Abstract
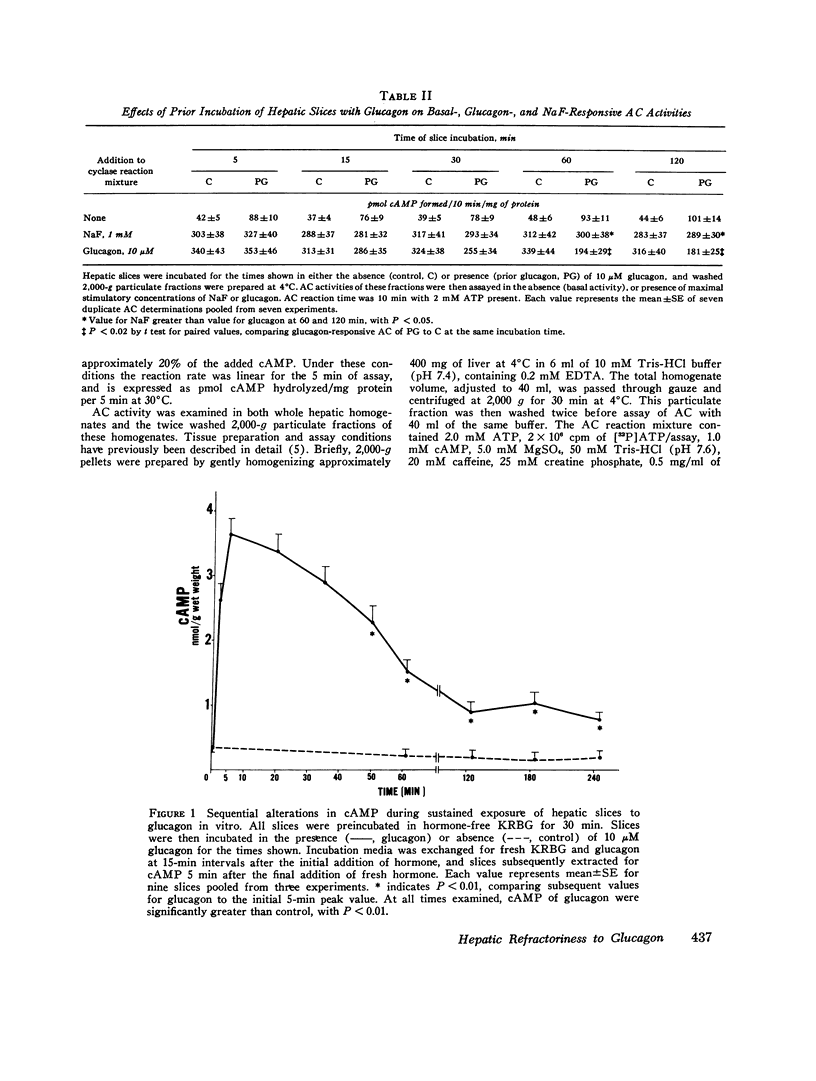
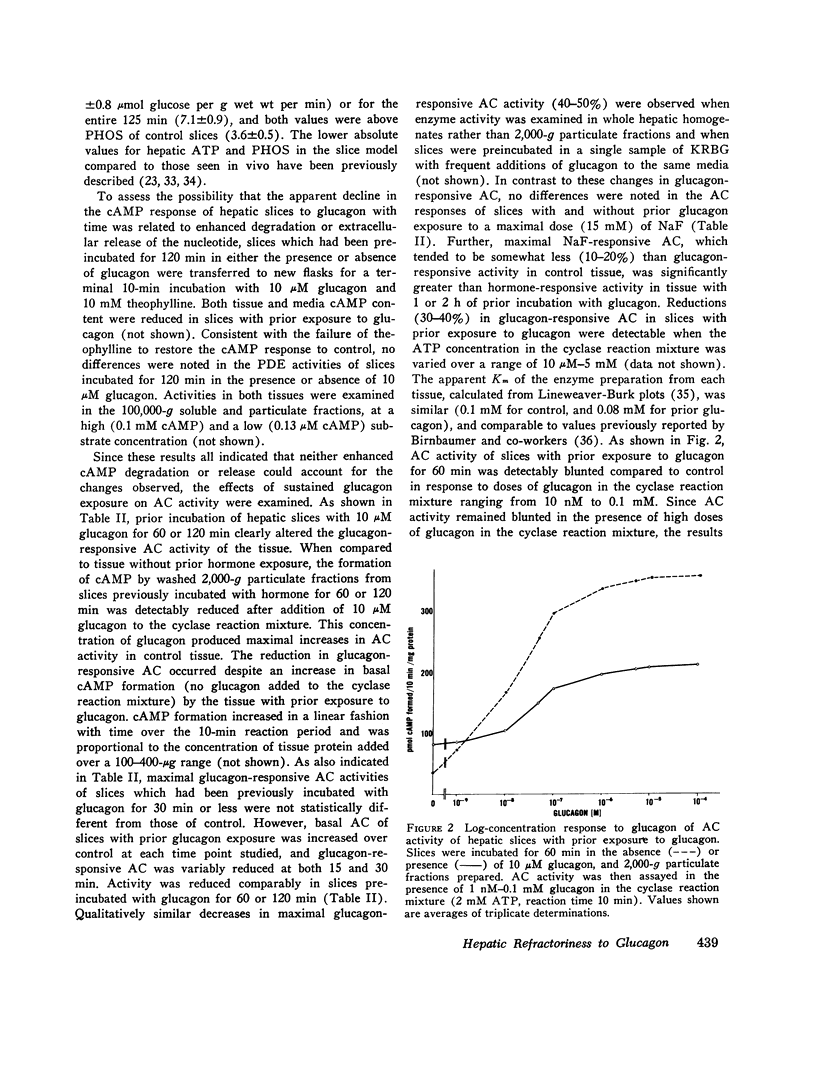
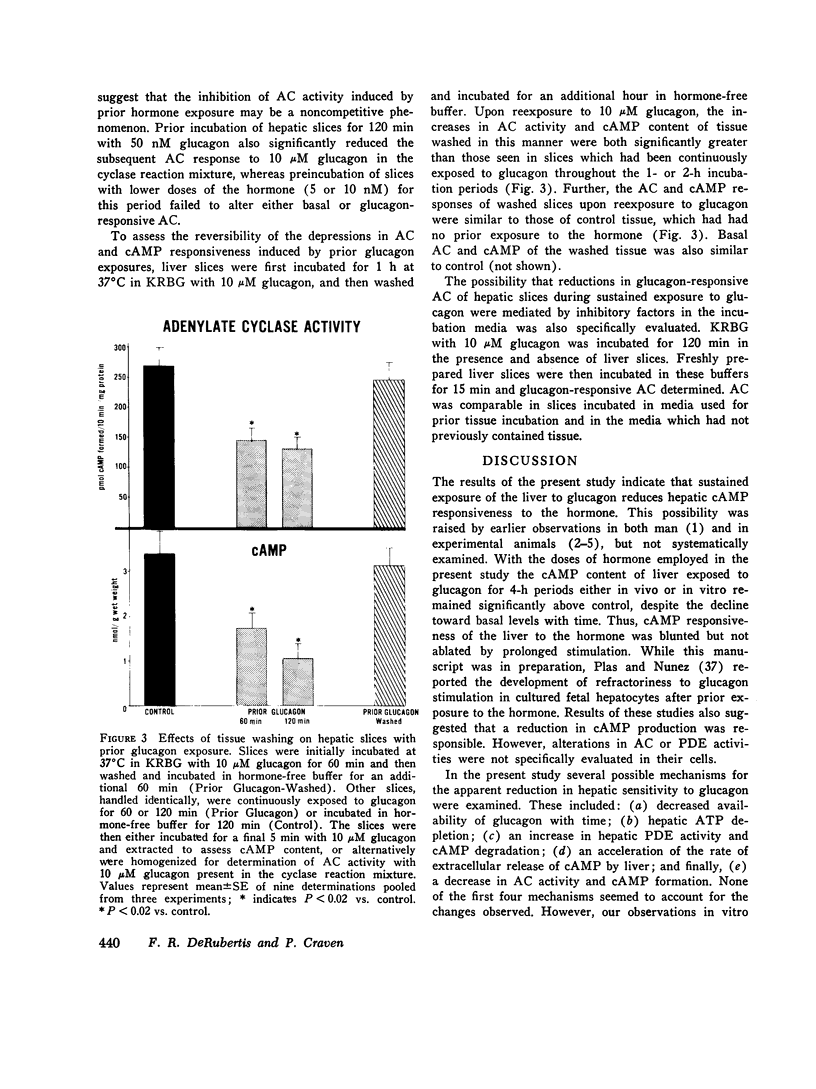
Hormone-induced desensitization of hormonal regulation of cyclic AMP (cAMP) content has been described in a number of tissues. In the present study, we examined responses of rat liver to glucagon after periods of sustained exposure to the hormone in vivo and in vitro. In intact anesthetized rats infused with glucagon (50 ng/min) for 1 h or more and in liver slices incubated with the hormone (10 muM) for this period, hepatic cAMP responsiveness to glucagon was significantly blunted compared with that of tissue exposed to the hormone for shorter periods. The reduction in hepatic cAMP responsiveness to glucagon appeared to be fully expressed by 2 h. With the doses of hormone employed, the sequential alterations in hepatic responsiveness seemed to be limited to the cAMP system, since other parameters of glucagon action did not wane with time. Diminished hepatic cAMP responsiveness during sustained hormonal exposure could not be attributed to decreased glucagon availability, accelerated extracellular release of cAMP, hepatic ATP depletion, or enhanced phosphodiesterase activity. Studies in vitro suggested that modulation of the cAMP response occurred at the level of adenylate cyclase (AC). During sustained exposure of hepatic slices to glucagon, reductions in glucagon-responsive AC correlated temporally with those in cAMP and both changes were reversible. Alterations in glucagon-responsive AC were demonstrated over a wide range of ATP (10 muM-0.1 mM) and glucagon (10 nM-5 MM) concentrations in the cyclase reaction mixture, and appeared to be a noncompetitive phenomenon relative to glucagon. Maximal NaF-responsive AC did not fall concomitantly with time. Thus, the reduction in glucagon-responsive AC was probably not related to a reduction in the catalytic unit of the enzyme, but could have been due to an alteration in glucagon binding to its receptor sites, or in the coupling mechanism involved in transmission of the hormonal signal to the catalytic unit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHMORE J., CAHILL G. F., Jr, HASTINGS A. B., ZOTTU S. Studies on carbohydrate metabolism in rat liver slices. IX. Ionic and hormonal effects on phosphorylase and glycogen. J Biol Chem. 1957 Jan;224(1):237–250. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M., Sundby F. The glucagon-sensitive adenylate cyclase system in plasma membranes of rat liver. VII. Hormonal stimulation: reversibility and dependence on concentration of free hormone. J Biol Chem. 1972 Apr 10;247(7):2038–2043. [PubMed] [Google Scholar]
- Bishop J. S., Goldberg N. D., Larner J. Insulin regulation of hepatic glycogen metabolism in the dog. Am J Physiol. 1971 Feb;220(2):499–506. doi: 10.1152/ajplegacy.1971.220.2.499. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Neufeld A. H., King R. A specific, reversible, macromolecular inhibitor of hepatic glucagon responsive adenyl cyclase. Endocrinology. 1971 Nov;89(5):1242–1249. doi: 10.1210/endo-89-5-1242. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Schwartz J. P., Breckenridge B. M. Norepinephrine-sensitive properties of C-6 astrocytoma cells. Mol Pharmacol. 1974 Jan;10(1):162–174. [PubMed] [Google Scholar]
- Buschiazzo H., Exton J. H., Park C. R. Effects of glucose on glycogen synthetase, phosphorylase, and glycogen deposition in the perfused rat liver. Proc Natl Acad Sci U S A. 1970 Feb;65(2):383–387. doi: 10.1073/pnas.65.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Curnow R. T. Inhibition of glucagon-mediated increases in hepatic cyclic adenosine 3', 5'-monophosphate by prostaglandin E1 and E2. Endocrinology. 1974 Jul;95(1):93–101. doi: 10.1210/endo-95-1-93. [DOI] [PubMed] [Google Scholar]
- DeVellis J., Brooker G. Reversal of catecholamine refractoriness by inhibitors of RNA and protein synthesis. Science. 1974 Dec 27;186(4170):1221–1223. doi: 10.1126/science.186.4170.1221. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Supersensitivity and subsensitivity of the beta-adrenergic receptor in pineal gland regulated by catecholamine transmitter. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2411–2414. doi: 10.1073/pnas.70.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Lewis S. B., Ho R. J., Robison G. A., Park C. R. The role of cyclic AMP in the interaction of glucagon and insulin in the control of liver metabolism. Ann N Y Acad Sci. 1971 Dec 30;185:85–100. doi: 10.1111/j.1749-6632.1971.tb45239.x. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Fain J. N., Pointer R. H., Ward W. F. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972 Nov 10;247(21):6866–6872. [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Hormone-induced desensitisation of hormonal control of cyclic AMP levels in human diploid fibroblasts. Nat New Biol. 1973 Dec 5;246(153):146–148. doi: 10.1038/newbio246146a0. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liljenquist J. E., Bomboy J. D., Lewis S. B., Sinclair-Smith B. C., Felts P. W., Lacy W. W., Crofford O. B., Liddle G. W. Effect of glucagon on net splanchnic cyclic AMP production in normal and diabetic men. J Clin Invest. 1974 Jan;53(1):198–204. doi: 10.1172/JCI107538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Makman M. H. Properties of adenylate cyclase of lymphoid cells. Proc Natl Acad Sci U S A. 1971 May;68(5):885–889. doi: 10.1073/pnas.68.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganiello V. C., Murad F., Vaughan M. Effects of lipolytic and antilipolytic agents on cyclic 3',5'-adenosine monophosphate in fat cells. J Biol Chem. 1971 Apr 10;246(7):2195–2202. [PubMed] [Google Scholar]
- Means A. R., MacDougall E., Soderling T. R., Corbin J. D. Testicular adenosine 3':5'-monophosphate-dependent protein kinase. Regulation by follicle-stimulating hormone. J Biol Chem. 1974 Feb 25;249(4):1231–1238. [PubMed] [Google Scholar]
- Mersmann H. J., Segal H. L. An on-off mechanism for liver glycogen synthetase activity. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1688–1695. doi: 10.1073/pnas.58.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey J., Tate R., Lefkowitz R. J. Subsensitivity of adenylate cyclase and decreased beta-adrenergic receptor binding after chronic exposure to (minus)-isoproterenol in vitro. J Biol Chem. 1975 Jul 25;250(14):5727–5729. [PubMed] [Google Scholar]
- Plas C., Nunez J. Glycogenolytic response to glucagon of cultured fetal hepatocytes. Refractoriness following prior exposure to glucagon. J Biol Chem. 1975 Jul 25;250(14):5304–5311. [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Birnbaumer L., Rodbell M. Inactivation of glucagon by plasma membranes of rat liver. J Biol Chem. 1972 Apr 25;247(8):2295–2301. [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W., WOSILAIT W. D. The relationship of epinephrine and glucagon to liver phosphorylase. III. Reactivation of liver phosphorylase in slices and in extracts. J Biol Chem. 1956 Jan;218(1):483–495. [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schimmel R. J. Responses of adipose tissue to sequential lipolytic stimuli. Endocrinology. 1974 May;94(5):1372–1380. doi: 10.1210/endo-94-5-1372. [DOI] [PubMed] [Google Scholar]
- Schultz J. Cyclic adenosine 3',5'-monophosphate in guinea pig cerebral cortical slices: possible regulation of phosphodiesterase activity by cyclic adenosine 3',5'-monophosphate and calcium ions. J Neurochem. 1975 Mar;24(3):495–501. doi: 10.1111/j.1471-4159.1975.tb07667.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Ramwell P. W. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J Biol Chem. 1968 Apr 10;243(7):1498–1503. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Zenser T. V., DeRubertis F. R., George D. T., Rayfield E. J. Infection-induced hyperglucagonemia and altered hepatic response to glucagon in the rat. Am J Physiol. 1974 Dec;227(6):1299–1305. doi: 10.1152/ajplegacy.1974.227.6.1299. [DOI] [PubMed] [Google Scholar]
- Zor U., Lamprecht S. A., Kaneko T., Schneider H. P., McCann S. M., Field J. B., Tsafriri A., Lindner H. R. Functional relations between cyclic AMP, prostaglandins, and luteinizing hormone in rat pituitary and ovary. Adv Cyclic Nucleotide Res. 1972;1:503–520. [PubMed] [Google Scholar]




