand of each type of hip replacement performed in England and Wales for the analysis, in order to control for brand heterogeneity within groups: (1) cemented (taper slip design), Exeter V40 stem/Contemporary cup (Stryker Orthopaedics, Mahwah, NJ) (23% of cemented implants); (2) cementless, Corail stem/Pinnacle cup (DePuy Ltd., Leeds UK) (31% of cementless); (3) hybrid, Exeter V40 stem/Trident cup (Stryker Orthopaedics) (33% of hybrids); and (4) resurfacing, Birmingham Hip Resurfacing (BHR) (Smith and Nephew, Memphis, TN) (55% of resurfacings) (England and Wales National Joint Registry 2012). These implants have been separately stratified into 2 groups based on revision risk of component options (Jameson et al. 2012a, b, 2013b, c). The “best”-performing component sets were: cemented Exeter with the Contemporary flanged cup and a 28-mm or 32-mm femoral head (metal or ceramic); hybrid Exeter with solid shell Trident cup and either a CoC bearing or a highly crosslinked PE liner (with either a metal head or a ceramic head); cementless Corail stem (size 11 or greater) with a Pinnacle cup and a PE liner (metal or ceramic head); and BHR using components with a head size of 48 mm or greater. All the remaining options had statistically significantly higher revision risk and were separately grouped as “others”: cemented Exeter with the Contemporary hooded cup and/or a head size less than 28 mm; hybrid Exeter with Trident multi-hole shell and/or a conventional PE liner; cementless small-stem Corail (less than size 11) and/or a Pinnacle cup with any hard-bearing liner; and BHR using head sizes of less than 48 mm (Figure).
Flow chart describing inclusion criteria.
The NJR has collected patient, implant, and surgeon data on all hip replacements performed in the public and private health systems in England and Wales since 2003. Submission of private health system data was mandatory from 2003, but public health providers were not obliged to submit data during the period of study. Despite this, compliance (the number of procedures recorded by the NJR compared with the number recorded by the NHS) rose from 60% in 2003 to 100% in 2010 (England and Wales National Joint Registry 2012).
In this study, all primary hip replacements were included if performed using the specified implants on patients under 60 years of age and submitted to the NJR between April 1, 2003 and December 31, 2010. There were a number of other exclusion criteria: all procedures with an indication other than OA (which represents only 7% of procedures (England and Wales National Joint Registry 2012); procedures with missing implant or patient data; and rarely used implant options. From data described in the original studies, between 4.0% and 14% of correctly specified procedures on patients with OA were excluded due to rarely used implant components or missing component data fields.
The national PROMs project was introduced in 2008 and uses validated measures of hip-specific function (Oxford hip score (OHS)) (Dawson et al. 1996) and general health status (EuroQol (EQ-5D-3L)) (group E 2009), collected preoperatively and around 6 months postoperatively (public health system patients only). By linking databases at the patient level, PROMs data can be combined with the corresponding demographic and operative details held in the NJR. To carry out linkage, we used a number of criteria: firstly, to ensure correct matching, 2 unique identifiers (NJR and procedure numbers) recorded in both datasets were used; secondly, the operation date recorded by the patient in the PROMs data had to be within ± 30 days of the operation date recorded in the NJR record, to ensure the patient was scoring the same procedure. Procedures with PROMs data that were missing, undated, dated more than 12 months prior to or following the operation, or non-identical duplicates were excluded; for identical duplicates, the first record was retained for analysis. Where the presence of a comorbidity was sought in the questionnaire but left blank by the patient, it was assumed to be absent. The study population is summarized in the Figure. The demographic, surgical, and implant-related variables available for analysis are listed in Table 1 (Supplementary data).
For this analysis, the patient-reported outcomes of interest were improvements between the preoperative and postoperative scores (the “change scores”). Change scores, being approximately normally distributed, are analytically preferable to postoperative scores (Browne et al. 2007). The OHS (score 0–48) has been shown to be a reliable, valid, and responsive outcome measure (Murray et al. 2007). A clinically relevant improvement in OHS is considered to be greater than 3 (Murray et al. 2007). The EQ-5D-3L index (where 0 is death, 1 is perfect health, and < 1 is “worse than death”) is a measure of health status that is used widely in clinical and economic evaluations. Patients are asked about comorbidities, general health, and self-reported disability as part of the preoperative PROMs questionnaire. These can be used to adjust for differences in health status between patient groups.
In the PROMs after surgery, patients are also requested to indicate their satisfaction with the outcome (excellent, very good, good, fair, or poor), and whether they deem surgery to have been a success (much better, a little better, about the same, a little worse, or much worse). While unadjusted values of success and satisfaction have been provided for information in this study, we made no attempt to adjust for baseline differences in these measures, as previous analyses have shown that the variables available in the NJR and PROMs databases are insufficient to explain any differences (i.e. the influence of unmeasured variables has a greater effect than the effect of the measured variables) (Browne et al. 2007, Hamilton et al. 2013).
24,709 procedures were available for analysis in the NJR dataset, comprising the most commonly used brands of cemented (1,552, 6.3%), hybrid (3,238, 13%), cementless (9,517, 39%), and resurfacing (10,402, 42%) replacements (Figure). Due to relatively poor compliance and fewer hip replacements performed in the early years of the registry, mean follow-up time was 2.7 years (median 2.4) despite the fact that the range was 0–8 years. Numbers of patients with 6-year survival data were: 153 best cemented, 212 other cemented, 212 best hybrid, 244 other hybrid, 107 best cementless, 381 other cementless, 1,573 best resurfacing, and 2,223 other resurfacing. Resurfacing procedures were more likely to have been performed in younger, fitter patients (Table 2). The majority of smaller (< 48-mm head size) resurfacing procedures (“other”) were performed in women (78%). Across the total hip replacement groups, patient variables were clinically very similar, although the “best” hybrid procedures were more likely to be performed in younger, fitter patients. The entire NJR population demographics profile was qualitatively similar to that of the smaller NJR-PROMs linked population (Tables 2 and 3).
Table 2.
Patient demographics for the National Joint Registry population studied, by implant group
| Cemented |
Hybrid |
Cementless |
Resurfacing |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Best | Others | Best | Others | Best | Others | Best | Others | p-value a | |
| Number (%) | 885 (4) | 667 (3) | 1,140 (5) | 2,098 (9) | 920 (4) | 8,597 (35) | 6,679 (27) | 3,723 (15) | |
| Age, median (range) | 56 (23–60) | 57 (26–60) | 54 (18–60) | 55 (20–60) | 57 (27–60) | 54 (16–60) | 52 (19–60) | 52 (19–60) | < 0.001 |
| Females, n (%) | 548 (62) | 412 (62) | 642 (56) | 1,340 (64) | 454 (49) | 4,880 (57) | 432 (7) | 2,914 (78) | < 0.001 |
| ASA, n (%) b | |||||||||
| 1 | 263 (30) | 193 (29) | 430 (38) | 677 (32) | 252 (27) | 2,815 (33) | 3,750 (56) | 1,969 (53) | < 0.001 |
| 2 | 543 (61) | 421 (63) | 664 (58) | 1,246 (59) | 603 (66) | 5,233 (61) | 2,748 (41) | 1,677 (45) | |
| 3+ | 79 (9) | 53 (8) | 46 (4) | 175 (8) | 65 (7) | 549 (6) | 181 (3) | 77 (2) | |
| BMI, mean (SD) (range) c | 30 (6) | 30 (5) | 30 (5) | 30 (6) | 30 (5) | 30 (5) | 29 (4) | 28 (5) | < 0.001 |
| (18–59) | (16–50) | (18–56) | (16–54) | (17–52) | (16–65) | (16–51) | (16–63) | ||
Differences between groups. Statistical notes: 1-way analysis of variance (ANOVA) was used for normally distributed data, Kruskal-Wallis test for non-normally distributed data, and chi-squared test for proportions;
ASA: American Society of Anaesthesiologists;
BMI: body mass index, kg/m2 (data based on 9,544 procedures (39%)).
Table 3.
Patient demographics for the National Joint Registry-PROMs a linked population studied, by implant group
| Cemented |
Hybrid |
Cementless |
Resurfacing |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Best | Others | Best | Others | Best | Others | Best | Others | p-value b | |
| Number (%) | 111 (6) | 52 (3) | 118 (6) | 213 (11) | 159 (8) | 1,117 (56) | 187 (9) | 57 (3) | |
| Age, median (range) | 56 (37–60) | 57 (48–60) | 54 (30–60) | 56 (28–60) | 57 (39–60) | 54 (25–60) | 52 (32–60) | 54 (35–60) | < 0.001 |
| Females, n (%) | 70 (63) | 37 (72) | 73 (62) | 147 (69) | 82 (52) | 671 (60) | 8 (4) | 41 (72) | < 0.001 |
| ASA c | |||||||||
| 1 | 34 (31) | 11 (21) | 44 (37) | 75 (35) | 40 (25) | 377 (34) | 84 (45) | 24 (42) | < 0.001 |
| 2 | 63 (57) | 33 (64) | 67 (57) | 123 (58) | 109 (69) | 687 (62) | 101 (54) | 31 (54) | |
| 3+ | 14 (13) | 8 (15) | 7 (6) | 15 (7) | 10 (6) | 53 (5) | 2 (1) | 2 (4) | |
| BMI, mean (SD) (range) d | 29 (6) | 30 (6) | 30 (5) | 30 (6) | 31 (5) | 30 (6) | 29 (4) | 29 (6) | |
| (19–59) | (19–49) | (20–47) | (18–50) | (17–46) | (18–65) | (18–45) | (19–43) | 0.7 | |
PROMs: patient-reported outcome measures;
Statistical notes: 1-way ANOVA was used for normally distributed data, Kruskal-Wallis test for non-normally distributed data, and chi-squared test for proportions;
ASA: American Society of Anaesthesiologists;
BMI: body mass index, kg/m2 (data based on 1,293 procedures (64%)).
Statistics
Implants were analyzed based on previously stratified revision risk; thus, we compared 8 groups (the optimal implant options were defined as “best”, while the remaining options were grouped as “other” for each of the 4 types of replacement) (Figure). Differences in baseline characteristics across the groups would be a source of confounding in any comparative analysis. Therefore, to test the hypothesis that there were no differences between groups, we employed the following tests: 1-way analysis of variance (ANOVA, normally distributed continuous data variables), the Kruskal-Wallis test (non-normally distributed continuous data variables), and the chi-squared test (categorical data variables).
Bivariable analysis was performed initially to identify variables potentially influencing each outcome, based on statistical rejection criteria of p > 0.1; these variables were then included in the multivariable models.
We used competing-risks regression models (CRR) to test adjusted differences in survival across the implant groups, where patient death prior to either revision or censoring was the competing risk. In contrast to Cox proportional hazards (CPH) models, death is treated as a permanent condition that prevents future revision from occurring (and so is a competing event to revision) rather than merely a censoring event. CPH analysis tends to overestimate the risk of revision, which progressively worsens over time, particularly when the risk of death is higher than the risk of revision (for example, in elderly patients). Although it could be argued that a young population is not particularly susceptible to this inaccuracy at medium-term follow-up, we felt this approach was the most suitable. We used the ‘stcrreg’ command in STATA to implement the competing-risks regression based on the proportional sub-hazards model of Fine and Gray (1999). CRR is semi-parametric in that the baseline sub-hazard of the event of interest is left unspecified, and the effects of covariates are assumed to be proportional. Although it is possible to allow the same covariate to have a different effect on the main risk and the competing risk, we felt that this was unnecessary given that the risk of death and revision was unlikely to vary greatly across the age range analyzed. Survival times for patients who had not undergone revision or had not died were censored at the study census date (December 31, 2010).
We used analysis of covariance (ANCOVA) for testing of differences in OHS and EQ5D index change scores. Time from implantation to questionnaire completion was included in models to evaluate whether differences in duration of follow-up influenced findings. Preoperative scores were included within all models, as recommended by the designers of the OHS (Murray et al. 2007).
The reliability of the multivariable statistical models was explored in a number of ways: covariates found not to be statistically significant were excluded from the model, based on statistical entry criteria (p < 0.1); the same covariates were fitted forward and reverse stepwise manually to ensure that findings were not qualitatively affected in the final model, with any inconsistency reported. We then re-evaluated the final models as a directly entered model (non-stepwise), assessed by exploring 2-way interactions between covariates and, for the survival analysis, assessed for the assumption of constant proportionality over time. Clustering of data may have an adverse effect on the results of one particular group, especially in registry studies where comparison groups are relatively small. For example, if a poorly performing hospital or surgeon contributes disproportionally to one group, the results of that group may be incorrectly poor. We did not adjust for clustering. However, previous registry analyses have found little difference in results when attempting this adjustment (Smith et al. 2012b).
The results of survival analysis are presented as hazard ratios (HRs). Statistical models for the change scores were evaluated with the margins function in STATA in order to provide predicted values separately for each of the implant groups. The p-values refer to statistical tests of the differences between the reference implant (cemented Exeter with a Contemporary flanged PE cup and 28- or 32-mm metal or ceramic head) and the 7 others. Significance was assumed at p < 0.05. Estimates are reported with 95% confidence intervals (CIs). All models were fitted using STATA software version 12. For the purpose of identification, parameter estimates with probabilities < 5% were considered significant, with further consideration of the clinical importance of magnitude of estimates.
Costs for specific implant combinations were provided by NHS Wales (all 7 units within Wales) and an NHS supply chain (buyers on behalf of 30 units within the English NHS). The highest and lowest prices paid for implants during 2012 were analyzed and a modal cost was provided for each of the implant components. These costs represent actual prices paid, after discounts but excluding value-added tax (VAT) at 20% and the NJR levy fee (£20, which is included in the cost of each implant). Costs presented also include acetabular screws (for cementless cup fixation) when used, the commonest cement used for each implant type, femoral cement restrictors, and all the equipment required to mix and perform pressurized cementation. For the purposes of this analysis, we assumed that theater use and length of stay were similar for all types of replacement; thus, differences between implant combination costs approximate to differences in NHS costs. £1 is equivalent to □1.22 and to $1.70 (correct as of May 7, 2014).
Explicit patient consent was taken for both the NJR and PROMs data collection. Further ethics approval is not required for registry studies in the UK.
Results
Patient-reported outcomes were available for 2014 procedures (8.2%), comprising cemented (163, 11% of NJR data), hybrid (331, 10%), cementless (1,276, 13%), and resurfacing (244, 2.3%) replacements (Table 4). Preoperative OHS and EQ5D indices were similar across implant groups, except the large-head resurfacing group (“best”) where patients had a 3.3 to 6.0 times higher preoperative OHS. Postoperative OHS values were generally lower in the cemented group, but postoperative EQ5D indices were similar in all groups. There were no statistically significant differences between groups regarding those who reported their satisfaction with the procedure and those who reported that the operation had been successful (Table 4).
Table 4.
Patient-reported outcomes for populations studied, by implant group
| Cemented |
Hybrid |
Cementless |
Resurfacing |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Best | Others | Best | Others | Best | Others | Best | Others | p-value a | |
| Number (%) | 111 (6) | 52 (3) | 118 (6) | 213 (11) | 159 (8) | 1,117 (56) | 187 (9) | 57 (3) | |
| Oxford hip scores | |||||||||
| Preoperative | |||||||||
| mean (SD) | 19 (9) | 16 (7) | 19 (8) | 18 (8) | 17 (7) | 18 (8) | 22 (8) | 19 (8) | 0.02 |
| range | 3–40 | 3–33 | 4–37 | 1–36 | 2–39 | 2–46 | 4–43 | 4–37 | 0.02 |
| Postoperative, | |||||||||
| median (range) | 41 (0–48) | 40 (4–48) | 43 (8–48) | 43 (7–48) | 42 (4–48) | 43 (2–48) | 46 (2–48) | 43 (5–48) | < 0.001 |
| EQ5D index | |||||||||
| Preoperative, | |||||||||
| mean (SD) | 0.40 (0.30) | 0.28 (0.32) | 0.41 (0.32) | 0.35 (0.33) | 0.32 (0.31) | 0.35 (0.32) | 0.47 (0.31) | 0.38 (0.34) | 0.3 |
| range | -0.24–0.85 | -0.18–0.80 | -0.24–0.80 | -0.59–0.81 | -0.35–0.73 | -0.25–1 | -0.24–1 | -0.24–0.81 | |
| Postoperative, | |||||||||
| median | 0.81 | 0.73 | 0.81 | 0.82 | 0.80 | 0.82 | 1.00 | 0.81 | < 0.001 |
| range | -0.59–1 | -0.02–1 | -0.24–1 | -0.35–1 | -0.07–1 | -0.24–1 | -0.35–1 | -0.24–1 | < 0.001 |
| Satisfaction (n, %) | |||||||||
| Good to excellent | 99 (89) | 44 (85) | 110 (93) | 198 (93) | 150 (94) | 1,033 (93) | 170 (91) | 50 (88) | 0.3 |
| Poor/fair | 12 (11) | 8 (15) | 8 (7) | 15 (7) | 9 (6) | 84 (8) | 17 (9) | 7 (12) | |
| Success (n, %) | |||||||||
| Better | 105 (95) | 47 (90) | 113 (96) | 207 (98) | 155 (98) | 1,072 (96) | 181 (97) | 53 (93) | 0.3 |
| About the same | |||||||||
| or worse | 6 (5) | 5 (10) | 5 (4) | 6 (3) | 4 (3) | 45 (4) | 6 (3) | 4 (7) | |
| Time from op. to | |||||||||
| PROMs complete, | |||||||||
| mean days (SD) | 213 (32) | 219 (37) | 211 (26) | 210 (28) | 210 (30) | 210 (28) | 272 (45) | 269 (49) | < 0.001 |
| range | 188–343 | 186–329 | 186–315 | 183–332 | 187–350 | 183– 361 | 186–360 | 186–353 | |
PROMs: patient-reported outcome measures.
Statistical notes: 1-way ANOVA was used for normally distributed data, Kruskal-Wallis test for non-normally distributed data, and chi-squared test for proportions.
In women, revision was higher in “other” (hard-bearing or small-stem) cementless implants (HR = 3.4, CI: 1.1–11) and resurfacings (“best”, large head: HR = 5.0, CI: 1.5–17; “other”, small head: HR = 6.7, CI: 2–30) when compared to the reference (cemented) group. The “best” hybrid group (solid shell with a CoC or metal/ceramic on highly crosslinked PE) had similar implant survival (HR = 1.4, CI: 0.3–6) (Table 5). Greater improvements in OHS were seen in the hybrid groups (“best”: 22, CI: 20–24; “other”, multi-hole shells or standard PE liner: 22, CI: 20–24) and the cementless groups (“best”, PE liners: 21, CI: 19–23; “other”: 22, CI: 22–23) when compared with the “best” cemented (18, CI: 16–20). The “best” resurfacing procedures showed good results, but this was based on only 8 procedures (Table 6). EQ5D indices were similar in all groups.
Table 5.
Risk of revision following hip replacement in patients aged < 60 years of age (simple and multivariable analyses)
| Simple | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Females (n = 11,622) | ||||||
| Best cemented (n = 548) | 1 | 1 | ||||
| Other cemented (n = 412) | 3.06 | 0.81–11.5 | 0.1 | 3.12 | 0.83–11.8 | 0.1 |
| Best hybrid (n = 642) | 1.42 | 0.34–5.94 | 0.6 | 1.39 | 0.33–5.78 | 0.7 |
| Other hybrid (n = 1,340) | 2.87 | 0.85–9.65 | 0.1 | 2.78 | 0.83–9.35 | 0.1 |
| Best cementless (n = 454) | 1.47 | 0.30–7.24 | 0.6 | 1.50 | 0.30–7.38 | 0.6 |
| Other cementless (n = 4,880) | 3.45 | 1.10–10.9 | 0.03 | 3.35 | 1.06–10.6 | 0.04 |
| Best resurfacing (n = 432) | 5.12 | 1.50–17.5 | 0.009 | 4.95 | 1.45–16.9 | 0.01 |
| Other resurfacing (n = 2,914) | 6.57 | 2.09–20.6 | 0.001 | 6.36 | 2.02–30.0 | 0.002 |
| Males (n = 13,087) | ||||||
| Best cemented (n = 337) | 1 | 1 | ||||
| Other cemented (n = 255) | 0.78 | 0.17–3.46 | 0.7 | 0.77 | 0.17–3.45 | 0.7 |
| Best hybrid (n = 498) | 0.48 | 0.11–2.16 | 0.3 | 0.48 | 0.11–2.16 | 0.3 |
| Other hybrid (n = 758) | 1.40 | 0.45–4.35 | 0.6 | 1.34 | 0.43–4.17 | 0.6 |
| Best cementless (n = 466) | 0.62 | 0.14–2.79 | 0.5 | 0.62 | 0.14–2.78 | 0.5 |
| Other cementless (n = 3,717) | 1.51 | 0.55–4.18 | 0.4 | 1.46 | 0.53–4.05 | 0.4 |
| Best resurfacing (n = 6,247) | 1.01 | 0.37–2.76 | 1.0 | 1.02 | 0.37–2.78 | 1.0 |
| Other resurfacing (n = 809) | 2.08 | 0.72–6.00 | 0.2 | 2.06 | 0.71–5.97 | 0.2 |
Table 6.
Patient-reported outcome change scores following hip replacement in female patients < 60 years of age (simple and multivariable analyses)
| Simple | Multivariable | |||||
|---|---|---|---|---|---|---|
| Value | 95% CI | p-value | Value | 95% CI | p-value | |
| Change in Oxford hip score (n = 1,129) | ||||||
| Best cemented (n = 70) | 18.0 | 15.6–20.4 | Ref. | 18.2 | 16.1–20.3 | Ref. |
| Other cemented (n = 37) | 22.2 | 18.8–25.5 | 0.051 | 21.7 | 18.8–24.6 | 0.052 |
| Best hybrid (n = 73) | 21.2 | 18.8–23.6 | 0.06 | 22.3 | 20.2–24.3 | 0.006 |
| Other hybrid (n = 147) | 22.2 | 20.5–23.9 | 0.006 | 21.9 | 20.4–23.3 | 0.005 |
| Best cementless (n = 82) | 23.1 | 20.8–25.3 | 0.003 | 21.3 | 19.4–23.3 | 0.03 |
| Other cementless (n = 671) | 22.1 | 21.3–22.9 | 0.002 | 22.2 | 21.6–22.9 | < 0.001 |
| Best resurfacing (n = 8) | 29.4 | 22.2–36.5 | 0.003 | 26.6 | 20.2–33.0 | 0.01 |
| Other resurfacing (n = 41) | 22.0 | 18.8–25.2 | 0.05 | 21.0 | 18.1–24.0 | 0.1 |
| Change in EQ5D index (n = 1,129) | ||||||
| Best cemented (n = 70) | 0.367 | 0.280–0.453 | Ref. | 0.407 | 0.347–0.466 | Ref. |
| Other cemented (n = 37) | 0.458 | 0.334–0.581 | 0.2 | 0.432 | 0.348–0.517 | 0.6 |
| Best hybrid (n = 73) | 0.462 | 0.375–0.548 | 0.1 | 0.486 | 0.428–0.545 | 0.06 |
| Other hybrid (n = 147) | 0.462 | 0.401–0.523 | 0.1 | 0.453 | 0.411–0.495 | 0.2 |
| Best cementless (n = 82) | 0.453 | 0.372–0.535 | 0.2 | 0.430 | 0.372–0.487 | 0.6 |
| Other cementless (n = 671) | 0.440 | 0.412–0.468 | 0.1 | 0.438 | 0.418–0.457 | 0.3 |
| Best resurfacing (n = 8) | 0.623 | 0.377–0.870 | 0.054 | 0.517 | 0.338–0.696 | 0.2 |
| Other resurfacing (n = 41) | 0.454 | 0.341–0.567 | 0.2 | 0.421 | 0.338–0.503 | 0.8 |
In men, improvements in revision (Table 5) and PROMs were equivalent in all groups when compared to the reference (Table 7).
Table 7.
Patient-reported outcome scores following hip replacement in male patients < 60 years of age (simple and multivariable analyses)
| Simple | Multivariable | |||||
|---|---|---|---|---|---|---|
| Value | 95% CI | p-value | Value | 95% CI | p-value | |
| Change in Oxford hip score (n = 885) | ||||||
| Best cemented (n = 41) | 18.4 | 15.4–21.4 | Ref. | 20.1 | 17.6–22.7 | Ref. |
| Other cemented (n = 15) | 17.9 | 12.9–22.9 | 0.8 | 17.9 | 13.7–22.1 | 0.4 |
| Best hybrid (n = 45) | 21.0 | 18.1–23.8 | 0.2 | 21.0 | 18.5–23.4 | 0.6 |
| Other hybrid (n = 66) | 21.6 | 19.2–24.0 | 0.1 | 20.8 | 18.8–22.8 | 0.7 |
| Best cementless (n = 77) | 20.4 | 18.2–22.6 | 0.3 | 19.8 | 17.9–21.6 | 0.8 |
| Other cementless (n = 446) | 20.9 | 19.9–21.7 | 0.1 | 20.3 | 19.6–21.1 | 0.9 |
| Best resurfacing (n = 179) | 19.7 | 18.3–21.2 | 0.5 | 20.8 | 19.5–22.1 | 0.6 |
| Other resurfacing (n = 16) | 18.4 | 13.6–23.3 | 1.0 | 20.0 | 15.8–24.2 | 1.0 |
| Change in EQ5D index (n = 885) | ||||||
| Best cemented (n = 41) | 0.368 | 0.262–0.475 | Ref. | 0.392 | 0.318–0.467 | Ref. |
| Other cemented (n = 15) | 0.397 | 0.219–0.574 | 0.8 | 0.336 | 0.212–0.459 | 0.4 |
| Best hybrid (n = 45) | 0.255 | 0.157–0.354 | 0.1 | 0.325 | 0.255–0.394 | 0.2 |
| Other hybrid (n = 66) | 0.419 | 0.340–0.498 | 0.5 | 0.413 | 0.357–0.469 | 0.7 |
| Best cementless (n = 77) | 0.395 | 0.320–0.470 | 0.7 | 0.388 | 0.335–0.442 | 0.9 |
| Other cementless (n = 446) | 0.410 | 0.379–0.441 | 0.5 | 0.396 | 0.374–0.417 | 0.9 |
| Best resurfacing (n = 179) | 0.370 | 0.321–0.419 | 1.0 | 0.398 | 0.361–0.435 | 0.9 |
| Other resurfacing (n = 16) | 0.307 | 0.142–0.472 | 0.5 | 0.357 | 0.238–0.476 | 0.6 |
Tests for interaction (multiplicative) between covariates and for time-dependency were not statistically significant. Forward and reverse stepwise model construction led to the same final models. Body mass index (BMI) was selected as a variable within the competing-risks survival model for men. However, this approach excluded 63% of data. BMI was therefore excluded and the model was constructed with age and ASA group. The output from these models (simple and multivariable with either BMI or age and ASA group included) is shown in Table 8 (Supplementary data). Variables included in the statistical models, and their significance levels within the final models, are shown in Tables 910 (Supplementary data).
Implant cost data showed the standard cemented replacement in this analysis to be the cheapest (median and modal price: £928, with a range from £899 to £1,250). Resurfacing implants ranged from £1,662.01 to £2,472.34. A cementless 36-mm CoC implant cost the NHS between £2,064 and £3,551 (Table 11). Cost data were obtained from units across England and Wales.
Table 11.
Cost of specific hip implants (NHS costs 2011/12). The figures are based on actual implant costs paid to manufacturers by NHS Wales (7 Trusts) and NHS supply chain (30 Trusts in England), excluding value-added tax (VAT, 20%) and NJR levy costs (£20). £1 is equivalent to 1.22 and to $1.70 (correct as of May 7, 2014)
| Costs (£) | |||
|---|---|---|---|
| Modal | Low | High | |
| Best cemented | |||
| Exeter stem / 28-mm metallic head / flanged Contemporary cup a | 928 | 899 | 1,250 |
| Exeter stem / 32-mm ceramic head / flanged Contemporary cup a | 1,343 | 1,183 | 1,580 |
| Other cemented | |||
| Exeter stem / 26-mm metallic head / hooded Contemporary cup a | 928 | 899 | 1,250 |
| Best hybrid | |||
| Exeter stem / 32-mm metallic head / highly crosslinked polyethylene liner / solid-back Trident shell a | 1,465 | 1,440 | 2,092 |
| Exeter stem / 36-mm ceramic head / ceramic liner / solid-back Trident shell a | 1,780 | 1,780 | 2,619 |
| Other hybrid | |||
| Exeter stem / 28-mm metallic head / conventional polyethylene liner / multi-hole Trident shell a | 1,405 | 1,405 | 2,040 |
| Best cementless | |||
| Corail stem / 28-mm metallic head / conventional polyethylene liner / Pinnacle shell | 1,587 | 1,587 | 2,722 |
| Other cementless | |||
| Corail stem / 36-mm metallic head / metallic liner / Pinnacle shell | 1,791 | 1,703 | 2,924 |
| Corail stem / 36-mm ceramic head / ceramic liner / Pinnacle shell | 2,210 | 2,064 | 3,551 |
| Best resurfacing | |||
| Birmingham Hip Resurfacing using head size ≥ 48 mm a | 1,944 | 1,662 | 2,472 |
| Other resurfacing | |||
| Birmingham Hip Resurfacing using head size < 48 mm a | 1,944 | 1,662 | 2,472 |
Including cement (cemented implants, 4 mixes of Heraeus Palacos R+G at £26.75 per mix; hybrid, 2 mixes of Palacos R+G; resurfacing, 1 mix of Stryker Antibiotic Simplex at £27.72), mixing set (Optivac £44.29, 2 sets for fully cemented), cement restrictor (Hardinge £22.00, not resurfacing). For multi-hole Trident shells, costs of 2 Stryker screws are included (£40 per screw). For Pinnacle shells, the cost of 2 screws for half of the implants is included (£54.05 per screw).
Note: Exeter stems 44/5, 44/6, and all 50 offsets increase cost by £614.27 (< 5% Exeter stems) (Jameson et al. 2012).
Discussion
This large cohort study using medium-term stratified revision-risk NJR-PROMs linked data comparing types of hip replacement in patients below 60 years of age showed no advantage of resurfacing or cementless implants over standard cemented hip replacement in male patients. For women, functional outcome was better with hybrid and cementless implants. Although revision risk was similar to that of cemented for the best cementless and hybrid implants, the risk was 3.5 times higher with the commonly used hard-bearing cementless implants. Material costs, approximating to NHS costs, were lowest with a standard cemented hip replacement and highest with hard-bearing cementless implants. These findings are important for clinicians and healthcare providers to determine the most suitable and cost-effective hip implants for young patients with osteoarthritis.
We have not found any previous analyses describing stratified implant revision-risk data, patient-reported outcomes, and material costs for specific implants in young patients requiring hip replacement. However, the findings may have some limitations. As with all database analyses, the study design was observational and therefore vulnerable to omitted variables, which may have confounded our findings. Potentially important variables such as race, socioeconomic status, patient experiences, and levels of perioperative pain were unavailable, yet they are known to influence certain patient outcomes such as satisfaction (Hamilton et al. 2013). In addition, important clinical information such as radiological data was not available.
A decision about a particular patient’s surgical treatment is based on patient-related, surgical, and unit factors, and is not randomly determined. Patients who receive cementless or resurfacing implants may be more aware of implant choice and they may be more highly educated. Although statistical adjustment can help, a large proportion of variation within the models remains unexplained. There is also the possibility of over-adjustment, which may influence the precision of the results. However, despite the inherent limitations of statistical adjustment in cohort studies, the variables we selected in the models appear logical. Some surgical factors such as volume, grade of surgeon, and approach have been included in analyses, but analysis of the effects of data clustering (in terms of surgeon and unit) was not possible.
As a result of limiting the study to specific brands and stratifying implant options, the numbers in some groups were low and there may have been bias. The PROMs feasibility pilot indicated that the minimum numbers of PROMs required within each comparison group were in the order of 150 for identification of meaningful differences (Browne et al. 2007). The analyses were possibly underpowered to detect differences between implants, and this might—in isolation—explain the lack of significant findings in men. However, similar numbers gave clearly significant findings in women. Qualitatively, this is unlikely to have occurred by chance given the consistent interaction with gender, although this analysis could usefully be replicated in other future database analyses to correlate theses findings.
The NJR currently only covers medium-term survival; many procedures have short follow-up. Polyethylene wear-associated revision may occur in greater numbers beyond 10 years, and hard bearings may ultimately have greater longevity, but there is currently no evidence to support this. A systematic review of worldwide registry and cohort study data failed to show any benefit of other bearings over metal-on-PE (MoP) bearings (Sedrakyan et al. 2011). Furthermore, Australian joint registry data suggest that metal on highly crosslinked PE has the lowest 10-year revision risk (Australian National-Joint Registry 2012) and dislocation rates are higher with CoC bearings (Sexton et al. 2009). In England and Wales, the use of MoM has declined dramatically over the last 5 years due to concerns about metal wear debris reactions (Medicines and Healthcare products Regulatory Agency 2011, England and Wales National Joint Registry 2012).
The validity of NJR data has been questioned, with loss of data or under-reporting of revision numbers a possibility, although this should affect each group equally. The PROMs data are recorded at 6 months only. This may be too early for determination of the success of a joint replacement. However, the Oxford group has published data showing that PROMs improve to 12 months, with the greatest improvement in the first 3 months. No improvements were seen between 12 months and 5 years, suggesting that the results of our short-term study are a reliable indication of longer-term outcome (Andrew et al. 2008, Judge et al. 2013). There may be selection bias in the PROMs data, as response rates may be differ in patients of different ages, different socioeconomic groups, and different races. However, we could not assess whether there was any bias in completion and return of PROMs, as no details were available regarding the number of questionnaires sent out or returned. The point at which a patient undergoes a hip procedure may also be different (reflecting the need to adjust for preoperative scores) depending on age, expectations, and occupation. Patients undergoing resurfacing tended to have higher preoperative scores. This may in turn limit their ability to improve after joint replacement, due to the ceiling effect in the OHS and EQ5D index.
The discrepancy between the ratio of NJR-PROMS linked episodes to total NJR episodes across implants (1:10 for cemented vs. 1:50 for resurfacing) is difficult to explain, but may be due to a generally younger resurfacing population, or because there was a higher proportion of resurfacings in the private sector (for which PROMs are not available). This may limit the conclusions that can be drawn from the resurfacing data.
Pennington et al. (2013) recently published a paper on cost effectiveness using NJR, PROMs, and implant cost data, which compared types of hip replacement. Hybrid implants were found to have the most cost-effective profile. As in our study, the authors found that cementless implants offered no net advantage while being more costly. However, there were a number of limitations, which may have influenced the reliability of their results: resurfacings were not included; all brands within each group were analyzed together, with no adjustment for the heterogeneity of implants; and analyses were limited to MoP bearings only.
Although hybrid implants appeared to offer a balance between implant survival and functional benefit for young women in the present study, it must be stressed that this requires adequate fixation with a solid acetabular shell. Analysis of the hybrid data previously demonstrated that multi-hole shell (with screw fixation) had poorer survival ( Jameson et al. 2013b). The risk of revision in women with this combination was 3 times greater than for the best cemented implant in our data, which approached significance (p = 0.1). While a cemented procedure will have reproducible results, the success of a cementless cup is reliant on adequacy of the press-fit. In addition, there is no obvious explanation for the difference in the effect of implant type on men and women in our study. It is reasonable to suggest, therefore, that a surgeon using cemented implants in the majority of patients could also use the same implants in young women with acceptable and reproducible results.
Despite the poor results of cemented implants during the 1980s, more contemporary analyses have shown equivalent or better survival compared to cementless implants (Busch et al. 2010, Pakvis et al. 2011, Schmitz et al. 2013, Toossi et al. 2013), supporting the encouraging results of registry data. The findings from the earlier studies may have been influenced by previous generations of implants and poor cementation techniques. Data from our previous study suggest that the Exeter Contemporary system using the flanged cup design and a head size of 28 mm or greater had good and reproducible results in all patients, from all surgeons across England and Wales (Jameson et al. 2012a). Moreover, no additional survival benefit was seen when 32-mm and/or ceramic heads were used in place of 28-mm metal heads. In addition, head size and bearing type appear to have no influence on PROMs and complications across a range of implant options ( Jameson et al. 2014).
Although our study found no benefit of a resurfacing procedure in young men over a standard cemented replacement (despite inclusion of only the best-performing brand and use of large femoral head sizes), there may be long-term implant survival benefit. However, it is known that high-volume surgeons have lower revision rates in complex procedures (Jameson et al. 2012b, Baker et al. 2013), and there remain concerns regarding the local and systemic complications associated with MoM bearings (Haddad et al. 2011); the regulatory body in the United Kingdom currently stipulates that all MoM implants should be reviewed on an annual basis (Medicines and Healthcare products Regulatory Agency 2011). In addition, Costa et al. (2012) found no evidence of benefit at 12 months when patients were randomized to resurfacing or hip replacement. A cost analysis performed on the same cohort found that resurfacing offered only very short-term efficiency benefits over THA in a selected patient group (Edlin et al. 2012). A dramatic fall in the use of resurfacings, with clustered use predominantly in the young male group during 2011, would suggest that surgeons in England and Wales are responding to the evidence (England and Wales National Joint Registry 2012).
Cementless implants with mid/large stems and MoP or CoP performed well in young women with equivalent survival and better improvement in OHS compared to cemented implants. However, this group represented only 8.5% of cementless implants used in women (454 of 5,334). Moreover, 39% of females required a small stem size (7,932 of 20,166) in a previous analysis (Jameson et al. 2013a) and implant failure increased with higher BMI, suggesting that the group of women that could benefit is small. Proponents of fully cementless procedures argue that operative time is also shorter, increasing patient turnover and theater use. However, there is no good evidence of this. Such an efficiency benefit is relevant only when implants offer equivalent clinical benefit and material costs, a finding that is not supported by our analysis. The use of cement on the femoral side has many advantages that outweigh the disadvantage of a slightly longer operating time (Murray 2011).
We found no advantage in the use of fully cementless or resurfacing implants in young patients when compared with a standard cemented hip replacement. For young women, hybrid implants that employ adequate press-fit acetabular fixation and either highly crosslinked PE or ceramic bearings may provide the best balance of early improvement in outcome, revision risk, and cost.
Acknowledgments
SJ, MR, and MP developed the concept. SJ analyzed the data with assistance from JM and PB. SJ wrote the first draft of the paper, with contributions and editing from PB, JM, PG, DD, and MR.
We thank the patients and staff of all the hospitals in England and Wales who have contributed data to the National Joint Registry. We are grateful to the Healthcare Quality Improvement Partnership (HQIP), the NJR Steering Committee, and the staff of the NJR center for facilitating this work. We also thank Andrew Smallwood (NHS Wales) and Philip Lewis (NHS supply chain) for their help with the implant cost data.
The National Joint Registry for England and Wales is funded through a levy raised on the sale of hip and knee replacement implants. The cost of the levy is set by the NJR Steering Committee. The NJR Steering Committee is responsible for data collection. This work was funded by a fellowship from the National Joint Registry. The authors have conformed to the NJR’s standard protocol for data access and publication. The views expressed are those of the authors and do not necessarily reflect those of the NJR Steering Committee or the Health Quality Improvement Partnership (HQIP), who do not vouch for how the information is presented.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
This study, which was presented at the Second International Society of Arthroplasty Registries Meeting, was given the Acta Orthopaedica award for the best study at the meeting.
Supplementary data
Tables 1 and 8–10 are available at Acta’s website (www.actaorthop.org), identification number 6590.
References
- Andrew JG, Palan J, Kurup HV, Gibson P, Murray DW, Beard DJ. Obesity in total hip replacement . J Bone Joint Surg (Br) 2008;90(4):424–9. doi: 10.1302/0301-620X.90B4.20522. [DOI] [PubMed] [Google Scholar]
- Australian National Joint Registry “Australian Orthopaedic Association, National Joint Replacement Register.” 2010. http://www.dmac.adelaide.edu.au/aoanjrr/index.jsp Retrieved 13th June 2011, from.
- Australian National Joint Registry “Australian National Joint Replacement Registry Annual Report 2012.”. https://aoanjrr.dmac.adelaide.edu.au/documents/10180/60142/Annual%20Report%202012?version=1.3&t=1361226543157 2012. from.
- Baker P, Jameson S, Critchley R, Reed M, Gregg P, Deehan D. Center and surgeon volume influence the revision rate following unicondylar knee replacement: an analysis of 23,400 medial cemented unicondylar knee replacements . J Bone Joint Surg (Am) 2013;95(8):702–9. doi: 10.2106/JBJS.L.00520. [DOI] [PubMed] [Google Scholar]
- Browne J, Jamieson L, Lewsey J. “Patient reported outcome measures (PROMs) in elective surgery: report to the Department of Health, 2007.”. http://www.lshtm.ac.uk/php/hsrp/research/proms_report_12_dec_07.pdf 2007. Retrieved 29/08/2012, from.
- Busch V, Klarenbeek R, Slooff T, Schreurs BW, Gardeniers J. Cemented hip designs are a reasonable option in young patients . Clin Orthop. 2010;(468)((12)):3214–20. doi: 10.1007/s11999-010-1355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study . J Bone Joint Surg (Am) 1981;63(9):1426–34. [PubMed] [Google Scholar]
- Collis DK. Cemented total hip replacement in patients who are less than fifty years old . J Bone Joint Surg (Am) 1984;66(3):353–9. [PubMed] [Google Scholar]
- Costa ML, Achten J, Parsons NR, Edlin RP, Foguet P, Prakash U, Griffin DR. Total hip arthroplasty versus resurfacing arthroplasty in the treatment of patients with arthritis of the hip joint: single centre, parallel group, assessor blinded, randomised controlled trial. BMJ. 2012. p. 344 e2147. [DOI] [PMC free article] [PubMed]
- Cuckler JM, Moore KD, Lombardi AV, Jr., McPherson E, Emerson R. Large versus small femoral heads in metal-on-metal total hip arthroplasty . J Arthroplasty (Suppl 3) 2004;19(8):41–4. doi: 10.1016/j.arth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement . J Bone Joint Surg (Br) 1996;78(2):185–90. [PubMed] [Google Scholar]
- Delaunay CP, Bonnomet F, Clavert P, Laffargue P, Migaud H. THA using metal-on-metal articulation in active patients younger than 50 years. Clin Orthop. 2008;(466)((2)):340–6. doi: 10.1007/s11999-007-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr LD, Takei GK, Conaty JP. Total hip arthroplasties in patients less than forty-five years old . J Bone Joint Surg (Am) 1983;65(4):474–9. [PubMed] [Google Scholar]
- Edlin R, Tubeuf S, Achten J, Parsons N, Costa M. Cost-effectiveness of total hip arthroplasty versus resurfacing arthroplasty: economic evaluation alongside a clinical trial . BMJ Open. 2012;2(5):e001162. doi: 10.1136/bmjopen-2012-001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England and Wales National Joint Registry “National Joint Registry for England and Wales 9th Annual Report.”. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/9th_annual_report/NJR%209th%20Annual%20Report%202012.pdf (2012). from.
- Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- Finnish National Arthoplasty Registry “Annual Report 2006. Finnish National Arthroplasty Register.”. http://www.nam.fi/english/publications 2006. from.
- group E “EuroQol (EQ5D Score).”. http://www.euroqol.org/ 2009. Retrieved 29/08/2012, from.
- Haddad FS, Thakrar RR, Hart AJ, Skinner JA, Nargol AV, Nolan JF, Gill HS, Murray DW, Blom AW, Case CP. Metal-on-metal bearings: the evidence so far . J Bone Joint Surg (Br) 2011;93(5):572–9. doi: 10.1302/0301-620X.93B4.26429. [DOI] [PubMed] [Google Scholar]
- Hamilton DF, Lane JV, Gaston P, Patton JT, Macdonald D, Simpson AH, Howie CR. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement . BMJ Open. 2013;3(4):e002525. doi: 10.1136/bmjopen-2012-002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SS, Baker PN, Mason J, Gregg PJ, Brewster N, Deehan DJ, Reed MR. The design of the acetabular component and size of the femoral head influence the risk of revision following 34 721 single-brand cemented hip replacements: A retrospective cohort study of medium-term data from a National Joint Registry . J Bone Joint Surg (Br) 2012a;94(12):1611–7. doi: 10.1302/0301-620X.94B12.30040. [DOI] [PubMed] [Google Scholar]
- Jameson SS, Baker PN, Mason J, Porter ML, Deehan DJ, Reed MR. Independent predictors of revision following metal-on-metal hip resurfacing: a retrospective cohort study using National Joint Registry data . J Bone Joint Surg (Br) 2012b;94(6):746–54. doi: 10.1302/0301-620X.94B6.29239. [DOI] [PubMed] [Google Scholar]
- Jameson SS, Baker PN, Mason JM, Rymaszewska M, Gregg PJ, Deehan DJ, Reed MR. Independent predictors of failure up to 7.5 years after 35 386 single-brand cementless total hip replacements . Bone Joint J. 2013a;95-B((6)):747–57. doi: 10.1302/0301-620X.95B6.31378. [DOI] [PubMed] [Google Scholar]
- Jameson SS, Mason JM, Baker PN, Jettoo P, Deehan DJ, Reed MR. Factors influencing revision risk following 15 740 single-brand hybrid hip arthroplasties: A cohort study from a National Joint Registry . J Arthroplasty. 2013b;28(7):1152–9. doi: 10.1016/j.arth.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Jameson SS, Baker PN, Mason JM, Gregg PJ, Deehan DJ, Reed MR. No functional benefit of larger femoral heads and alternative bearings in primary hip replacement: A cohort study using linked National Joint Registry and Patient Reported Outcome Measures (PROMs) data. Acta Orthop. 2014 doi: 10.3109/17453674.2014.972259. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge A, Arden NK, Batra RN, Thomas G, Beard D, Javaid MK, Cooper C, Murray D. The association of patient characteristics and surgical variables on symptoms of pain and function over 5 years following primary hip-replacement surgery: a prospective cohort study . BMJ Open. 2013;3(3):e002453. doi: 10.1136/bmjopen-2012-002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GA, Hardy JR, Kummer FJ. An uncemented total hip replacement: experimental study and review of 300 madreporique arthroplasties . Clin Orthop. 1979;141:2–16. [PubMed] [Google Scholar]
- Medicines and Healthcare products Regulatory Agency “Medical Device Alert: All metal-on-metal (MoM) hip replacements (MDA/2012/008).”. http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON143782 2011. Retrieved 21/03/2012, from.
- Murray DW. Cemented femoral fixation: the North Atlantic divide . Orthopedics. 2011;34(9):e462–3. doi: 10.3928/01477447-20110714-25. [DOI] [PubMed] [Google Scholar]
- Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, Dawson J. The use of the Oxford hip and knee scores . J Bone Joint Surg (Br) 2007;89(8):1010–4. doi: 10.1302/0301-620X.89B8.19424. [DOI] [PubMed] [Google Scholar]
- New Zealand National Joint Registry “Annual Report 2008, 8 year report. New Zealand National Joint Registry.”. http://www.cdhb.govt.nz/njr/reports/A2D65CA3.pdf 2008. Retrieved 23/03/2012, from.
- Norwegian Arthroplasty Registry “Annual Report 2008. Norwegian Arthroplasty Register.”. http://www.haukeland.no/nrl/eng/default.htm 2008. Retrieved 23/03/2012, from.
- Pakvis D, van Hellemondt G, de Visser E, Jacobs W, Spruit M. Is there evidence for a superior method of socket fixation in hip arthroplasty? A systematic review . Int Orthop. 2011;35(8):1109–18. doi: 10.1007/s00264-011-1234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington M, Grieve R, Sekhon JS, Gregg P, Black N, van der Meulen JH. Cemented, cementless, and hybrid prostheses for total hip replacement: cost effectiveness analysis . BMJ. 2013;346:f1026. doi: 10.1136/bmj.f1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranawat CS, Atkinson RE, Salvati EA, Wilson PD., Jr Conventional total hip arthroplasty for degenerative joint disease in patients between the ages of forty and sixty years . J Bone Joint Surg (Am) 1984;66(5):745–52. [PubMed] [Google Scholar]
- Schmitz MW, Busch VJ, Gardeniers JW, Hendriks JC, Veth RP, Schreurs BW. Long-term results of cemented total hip arthroplasty in patients younger than 30 years and the outcome of subsequent revisions . BMC Musculoskelet Disord. 2013;14:37. doi: 10.1186/1471-2474-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel L, Kerboull L, Christel P, Meunier A, Witvoet J. Alumina-on-alumina hip replacement. Results and survivorship in young patients . J Bone Joint Surg (Br) 1990;72(4):658–63. doi: 10.1302/0301-620X.72B4.2380223. [DOI] [PubMed] [Google Scholar]
- Sedrakyan A, Normand SL, Dabic S, Jacobs S, Graves S, Marinac-Dabic D. Comparative assessment of implantable hip devices with different bearing surfaces: systematic appraisal of evidence . BMJ. 2011;343:d7434. doi: 10.1136/bmj.d7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton SA, Walter WL, Jackson MP, De Steiger R, Stanford T. Ceramic-on-ceramic bearing surface and risk of revision due to dislocation after primary total hip replacement . J Bone Joint Surg (Br) 2009;91(11):1448–53. doi: 10.1302/0301-620X.91B11.22100. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Porter KM. The Charnley total hip arthroplasty in patients under age 40 . Clin Orthop. 1985;201:51–6. [PubMed] [Google Scholar]
- Smith AJ, Dieppe P, Howard PW, Blom AW. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales . Lancet. 2012a;380(9855):1759–66. doi: 10.1016/S0140-6736(12)60989-1. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales . Lancet. 2012b;379(9822):1199–204. doi: 10.1016/S0140-6736(12)60353-5. [DOI] [PubMed] [Google Scholar]
- Spencer RF. Evolution in hip resurfacing design and contemporary experience with an uncemented device . J Bone Joint Surg (Am) (Suppl 2) 2011;93:84–8. doi: 10.2106/JBJS.J.01716. [DOI] [PubMed] [Google Scholar]
- Swedish Hip Registry “Annual report 2010. Swedish Hip Registry.”. http://www.shpr.se/Libraries/Documents/AnnualReport-2010-2-eng.sflb.ashx 2010. from.
- Toossi N, Adeli B, Timperley AJ, Haddad FS, Maltenfort M, Parvizi J. Acetabular components in total hip arthroplasty: is there evidence that cementless fixation is better? . J Bone Joint Surg (Am) 2013;95(2):168–74. doi: 10.2106/JBJS.K.01652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.