Abstract
Background and purpose
Use of bisphosphonates in women is associated with higher risk of atypical femoral fractures. The risk in terms of timing of use and type of bisphosphonate, and in men, remains unclear.
Patients and methods
We reviewed radiographs of 5,342 Swedish women and men aged 55 years or more who had had a fracture of the femoral shaft in the 3-year period 2008–2010 (97% of those eligible), and found 172 patients with atypical fractures (93% of them women). We obtained data on medication and comorbidity. The risk of atypical fracture associated with bisphosphonate use was estimated in a nationwide cohort analysis. In addition, we performed a case-control analysis with comparison to 952 patients with ordinary shaft fractures. A short report of the findings has recently been presented (Schilcher et al. 2014a). Here we provide full details.
Results
The age-adjusted relative risk (RR) of atypical fracture associated with bisphosphonate use was 55 (95% CI: 39–79) in women and 54 (CI: 15–192) in men. In bisphosphonate users, women had a 3-fold higher risk than men (RR = 3.1, CI: 1.1–8.4). Alendronate users had higher risk than risedronate users (RR = 1.9, CI: 1.1–3.3). The RR after 4 years or more of use reached 126 (CI: 55–288), with a corresponding absolute risk of 11 (CI: 7–14) fractures per 10,000 person-years of use. The risk decreased by 70% per year since last use.
Interpretation
Women have a higher risk of atypical femoral fracture than men. The type of bisphosphonate used may affect risk estimates and the risk decreases rapidly after cessation.
In efficacy trials, bisphosphonates have reduced the risk of osteoporotic fractures. However, recent studies (Schilcher and Aspenberg 2009, Dell et al. 2012, Meier et al. 2012,), although not all (Feldstein et al. 2012, Gedmintas et al. 2013), have found a strong and duration-dependent association between bisphosphonate use and atypical fractures of the femoral shaft. We have already analyzed cessation of use and found that the risk decreased rapidly after stopping treatment (Schilcher et al. 2011). This time dependency may be because bisphosphonates increase the risk of atypical fracture by impairing the healing of naturally occurring bone microcracks (Schilcher et al. 2011, Ettinger et al. 2013). However, concerning the change in risk after cessation, the numbers in our previous publication were small and the finding needed confirmation.
Whether men also have a higher risk of atypical femoral fracture when using bisphosphonates is unclear. Ordinary stress fractures in athletes may occur more frequently in women due to a narrower bone (Seeman 2008): the bending strength of the femoral shaft is proportional to the third power of the radius. In addition, women tend to have a higher prevalence of microcrack accumulation (Seeman 2008). Thus, bisphosphonate use may mean a higher risk of atypical fracture in women than in men. Moreover, bisphosphonates with different dose regimens and pharmacodynamic properties may give different risks of atypical fracture (Ebetino et al. 2011).
To better understand how bisphosphonate use and risk of atypical fracture are related to timing of use, sex, age, and the type of bisphosphonate, we have now extended our previous nationwide study (Schilcher et al. 2011), and also included men. Clinical decision-making can be improved with better knowledge of the extent of possible harm, as evaluated in a population-based study. A short report of the findings has recently been presented (Schilcher et al. 2014a).
Methods
Study population
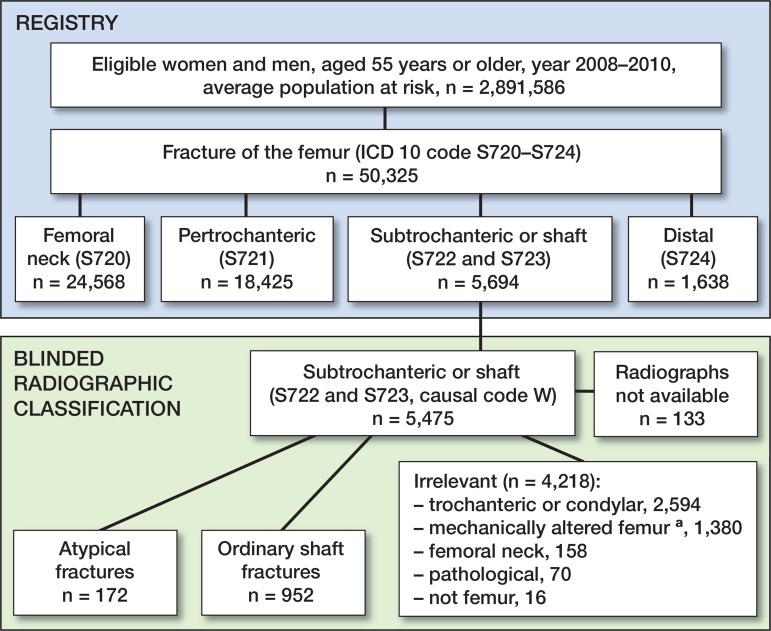
We performed a nationwide retrospective analysis of all patients, 55 years of age or older, who were admitted to any hospital in Sweden because of a fractured femur (ICD-10 diagnosis codes S720 to S724) according to the Swedish National Patient Register. The positive predictive value in the Patient Register ranges from 85% to 100% depending on the diagnosis (Ludvigsson et al. 2011). The accuracy of coding of lower extremity fractures in general is also high (96% to the third position of the ICD code) (Bergstrom et al. 2011). The study period was extended beyond 2008: from January 1, 2008 through December 31, 2010. In the 2008 study, only women were considered (Schilcher et al. 2011). During the 3-year period, 38,747 women and 11,578 men with a fracture of the femur were identified (Figure 1). Of these, 5,694 patients (4,446 women and 1,248 men) had a femoral subtrochanteric or shaft fracture (ICD-10 code S722 or S723). Patients with multiple femoral fractures during the study period, ipsilateral or bilateral, were only considered for their first fracture event. Patients with injuries and ICD-10 V-diagnosis codes associated with motor vehicle accidents and patients with falls from higher than standing height were not included. Thus, 5,475 patients remained. Digitized radiographs from 5,342 patients (97%) were obtained from 76 hospitals. The remaining 133 patients were excluded because the quality of the radiographs was insufficient or because radiographs were not available.
Figure 1.
Identification of atypical femoral fractures in the study population. Patients from 2008 were women only. aMechanically altered femurs include patients with knee and hip prostheses, retained plates, screws, intramedullary nails, joint arthrodeses, and other conditions.
Classification of fractures
Patients with subtrochanteric or shaft fractures, according to the National Swedish Patient Register, were categorized into 3 groups after individual radiographic review by VK, who was blinded regarding patients’ previous drug history and comorbidities. The first group included 172 patients with atypical shaft fractures according to the revised criteria from the American Society of Bone and Mineral Research task force (transverse on the lateral side and with focal cortical thickening (callus formation) at the fracture site (Schilcher 2013, Shane et al. 2014). The femur showed no signs of previous surgery and the fracture was not pathological. In contrast to the classification in the Swedish 2008 study (Schilcher et al. 2011), intermediate fragments were allowed but not fracture comminution. The second group included 952 patients with common shaft fractures that were relevant for comparison with atypical fracture cases. None of the common shaft fractures were transverse on the lateral side. There were no implants or pathological fractures. The third group included 4,218 patients with fractures that were not relevant for comparison with atypical fractures (Figure 1).
Before retrieval of registry information, a sample of 50 atypical fractures and 50 control fractures was randomly selected for reclassification. JS, who performed the reclassification, was not informed about patient background characteristics and the previous categorization. A kappa value of 0.96 was achieved.
Registry information
After fracture classification, data on drug use, comorbidities, and previous medical history were obtained from registries of the Swedish National Board of Health and Welfare.
The Swedish Prescription Register contains data on all prescriptions dispensed to the entire Swedish population from July 2005 onward. The drugs are normally dispensed every third month. The total duration of use was calculated as the difference between the first day of dispensing and the last day of dispensing plus 3 months, excluding gaps in treatment.
Discharge diagnoses were collected from the Swedish National Patient Register, from 1987 onwards. For outpatients, data from 2001 and onwards were collected. All diagnoses were included: primary and secondary diagnoses as well as inpatient and outpatient data. Detailed information about the registries can be found in our previous article (Schilcher et al. 2011). Complete linkage between the registries is possible through the use of the personal identification number provided to all Swedish citizens.
Statistics
Cohort study—According to data from Statistics Sweden, on average 1.54 million women and 1.35 million men over 55 years of age lived in Sweden between 2008 and 2010. Of these, approximately 85,300 women and 12,900 men used bisphosphonates. Age (5-year strata) and sex-stratified incidence proportions of atypical fractures were calculated for patients who received bisphosphonates and for those with no reported use of bisphosphonates. These calculations were used to estimate both the age-adjusted RR and the absolute risk (together with 95% confidence intervals (CIs)) of atypical fracture in women and men (Greenland and Rothman 2008). The risk with duration of use in women was estimated in categories (< 1 year, 1–1.9 years, 2–2.9 years, 3–3.9 years, and ≥ 4 years). Moreover, the age-adjusted RR for women compared to men was calculated for patients on bisphosphonates and for those who were not. In addition, the age-adjusted risk in alendronate users was compared with the risk in risedronate users.
Case-control study—Patients with atypical fractures (cases) were compared with 952 control patients with ordinary shaft fractures. All drug exposures were analyzed in relation to the date of fracture. Use of any type of bisphosphonate before the fracture and use by type of bisphosphonate were considered. The total duration of bisphosphonate use was estimated according to defined daily doses from the first registered prescription to the last prescription or to the date of fracture, whichever came first, and excluding periods of non-use. Duration of “ever use” was analyzed as a continuous variable. Additionally, duration of current bisphosphonate use was considered in categories (< 1.0 year, 1–1.9 years, 2–2.9 years, 3–3.9 years, and 4–4.9 years). In this particular analysis, we excluded patients with first-time use between July and October, 2005, thereby reducing the possibility of erroneously registering previous users as first-time users.
The time since last use was treated as a continuous variable. The use or non-use of systemic glucocorticoids, estrogen-replacement therapy, selective estrogen-receptor modulators, antiepileptic drugs, antidepressants, or proton-pump inhibitors was identified from the National Prescription Register. Comorbidities, diagnosed between 1987 and the date of fracture, were identified by ICD-10 codes from the Patient Register. This information was used to calculate the Charlson comorbidity index according to the original description (Charlson et al. 1987). ICD-10 codes (Quan et al. 2005) for indexation are given in Table 1 (see Supplementary data). Odds ratios with 95% confidence intervals (CIs), used as measures of association for the RR of atypical fracture with bisphosphonate use, were calculated by means of unconditional logistic regression using the PROC GENMOD procedure in SAS version 9.4. Age- and sex-adjusted odds ratios (with age as a continuous variable) and odds ratios additionally adjusted according to glucocorticoid use (dichotomous) and the Charlson score (a continuous variable) were estimated. Year of investigation was included in the model as a frailty component by generalized estimating equations, using the PROC GENMOD procedure to handle year-specific dependencies. We also performed stratified analyses according to previous fracture (yes or no), age (< 80 years or > 80 years), the use or non-use of glucocorticoids, and the use or non-use of proton-pump inhibitors.
Ethics
The study was approved by the regional ethics review board in Linköping (2011/358-31).
Results
Cohort study
Of the female case patients, 81% (130 women) had used some kind of bisphosphonate, while only 4 of the 12 male patients had. This can be compared to the population prevalence of bisphosphonate use of 5% in women and 1% in men. These proportions gave an overall age-adjusted RR of 55 (CI: 39–79) for women and 54 (CI: 15–192) for men (Table 2). For women, the higher risk was already evident after 1 year of use, but increased for every year of use and reached 126 (CI: 55–288) after 4 or more years (Figure 2). This corresponded to an increase in absolute risk for bisphosphonate users of 11 (CI: 7–14) fractures per 10,000 patient-years. In women younger than 80 years of age with use for 4 or more years, the age-adjusted RR was 100 (CI: 40–253) and the absolute risk was 10 (CI: 5–15) per 10,000 patient-years of treatment. The corresponding RR in older women (≥ 80 years) was 163 (CI: 39–687), with an absolute risk of 11 (CI: 7–16) per 10,000 patient-years.
Table 2.
Age-adjusted relative risk (RR) and absolute risk (AR), with 95% confidence intervals (95% CIs), of atypical femoral fracture associated with use of bisphosphonate in women and men 55 years of age or older, during the 3-year period 2008–2010
| Bisphosphonate use | No. of atypical fractures | Crude incidence n/10,000 patient-years | Age-adjusted RR (95% CI) | Age-adjusted AR (95% CI) |
|---|---|---|---|---|
| Women | ||||
| Never use | 30 | 0.08 | Reference | Reference |
| Any type of bisphosphonate | 130 | 5.0 | 55 (39–79) | 0.0005 (0.0004–0.0006) |
| Alendronate | 117 | 5.9 | 63 (41–97) | 0.0006 (0.0005–0.0007) |
| Risedronate | 15 | 5.4 | 35 (19–66) | 0.0003 (0.0001–0.0006) |
| Men | ||||
| Never use | 8 | 0.03 | Reference | Reference |
| Any type of bisphosphonate | 4 | 1.6 | 54 (15–192) | 0.0002 (0.0000–0.0003) |
| Women compared to men a | ||||
| Men, users of bisphosphonate | 4 | 1.6 | Reference | Reference |
| Women, users of bisphosphonate | 86 | 5.0 | 3.1 (1.1–8.4) | 0.0003 (0.0001–0.0005) |
| Men, non-users | 8 | 0.030 | Reference | Reference |
| Women, non-users | 21 | 0.072 | 2.3 (1.0–5.3) | < 0.000001 |
Based on data from 2009 and 2010.
Figure 2.
Age-adjusted relative risk of atypical femoral fracture in women, by duration of use compared to non-use. Relative risk estimates (dots) with error bars representing 95% CIs.
In both non-users and users of bisphosphonates, the age-adjusted relative risk was higher for women than for men, with an RR of 2.3 (CI: 1.0–5.3) in non-users and an RR of 3.1 (CI: 1.1–8.4) in users. In women, the mean absolute increase in risk with bisphosphonate use was 5 per 10,000 (CI: 4–6) patient-years. In men, it was 2 per 10,000 (CI: 0–3) patient-years.
In bisphosphonate users, the majority of atypical femoral fractures (120 patients) were associated with alendronate use, while 16 patients had used risedronate. Compared to risedronate users, alendronate users had a 2-fold higher age-adjusted risk of an atypical fracture (RR = 1.9, CI: 1.1–3.3).
Case-control study
Case patients were generally younger than controls. Patients with atypical fractures had been less frequently diagnosed with endocrine, neurological, and psychiatric disorders. Irrespective of fracture type, bisphosphonate users had more frequent use of corticosteroids and estrogens, and a more frequent diagnosis of osteoporosis (Table 3).
Table 3.
Characteristics of stress fracture cases and controls with ordinary femoral shaft fractures
| Cases (n = 172) Bisphosphonate |
Controls (n = 952) Bisphosphonate |
|||
|---|---|---|---|---|
| users | non-users | users | non-users | |
| n = 134 | n = 38 | n = 110 | n = 842 | |
| Age a | 77 (7.8) | 75 (9.3) | 81 (8.5) | 82 (9.8) |
| Women b | 130 (97) | 30 (79) | 104 (95) | 670 (80) |
| Men b | 4 (3) | 8 (21) | 6 (5) | 172 (20) |
| Drug use b | ||||
| Cortisone | 48 (36) | 5 (13) | 35 (32) | 116 (14) |
| Estrogen | 43 (32) | 7 (18) | 34 (31) | 141 (17) |
| SERM | 1 (1) | 0 | 2 (2) | 1 (0) |
| Antidepressives | 28 (21) | 8 (21) | 41 (37) | 283 (34) |
| Antiepileptics | 8 (6) | 3 (8) | 6 (5) | 76 (9) |
| Proton-pump inhibitors | 56 (42) | 9 (24) | 37 (34) | 251 (30) |
| Previous diseases b | ||||
| Any fracture c | 59 (44) | 17 (45) | 57 (52) | 341 (41) |
| Osteoporotic fracture d | 42 (31) | 10 (26) | 50 (45) | 291 (35) |
| Hip fracture | 11 (8) | 5 (13) | 18 (16) | 106 (13) |
| Musculoskeletal disease | 86 (64) | 18 (47) | 73 (66) | 321 (38) |
| Inflammatory joint disease | 6 (4) | 0 (0) | 6 (5) | 15 (2) |
| Osteoporosis | 21 (16) | 0 (0) | 16 (14) | 18 (2) |
| Cardiovascular disease | 65 (49) | 14 (37) | 74 (67) | 493 (59) |
| Ischemic heart disease | 27 (20) | 6 (16) | 22 (20) | 179 (21) |
| Stroke | 5 (4) | 0 (0) | 12 (11) | 107 (13) |
| Endocrine disorder | 24 (18) | 9 (24) | 33 (30) | 261 (31) |
| Diabetes mellitus | 5 (4) | 2 (4) | 15 (14) | 136 (16) |
| Malignancy | 18 (13) | 6 (16) | 19 (17) | 160 (19) |
| Neurological disorder | 13 (10) | 1 (3) | 21 (19) | 205 (24) |
| Psychiatric disease | 9 (7) | 3 (8) | 26 (24) | 201 (24) |
| Kidney or urinary disease | 43 (32) | 10 (26) | 53 (48) | 318 (38) |
| Gastrointestinal disease | 42 (31) | 15 (40) | 46 (42) | 283 (34) |
| Respiratory disease | 32 (24) | 8 (21) | 19 (17) | 199 (24) |
mean (SD)
n (%)
Including ICD-10 codes: S12, S22, S32, S42, S52, S62, S72, S82, S92.
Including ICD-10 codes: hip S720, S721, S722; proximal humerus S422; distal forearm: S524, S525, S526; spine S220, S320.
Bisphosphonates were used in 78% (including both women and men) of the case patients and in 12% of the controls. This relationship led to a multivariable-adjusted odds ratio of 26 (CI: 18–38). The corresponding multivariable-adjusted odds ratios for women and men were 29 (CI: 20–41) and 19 (CI: 9–36) (Table 4). A direct comparison of bisphosphonate users revealed a higher odds of an atypical fracture for women than for men (multivariable-adjusted odds ratio = 3.6, CI: 2.5–5.3). This estimate was not attenuated after additional adjustment for duration of bisphosphonate use (OR = 4.2, CI: 1.7–10).
Table 4.
Odds ratios (ORs), with 95% confidence intervals (CIs), for femoral stress fractures associated with bisphosphonate use
| Bisphosphonate use | Cases Controls | Age- and sex-adjusted OR (CI) | Multi variable-adjusted a OR (CI) | |
|---|---|---|---|---|
| Never use | 38 | 842 | 1.0 (reference) | 1.0 (rreference) |
| Ever use | 134 | 110 | 26 (20–34) | 26 (18–38) |
| (78%) | (12%) | |||
| Alendronate use | 120 | 73 | 36 (29–44) | 36 (28–46) |
| Any other oral bisphosphonate | 18 | 39 | 7.8 (4.1–15) | 8.0 (4.0–16) |
| Risedronate use | 16 | 26 | 12 (6.5–21) | 13 (6.1–26) |
| Etidronate use | 0 | 13 | NA | NA |
| Ibandronate use | 2 | 0 | NA | NA |
| Zoledronate use | 0 | 2 | NA | NA |
| Per year since last use | 0.31 (0.28–0.36) | 0.31 (0.27–0.35) | ||
| Per year of use | 2.6 (2.1–3.2) | 2.5 (2.0–3.1) | ||
| Current use (within the last year) | 129 | 79 | 34 (26–46) | 35 (23–51) |
| Years of use b in current users | ||||
| 0–1 | 1 | 10 | 1.3 (0.1–16) | 1.7 (0.2–19) |
| 1–2 | 5 | 11 | 8.4 (2.5–28) | 8.2 (2.5–27) |
| 2–3 | 17 | 11 | 27 (25–30) | 29 (26–32) |
| 3–4 | 12 | 5 | 47 (22–99) | 40 (17–91) |
| 4–5 | 13 | 3 | 81 (40–164) | 116 (58–234) |
| > 5 | 11 | 5 | 85 (66–111) | 93 (66–132) |
| Women | ||||
| Never use | 30 | 670 | 1.0 (reference) | 1.0 (reference) |
| Ever use | 130 | 104 | 26 (21–33) | 29 (21–41) |
| (81%) | (13%) | |||
| Per year of use | 2.6 (2.2–3.2) | 2.6 (2.1–3.3) | ||
| Per year since last use | 0.30 (0.28–0.33) | 0.29 (0.26–0.32) | ||
| Men | ||||
| Never use | 8 | 172 | 1.0 (reference) | 1.0 (reference) |
| Ever use | 4 | 6 | 19.1 (8.0–46.0) | 19.0 (9.9–36.6) |
| (33%) | (3%) | |||
| Per year of use | 2.1 (1.3–3.3) | 2.4 (2.1–2.7) | ||
| Per year since last use | 0.37 (0.28–0.50) | 0.37 (0.30–0.47) |
Adjusted by age (continuous), sex, cortisone use (yes/no), and Charlson’s co-morbidity index (continuous).
First use after October 1, 2005 to ascertain non-use in the period July through September 2005. The national Swedish Prescription Register started on July 1, 2005, and provides complete national data on individuals exposed to dispensed drugs in the Swedish population; the drugs are normally dispensed every third month.
NA: not applicable.
The odds ratio for atypical fracture with alendronate use was 36 (CI: 28–46), and it was 13 (CI: 6–26) with risedronate use. Total duration of use was similar for alendronate users and for risedronate users (mean difference = 31 days, CI: 48–211 days). Etidronate and zolendronate were used by 13 and 2 control patients, respectively, but none of etidronate or zolendronate users developed an atypical femoral fracture during the study period. Ibandronate was used by 2 patients with atypical femoral fracture, but none of the controls used it.
The odds of atypical femoral fracture increased with duration of treatment (multivariable-adjusted odds ratio = 2.5, CI: 2.0–3.1 per year of use) (Table 4). Even during the first years of treatment, the multivariable-adjusted odds ratio verged toward an increased risk. After 4–5 years of treatment, the multivariable-adjusted odds ratio had increased to 116 (CI: 58–234). The risk of fracture decreased rapidly after cessation of treatment. A reduction in risk of 70% occurred with every year passed since last use (multivariable-adjusted odds ratio = 0.31, CI: 0.27–0.35).
The odds of having an atypical fracture associated with bisphosphonate use were higher in patients with no previous fracture than in patients with a previous fracture (p-value for homogeneity = 0.02), whereas small differences in odds ratios were detected between categories of other potential effect modifiers (Table 5, see Supplementary data).
Discussion
We found a strong time dependency between bisphosphonate use and atypical fractures. The risk increased steadily with longer duration of treatment and decreased rapidly after cessation of treatment. Women appeared to be more vulnerable regarding atypical femoral fracture, and alendronate use conferred a higher risk than risedronate use.
The rapid decrease in risk after cessation supports the theory that bisphosphonates cause atypical fractures by hampering the targeted remodeling of bone sites damaged by microcracks (Schilcher et al. 2011). Bisphosphonates in the circulation after dosing will localize at these sites. When dosing ceases, the effects on these sites will diminish, and crack growth can be stopped by localized resorption and remodeling (Schilcher 2013). This challenges the pathogenetic theory of increased general brittleness as a main cause of atypical fractures (Odvina et al. 2005). Even if ongoing use were the only risk factor, the risk cannot be expected to disappear instantly after cessation of treatment. Incomplete atypical fractures show slow healing even after cessation of bisphosphonate use, and they can remain undiagnosed for months until they lead to complete fracture and diagnosis (Kharazmi et al. 2014, Schilcher et al. 2014b). The risk after cessation could, at least in part, reflect delayed presentation of pre-cessation conditions: the bisphosphonate-associated risk would disappear first when all incomplete factures have become diagnosed or complete.
The association between bisphosphonate treatment and atypical fractures of the femur has now been shown in several observational studies with similar methodologies, and is supported by the current findings. Conflicting results in some studies can largely be explained by differences in the radiographic definition of atypical fractures (Feldstein et al. 2012, Rydholm 2012) and lack of statistical power (Black et al. 2010). In spite of the strong and most likely causative association between bisphosphonates and atypical fractures, 22% of the patients suffering from an atypical femoral fracture had never used bisphosphonates. In this context, the long disputed relationship between smoking and lung cancer springs to mind (Talley et al. 2004). Smoking, a main established cause of small cell and non-small cell lung cancer, contributes to 80% and 90% of lung cancer deaths in women and men (U.S. Department of Health and Human Services 2004). Men who smoke are 23 times more likely to develop lung cancer. Women are 13 times more likely than never-smokers (U.S. Department of Health and Human Services 2004). Nonetheless, RCTs on cessation of smoking have shown no benefit regarding mortality (Rose and Hamilton 1978). The current state of evidence for the link between bisphosphonates and atypical fractures has striking similarities, although no one would nowadays dispute that smoking is a cause of lung cancer.
The higher risk of atypical fractures in women than in men cannot be explained by differences in prescription rates or age. It may be related to differences in mechanical stress: women have a narrower bone and a broader pelvis, which both increase the stress in the lateral femoral cortex. Differences in stress might at least partly explain the higher burden of microcrack accumulation with increasing age in women than in men, and therefore their higher susceptibility to fatigue fracture (Norman and Wang 1997).
Risedronate was associated with a lower risk of atypical fracture than alendronate. This could be explained by different potency in relation to the recommended dose, and therefore might not influence the risk-benefit ratio. Risedronate has a lower affinity for the mineral (Ebetino et al. 2011), which results in a more even distribution in bone, shorter half-life, and a weaker effect on remodeling (Rosen et al. 2005). Differences in duration of use did not appear to explain the difference in risk associated with alendronate and risedronate use.
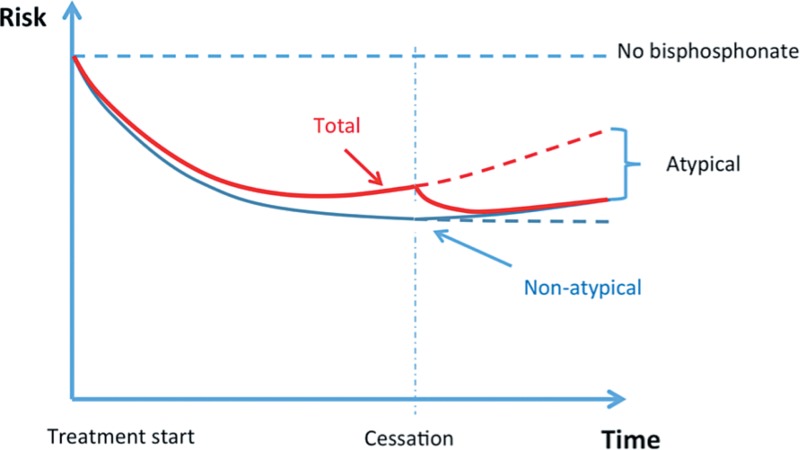
The risk of atypical femoral fracture increased steadily during the 5-year observation time. Dell et al. (2012) were able to show a steady increase in risk up until 10 years of treatment. The dramatic increase in risk of atypical fracture with longer duration of treatment has no corresponding decrease in risk of ordinary fracture over time. The risk-benefit ratio is quite favorable at the start of treatment in patients with a good indication, but prolongation beyond 5 years might not further reduce the absolute risk of ordinary fractures, as concluded by the U.S. Food and Drug Administration (Whitaker et al. 2012)—a conclusion that has been partly contested by some researchers in the field (Black et al. 2012). If there is no further risk reduction by prolongation, the risk-benefit ratio for prolongation after 5 years would be inverted: a risk for no gain. We have presented data on the increase in absolute risk of atypical fracture by prolonging treatment. Unfortunately, there are no published data available on the corresponding change in benefit. Such data would enable an estimation of the risk-benefit ratio pertaining to a decision to prolong treatment for another year. However, as such estimations only consider the year in question, they could underestimate the reasons for stopping bisphosphonate treatment, because the preventive effect is likely to last several years after cessation, whereas the risk of atypical fractures decreases rapidly (Figure 3). It may never be possible to acquire sufficient data to exactly specify the optimal duration of treatment, especially as this is dependent on the strength of the indication.
Figure 3.
Schematic graph of risk over time, with arbitrary units. Blue curve: risk of fragility fracture; red curve: total fracture risk; dashed red line: projected total risk without cessation. The curves are based on the assumption that the protective effect against ordinary (non-atypical) fractures has a longer half-life than the risk of atypical fracture.
In efficacy trials, bisphosphonates have reduced the risk of vertebral and non-vertebral fractures in women (< 80 years of age) with pre-existing vertebral fractures and osteoporosis (T-score < −2.5), in those with osteoporosis, and in those with a previous hip fracture (Lyles et al. 2007, Sambrook and Cooper 2006). Efficacy data for a reduction in clinical fracture risk by bisphosphonates in women with osteopenia (T-score between −1 and −2.5) or normal BMD (Cummings et al. 1998, Wells et al. 2008a,b,c) and in male patients (Valimaki et al. 1994) are meager or lacking. In addition, the population-based effectiveness of oral bisphosphonate therapy for the prevention of fractures in both women and men in clinical practice remains unclear (Feldstein et al. 2009, Sim Ie and Ebeling 2013), partly due to uncertainties surrounding optimal duration of use (Whitaker et al. 2012), differential age effects (Eastell et al. 2011), and low compliance (Silverman et al. 2011). Thus, with an unclear or weaker benefit—as with women older than 80 years (Curtis et al. 2008, Inderjeeth et al. 2009) or with a T-score above −2.5 SD (Wells et al. 2008)—the risks might outweigh the benefit.
The present study was based on a large population and on registries with high validity. The combination of registry data and review of individual radiographs made it possible to identify atypical femoral fractures and therefore to make risk calculations reliable. Information on individual prescriptions from registry data only became available from July, 2005, thus limiting the maximum measurable duration of bisphosphonate exposure to 5 years. To account for possible misclassification in the onset and duration of bisphosphonate use, we also performed a separate sensitivity analysis in the case-control study, where only patients with first-time drug dispensing at least 2 years after the start of the drug registry were included. We found a similar risk of atypical femoral fractures after 1 or 2 years of treatment (unpublished data) compared to the estimates based on analysis without data restriction.
We were not able to correct for compliance issues. Long-term adherence is generally low with bisphosphonate treatment. However, it is unlikely that patients would not have used the drug when it was dispensed continuously for several years. Collection without actual use of the drug would have led to an underestimation of the risk. Measurements of bone mineral density were not available. We limited the radiographic review to fractures registered as shaft or subtrochanteric fractures. Some ordinary shaft fractures and atypical femoral fractures may have been misdiagnosed and registered with other diagnostic codes, making them inaccessible for our analysis. Some confidence intervals were wide, making estimates in these specific duration-of-use categories less precise. We adjusted for several previously suggested covariates, although residual confounding is a possibility (but generally leading to conservatively biased estimates—given our design with non-differential assessment of exposure and covariate information). Genetic selection bias or individual vulnerability regarding atypical fractures is another possibility. There may be individuals in the population with a special, perhaps genetically determined, increased risk of atypical fractures, but such a risk profile has not yet been identified. The study was limited to individuals in a northern European country, and generalizability therefore might be limitied.
The risk of atypical femoral fracture has previously been thought to be associated with the use of glucocorticoids (Girgis et al. 2010) and proton-pump inhibitors (Giusti et al. 2010, Ing-Lorenzini et al. 2009). We found the association between bisphosphonate use and atypical fractures to be independent of these drugs. The treatment indications are linked, as glucocorticoid treatment is an indication for bisphosphonate use and proton-pump inhibitors may be prescribed due to gastrointestinal side effects of the bisphosphonate.
Bisphosphonates are effective in the primary prevention of symptomatic fragility fractures during the first few years of use in female patients with osteoporosis who are less than 80 years of age. Prolongation of bisphosphonate treatment, however, does not appear to improve the initial benefit any further, but the risk of atypical femoral fracture increases with every year of use. Bisphosphonate treatment should therefore be limited, regarding both treatment duration and indication for treatment.
Supplementary data
Tables 1 and 5 are available at Acta’s website (www.actaorthop.org), identification number 7825.
Acknowledgments
JS contributed to collecting and evaluating the radiographs, analyzing and interpreting the data, and drafting and revising the article. VK contributed to collecting and evaluating the radiographs and helped to revise the article. PA contributed to the interpretation of data, drafting, and revision of the article. KM performed the analysis and interpretation of data and revised the article. PA and KM contributed equally.
The study was funded by the Swedish Research Council (VR 2009-6725), Linköping University, Östergötland County Council, and the King Gustaf V and Queen Victoria Freemason Foundation.
PA has a patent on a process for coating metal implants with bisphosphonates, and has shares in a company trying to commercialize the principle (Addbio AB). PA has also received reimbursement for consultation and grants from Eli Lilly & Co. VK, JS, and KM have no competing interests to declare.
References
- Bergstrom MF, Byberg L, Melhus H, Michaelsson K, Gedeborg R. Extent and consequences of misclassified injury diagnoses in a national hospital discharge registry . Inj Prev. 2011;17(2):108–13. doi: 10.1136/ip.2010.028951. [DOI] [PubMed] [Google Scholar]
- Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur . N Engl J Med. 2010;362(19):1761–71. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? . N Engl J Med. 2012;366(22):2051–3. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation . J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial . JAMA. 1998;280(24):2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users . J Bone Miner Res. 2008;23(9):1435–41. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur . J Bone Miner Res. 2012;27(12):2544–50. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- Eastell R, Walsh JS, Watts NB, Siris E. Bisphosphonates for postmenopausal osteoporosis . Bone. 2011;49(1):82–8. doi: 10.1016/j.bone.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, et al. The relationship between the chemistry and biological activity of the bisphosphonates . Bone. 2011;49(1):20–33. doi: 10.1016/j.bone.2011.03.774. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures: lessons from materials research . Bone. 2013;55(2):495–500. doi: 10.1016/j.bone.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Feldstein AC, Weycker D, Nichols GA, Oster G, Rosales G, Boardman DL, et al. Effectiveness of bisphosphonate therapy in a community setting . Bone. 2009;44(1):153–9. doi: 10.1016/j.bone.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Feldstein AC, Black D, Perrin N, Rosales AG, Friess D, Boardman D, et al. Incidence and demography of femur fractures with and without atypical features . J Bone Miner Res. 2012;27(5):977–86. doi: 10.1002/jbmr.1550. [DOI] [PubMed] [Google Scholar]
- Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis . J Bone Miner Res. 2013;28(8):1729–37. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use . N Engl J Med. 2010;362(19):1848–9. doi: 10.1056/NEJMc0910389. [DOI] [PubMed] [Google Scholar]
- Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: A systematic review of case/case series studies . Bone. 2010;47(2):169–80. doi: 10.1016/j.bone.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Greenland SR, Rothman K. J. Introduction to Stratified Analysis In: Modern Epidemiology, Third Edition. In: Rothman KJG, S. Lash T. L., editors. Lippincott Williams and Wilkins; Philadelphia: 2008. [Google Scholar]
- Inderjeeth CA, Foo AC, Lai MM, Glendenning P. Efficacy and safety of pharmacological agents in managing osteoporosis in the old old: review of the evidence . Bone. 2009;44(5):744–51. doi: 10.1016/j.bone.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Ing-Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R. Low-energy femoral fractures associated with the long-term use of bisphosphonates . Drug Saf. 2009;32(9):775–85. doi: 10.2165/00002018-200932090-00002. [DOI] [PubMed] [Google Scholar]
- Kharazmi M, Michaelsson K, Hallberg P. Prodromal symptoms in patients with bisphosphonate-associated atypical fractures of the femur . J Bone Miner Metab. 2014 doi: 10.1007/s00774-014-0611-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register . BMC public health. 2011;11(450) doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use . Arch Intern Med. 2012;172(12):930–6. doi: 10.1001/archinternmed.2012.1796. [DOI] [PubMed] [Google Scholar]
- Norman TL, Wang Z. Microdamage of human cortical bone: incidence and morphology in long bones . Bone. 1997;20(4):375–9. doi: 10.1016/s8756-3282(97)00004-5. [DOI] [PubMed] [Google Scholar]
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy . J Clin Endocrinol Metab. 2005;90(3):1294–301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data . Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Rose G, Hamilton PJ. A randomised controlled trial of the effect on middle-aged men of advice to stop smoking . J Epidemiol Community Health. 1978;32(4):275–81. doi: 10.1136/jech.32.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study . J Bone Miner Res. 2005;20(1):141–51. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- Rydholm A. Highly different risk estimates for atypical femoral fracture with use of bisphosphonates - debate must be allowed! Acta Orthop . 2012;83(4):319–20. doi: 10.3109/17453674.2012.718517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilcher J. Epidemiology, radiology and histology of atypical femoral fractures . Acta Orthop Suppl. 2013;84(352):1–26. doi: 10.3109/17453674.2013.850008. [DOI] [PubMed] [Google Scholar]
- Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate . Acta Orthop. 2009;80(4):413–5. doi: 10.3109/17453670903139914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft . N Engl J Med. 2011;364(18):1728–37. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- Schilcher J, Koeppen V, Ranstam J, Skripitz R, Michaelsson K, Aspenberg P. Atypical femoral fractures are a separate entity, characterized by highly specific radiographic features. A comparison of 59 cases and 218 controls . Bone. 2013;52(1):389–92. doi: 10.1016/j.bone.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Schilcher J, Koeppen V, Aspenberg P, Michaelsson K. Risk of atypical femoral fracture during and after bisphosphonate use . N Engl J Med. 2014a;371(10):974–6. doi: 10.1056/NEJMc1403799. [DOI] [PubMed] [Google Scholar]
- Schilcher J, Sandberg O, Isaksson H, Aspenberg P. Histology of 8 atypical femoral fractures. Acta Orthop. 2014b. pp. 1–7. [DOI] [PMC free article] [PubMed]
- Seeman E. Bone quality: the material and structural basis of bone strength . J Bone Miner Metab. 2008;26(1):1–8. doi: 10.1007/s00774-007-0793-5. [DOI] [PubMed] [Google Scholar]
- Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the american society for bone and mineral research . J Bone Miner Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- Silverman SL, Schousboe JT, Gold DT. Oral bisphosphonate compliance and persistence: a matter of choice? . Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(1):21–6. doi: 10.1007/s00198-010-1274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim Ie W, Ebeling PR. Treatment of osteoporosis in men with bisphosphonates: rationale and latest evidence . Therapeutic advances in musculoskeletal disease. 2013;5(5):259–67. doi: 10.1177/1759720X13500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley C, Kushner HI, Sterk CE. Lung cancer, chronic disease epidemiology, and medicine , 1948-1964 . Journal of the history of medicine and allied sciences. 2004;59(3):329–74. doi: 10.1093/jhmas/jrh088. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Atlanta, GA; U.S.: 2004. The Health Consequences of Smoking: A Report of the Surgeon General. The Health Consequences of Smoking: A Report of the Surgeon General. [Google Scholar]
- Valimaki MJ, Karkkainen M, Lamberg-Allardt C, Laitinen K, Alhava E, Heikkinen J, et al. Exercise, smoking, and calcium intake during adolescence and early adulthood as determinants of peak bone mass. Cardiovascular Risk in Young Finns Study Group . BMJ (Clinical research ed) 1994;309(6949):230–5. doi: 10.1136/bmj.309.6949.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008a. CD004523. [DOI] [PubMed]
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, et al. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008b. CD003376. [DOI] [PMC free article] [PubMed]
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, et al. Alendronate for preventing fractures caused by osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2008c. CD001155. [DOI] [PMC free article] [PubMed]
- Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis--where do we go from here? . N Engl J Med. 2012;366(22):2048–51. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.