Abstract
Background
The thermic effect of food accounts for ~ 10% of daily energy expenditure. A reduction in the thermic effect of food, which has been variably observed in the older adults, could predispose to fat gain. We tested whether the thermic effect of food is reduced in older adults compared with young adults by analyzing our database of standardized studies conducted at the Mayo Clinic between 1999 and 2009.
Methods
Data was available from 136 older adults volunteers age 60 to 88 (56 females) and 141 young adults ages 18 to 35 years (67 female). Basal energy expenditure was measured by indirect calorimetry to assess basal metabolic rate. Body fat, fat free mass and visceral fat were measured using a combination of dual energy x-ray absorptiometry and an abdominal CT scan. We measured the thermic effect of food and postprandial insulinemia in 123 older adults (52 female) and 86 young (38 female) of these volunteers.
Results
Basal metabolic rate adjusted for fat free mass was less in older adults (P = 0.01) and the thermic effect of food was ~ 1% (P = 0.02) less in the older adults. After controlling for meal size and fat free mass, body fat and fat distribution did not predict the thermic effect of food.
Conclusions
Both basal metabolic rate and the thermic effect of food are less in older adults than young adults, even when they have similar amounts of lean tissue and consume a similar size meal. These factors contribute to lower daily energy expenditure in the older adults.
Keywords: Indirect calorimetry, resting metabolic rate, body composition, body fat, insulin
Introduction
Obesity is associated with an increased risk of metabolic disorders such as hyperlipidemia, metabolic syndrome, and type 2 diabetes in older adult population [1,2]. Excessive energy intake and/or insufficient expenditure will contribute to the storage of energy as fat with increasing age [3].
Total energy expenditure includes resting metabolic rates (RMR), the thermic effect of food (TEF), and the energy expenditure during physical activity. TEF is the increase in energy expenditure above resting associated with the ingestion of food and accounts for ~ 10% of the total daily energy expenditure [4]. It has been suggested that reduced TEF in the older adults [5,6] may contribute to the onset or maintenance of obesity over a long term.
The best predictor of RMR is fat-free mass (FFM) [7,8], and it has been reported that RMR relative to FFM is decreased in the older adults. However, whether TEF is reduced with increasing age and whether inter-individual differences in TEF with aging can be related to variations in body fat and body fat distribution is still unclear. We took advantage of our large database to examine the relationship of RMR and TEF with age. The second objective of the study was to further clarify the relationship between body composition, fat distribution and postprandial insulin responses with TEF.
Research Design and Methods
Participants
We used data collected from 277 volunteers (123 females), all of whom had standardized measurements of basal metabolic rate and body composition. Of these 277 volunteers, 209 (90 females) also underwent protocolized measures of TEF that employed a standardized approach to meal size and meal composition. These studies were conducted in the Mayo Clinic General Clinical Research Center (GCRC) between 1999 and 2009. All volunteers were in good health with no acute illness, recent tobacco use or diabetes. None of the volunteers used medications known to affect metabolic rate and all volunteers gave written informed content to these Mayo Clinic Institutional Review Board approved protocols.
Study protocol
A complete blood count, electrolytes, liver and kidney function studies were documented to be normal prior to the study. Body composition was measured prior to the RMR/TEF studies. Participants were admitted to the Mayo Clinic GCRC the evening before the study after at least 3 days of consuming a weight-maintenance, controlled diet provided by the GCRC Metabolic Kitchen. The morning of the study an intravenous catheter was placed in a hand vein for collecting arterialized blood using the heated (55°C) hand vein technique. At ~ 0700 h, after at least a 12 h fast, each volunteer’s RMR was measured as previously described [9]. This value was used to provide a meal containing 33% of their resting energy expenditure which was consumed at ~ 0800 h in 123 older and 86 young volunteers. The meals always consisted of a liquid formula (Ensure Plus, Ross Laboratories) that contains 57% carbohydrate, 27% fat (16 % saturated fat, 27% monounsaturated fat, 57 % polyunsaturated fat) and 15% protein. After consuming the meal, indirect calorimetry was performed hourly for 4 hours. The indirect calorimetry measurements were always done in the recumbent position, with modest elevation of the head of the bed as needed for volunteer comfort. The volunteers were permitted to get out of bed after consuming the test meal in order to void. Blood was sampled prior to the meal and hourly for 4 hours thereafter for measurement of plasma insulin concentrations.
Body composition measurement
Total body and regional fat and fat free mass was measured using dual energy x-ray absorptiometry (DXA) (DPX-IQ, Lunar Radiation Corp, Madison, WI). Visceral fat was measured using a combination of DXA and a single slice computed tomography (CT) scan of the abdomen at the L2-3 interspace [10].
Indirect calorimetry
Oxygen consumption and carbon dioxide production were always measured by indirect calorimetry using a DeltaTrac Metabolic Cart (Yorba Linda, CA). The initial indirect calorimetry measurement was 30 min in duration with a 5–10 min acclimatization period. Each subsequent measurement was 20 min in duration, including a 5 min interval to establish stable breathing. The precautions taken to assure good quality indirect calorimetry results have previously been reported [11]. We examined the test, re-test differences in TEF from one of our previous studies [12] and found it to be 21 ± 6% in healthy young men.
Analysis of samples
Insulin concentrations were measured using chemiluminescent sandwich assays (Sanofi Diagnostics, Chaska, MN). Plasma glucose was measured using a glucose analyzer (Beckman Instruments, Fullerton, CA).
Calculations and statistics
Oxygen consumption and carbon dioxide production data were converted to energy expenditure using the formulas outlined by Frayn [13]. The thermic effect of food was calculated as the area under the curve above baseline for the 4 hours after the meal. The data are presented as mean ± SEM unless otherwise stated. Skewed data were logarithmically transformed to ensure normal distribution. Statistical analyses were performed using unpaired two-sided Student t tests for group comparison. ANCOVA was used to adjust for differences in body composition and body fat distribution when BMR and TEF were compared between age groups. Univariate regression analysis was used to test for correlations between FFM and RMR. Pearson’s correlation coefficients were calculated to investigate the relationship between TEF and body composition, fat distribution. Stepwise regression analyses were used to assess independent predictors of TEF. All data were analyzed using JMP 9.0 (SAS Institute, Cary, NC). P value of <0.05 were considered statistically significant.
Results
Subject characteristics and measurement of RMR
The characteristics of volunteers participating in these studies are provided in Table 1, including body composition, body fat distribution. Older adult participants had significantly higher BMI and body fat than did the young subjects. FFM (kg) was not significantly different between the two groups.
Table 1.
Subject characteristics
| Young adults (n=141) | Older adults (n=136) | P Value | |
|---|---|---|---|
|
|
|||
| Age (yr) | 25±4 (18–35) | 69±6 (60–88) | - |
| Female: male | 67:74 | 56:80 | NS |
| Weight (kg) | 71±12 (45–103) | 80±13 (53–127) | <0.0001 |
| BMI (kg/m2) | 23±3 (18–31) | 27±3 (19–35) | <0.0001 |
| FM (%) | 24±8 (8–47) | 32±9 (7–53) | <0.0001 |
| FFM (kg) | 54±12 (31–85) | 54±12 (34–84) | NS |
| FM (kg) | 17±6 (6–35) | 25±8 (5–47) | <0.0001 |
| UBSQ (kg) | 8.6±3.2 (2.8–22.3) | 12.6±3.9 (2.4–22.4) | <0.0001 |
| LBSQ (kg) | 6.6±2.6 (2.2–16.1) | 8.4±3.4 (1.9–18.4) | <0.0001 |
| VAT (kg) | 1.5±0.9 (0.2–5.4) | 4.3±1.9 (0.9–11.1) | <0.0001 |
| RMR (kcal/24h) | 1566±260 (1019–2323) | 1525±294 (999–2628) | NS |
Values are mean ± SD (range). BMI – body mass index; FM – fat mass; FFM – fat free mass; UBSQ – upper body subcutaneous fat mass; LBSQ – lower body subcutaneous fat mass; VAT –visceral adipose tissue; RMR – resting metabolic rate; NS – not significant.
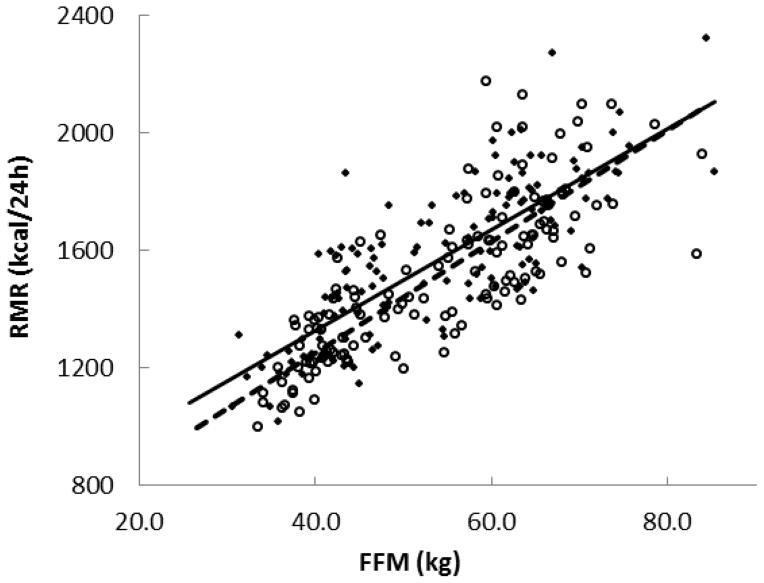
Although the RMR (kcal/24h) values were not significantly different between the older adults and young adults (P = 0.22), the relationship between RMR and FFM was different. RMR and FFM were strongly related in both groups (R2 = 0.64, P < 0.0001 in older adults and R2 = 0.65, P < 0.0001 in young group) (Figure 1), but after adjusting RMR for FFM, RMR was significantly less in older adults than young (P = 0.01). The slope of this relationship was not different, while the intercept was different (P = 0.01). This finding is in accordance with previous investigation [7,8].
Fig 1.
Resting metabolic rate (RMR) positively correlated with fat free mass (FFM) in the young adults (◆) (n=141) and older adults (○) (n=136) groups. Regression line of the young adults group (solid line): y=17.2x + 641 (R2 = 0.65, P < 0.0001); Older adults groups (dashed line): y=19.0x + 491 (R2 = 0.64, P < 0.0001). P = NS between the slope and P = 0.01 between the intercept of the two regression lines.
Measurement of TEF between older adults and young subjects
Table 2 provides the RMR, TEE (total energy expenditure) over 4 h, TEF, meal size and plasma insulin AUC measured in 123 older adults and 86 young volunteers. On average, RMR was not significantly different between the two groups. TEE (kcal/4h, P = 0.04) was less in older adults than young. Both total TEF (kcal/4h, P = 0.004) and TEF expressed as % of meal size (P = 0.02) were significantly less in the older adults than in the young. The peak TEF (kcal/min) was significantly less in the older adults than the young (P < 0.0001). Meal size was not different between older adults and young subjects and plasma insulin concentration AUC was almost double in the older adults (P < 0.0001) compared with the young. The TEF (% of meal size) was not significantly different between older adult women and men (5.9±0.3 vs. 6.8±0.3 %, respectively, P = 0.27) and of borderline difference between young women and men (6.9±0.5 vs. 7.6 ±0.4%, respectively, P = 0.05).
Table 2.
Resting metabolic rate, total energy expenditure, thermic effect of food, plasma insulin AUC and meal size during the TEF study
| Young adults (n=86) | Older adults (n=123) | P Value | |
|---|---|---|---|
|
|
|||
| Female: male | 38:48 | 52:71 | NS |
| RMR (kcal/24h) | 1574±29 | 1515±26 | NS |
| TEE (kcal/4h) | 301±6 | 285±5 | 0.04 |
| TEF (kcal/4h) | 38.2±1.8 | 32.0±1.3 | 0.004 |
| Peak TEF (kcal/min above baseline) | 0.30±0.01 | 0.23±0.008 | <0.0001 |
| TEF (%meal) | 7.3±0.3 | 6.4±0.2 | 0.02 |
| Meal size (kcal) | 524±10 | 500±9 | NS |
| Insulin AUC (pmol/4h) $ | 4476±306 | 8072±446 | <0.0001 |
Values are mean ± SEM. RMR – resting metabolic rate; TEE – total energy expenditure; TEF –thermic effect of food; Insulin AUC – postprandial plasma insulin concentration area under curve;
Statistics performed on log transformed data; NS – not significant.
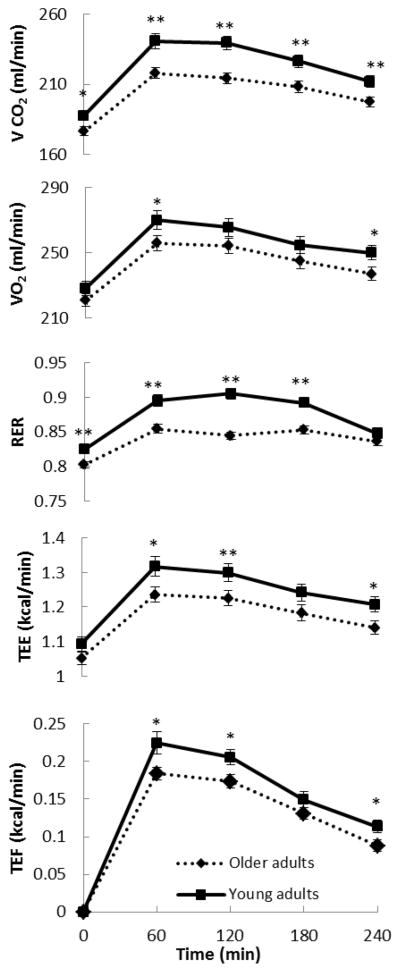
Figure 2 shows the curve of the rate of oxygen consumption (VO2), carbon dioxide production (VCO2), the respiratory exchange ratio (RER), TEE and TEF in the older adults and young. The peak postprandial VO2 (60 min) was significantly greater in young than older adults (P < 0.05) and the RER was greater from basal to180 min in young than the older adults (P < 0.01), indicating the young oxidize more carbohydrate as a source of energy. The postprandial TEE was greater in young than older adults (P < 0.05 at 60 and 240 min, P < 0.01 at 120 min) and the TEF was greater in young than older adults (P < 0.05 at 60, 120 and 240 min).
Fig 2.
Time course of the rate of carbon dioxide production (VCO2– first), oxygen consumption (VO2– second), respiratory exchange ratio (RER– third), total energy expenditure (TEE– fourth) and thermic effect of food (TEF– fifth) measured every 60 min for the young adults and older adults groups. **P < 0.01,*P < 0.05 between the two groups.
TEF associations with body composition and insulin concentrations
Table 3 provides the Pearson’s correlation coefficients between the TEF (kcal/4h) and a variety of parameters, including meal size, sex, body composition and body fat/body fat compartments, as well as plasma insulin AUC. Meal size (P < 0.0001 and P = 0.007, respectively) and FFM (kg) (P < 0.0001 and P = 0.006, respectively) positively correlated with TEF both in older adults and young, while FM (%) (P = 0.0002 and P = 0.07, respectively), LBSQ (%) (P = 0.0001 and P = 0.02, respectively), and UBSQ (%) (P = 0.0008 and P = 0.07, respectively) negatively correlated with TEF in older adults and young. VAT and total insulin AUC were not significantly associated with TEF in either group. Taking into consideration these significant parameters, we performed stepwise multivariate regression analyses. Only FFM in both older adults and young subject remained significant predictors of TEF. The difference in TEF between older adults and young remained (P = 0.02) after adjustment for meal size, FFM, FM, LBSQ and UBSQ.
Table 3.
Simple correlation coefficients between thermic effect of food and body composition variables
| Young adults | Older adults | |||
|---|---|---|---|---|
|
| ||||
| TEF | P value | TEF | P value | |
| Meal (kcal) | 0.29 | 0.007 | 0.37 | <0.0001 |
| FFM (kg) | 0.29 | 0.006 | 0.49 | <0.0001 |
| FM (%) | −0.20 | NS | −0.33 | 0.0002 |
| LBSQ (%) | −0.24 | 0.02 | −0.35 | 0.0001 |
| UBSQ (%) | −0.20 | NS | −0.30 | 0.0008 |
TEF – thermic effect of food (kcal/4h); FM – fat mass; FFM – fat free mass; UBSQ – upper body subcutaneous fat mass; LBSQ – lower body subcutaneous fat mass. Symbol % represents % of body weight; NS – not significant.
Discussion
Although averaging < 10% of daily energy expenditure, the wide inter-individual variation of TEF has been sufficiently interesting to provoke research into its regulation. We used a large data base of identically conducted test meal experiments to address the question of whether TEF is reduced in the older adults (age ≥ 60) compared with those ≤ 35 years of age. The novel findings from our results are that, although TEF is statistically significantly reduced in the older adults, it is only ~ 1% less in the older adults than the young. Furthermore, this reduction was primarily related to a lesser peak increase in postprandial energy expenditure rather than a more rapid offset of TEF. Finally, the postprandial increase in RER was blunted in the older adults, indicating a reduced carbohydrate oxidation in response to meals. After controlling for variables such as body composition and postprandial insulin responses (an index of insulin resistance), which also differed between the older adults and the young, age group continued to be a predictor of TEF.
Different groups have come to opposite conclusions as to whether TEF is reduced in the older adults. Some authors suggest that both RMR and TEF were lower in the older adults [5,6,14], while others found that only RMR was reduced in the older adults [15–17]. Some of the discrepant findings regarding whether TEF is reduced in the older adults may be the result of differences in sample size, duration of the post-meal measurements, energy and macronutrient composition of the test meals, and pre-study preparations. Because of the large intra-individual coefficient variations for TEF (about 30%) [18], studies with a small sample size will have limited statistical power to detect differences. The number of participants in some previous studies [5,6,14–17] may have been only a few dozen. We believe that our relatively large sample size improved our ability to detect small differences between the young and older adults. There are differences of opinion regarding the optimal duration of TEF measures [18–23]. Hill et al [22] reported that TEF had not returned to baseline at 3 h for following consumption of meals providing 500, 1000, or 1500 kcal. Reed et al [4] recommended the TEF be measured for more than 5 h. In contrast, Weststrate [18] concluded that TEF could be assessed accurately with 3 h of measures and reported that more prolonged experiments could increase participant restlessness. The 4 h duration of our measurement seems to be a reasonable compromise between acquiring the complete TEF AUC and avoiding restlessness.
Previous studies finding a reduced TEF in the older adults used an oral glucose load given on the basis of each participant’s FFM [6] or provided a fixed, 800 kcal high carbohydrate meal [5] to all volunteers. Our participants consumed a meal containing usual amounts of fat, protein and carbohydrate. The energy content of the mixed meal was adjusted based upon each individual’s RMR. This experimental design was developed to more closely mimic a typical meal and avoid potential, confounding variables that might distort TEF responses. Furthermore, by providing meals of similar macronutrient content for at least 3 days prior to the TEF measure, and by allowing our volunteers to sleep overnight in the GCRC prior to the study, we minimized pre-study issues of over- or under-consumption of various macronutrients that might distort postprandial substrate oxidation and eliminated the potential for RMR disturbances related to early morning travel.
We found that our older adults participants had reduced RMR adjusted for FFM compared with the young volunteers, which is in line with previous investigations [7,8,16]. This suggests our indirect calorimetry and body composition measures are comparable to other centers. After adjusting for FFM and meal size, we could not detect a relationship between body fat, fat distribution and TEF. Some reports indicate that TEF is inversely related to plasma insulin concentrations, perhaps explaining why the older adults might have lower TEF [24,25], but other investigators have come to opposite conclusions [26]. We did not find a significant, negative relationship between body fat or insulin AUC and TEF after adjusting for meal size, age, FFM and sex, although the body fat and insulin AUC were higher in older adults.
What might be the thermogenic implications of these results? The average RMR adjusted for FFM for those with 55 kg of FFM would be ~ 25 kcal/day less in the older adults than the young. If we use the 24 h energy intake of Vaughan et al (14) to calculate TEF (% meal size), there would be additional, average ~ 40 kcal/day TEF gap between the older adults and the young adults. Extrapolated over a year, the average older adult individual would expend 24,000 kcal less than a young adult even if they are matched for FFM and have similar activity levels. The reduced RMR and TEF with aging increase the risk of gaining an unhealthy amount of body fat unless food intake declines or activity increases.
Some limitations to our results must be acknowledged. Virtually all of our volunteers were Caucasian, and thus we cannot extrapolate these results to other racial groups. It is possible that if we had extended the TEF measures for a longer period of time we may have seen different results, however, examining the curves of TEF above baseline (Figure 2, bottom panel), we see the decline in oxygen consumption is parallel in the young and older adults, making it extremely unlikely the young would have returned to baseline before the older adults. In all likelihood, we have somewhat underestimated the TEF defect in the older adults relative to the young.
In summary, we found a clinically and statistically significant reduction in both TEF and RMR in older adults compared with young adults. The difference could not be related to body fat content or postprandial insulin if we took into account meal size and FFM. The lesser energy expenditure in the older adults may contribute to a greater tendency to gain fat with age and increase the risk of obesity-related diseases.
References
- 1.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, Kessler RC, Kling JR, Lindau ST, Whitaker RC, McDade TW. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med. 2011;365:1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fradkin JE, Roberts BT, Rodgers GP. What’s preventing us from preventing type 2 diabetes? N Engl J Med. 2012;367:1177–1179. doi: 10.1056/NEJMp1208169. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63:164–169. doi: 10.1093/ajcn/63.2.164. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RS, Jaeger LF, Veith RC. The thermic effect of feeding in older men: the importance of the sympathetic nervous system. Metabolism. 1990;39:733–737. doi: 10.1016/0026-0495(90)90109-p. [DOI] [PubMed] [Google Scholar]
- 6.Jones PP, Van Pelt RE, Johnson DG, Seals DR. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. J Clin Endocrinol Metab. 2004;89:5138–5144. doi: 10.1210/jc.2004-0101. [DOI] [PubMed] [Google Scholar]
- 7.Poehlman ET. Energy intake and energy expenditure in the elderly. Am J Hum Biol. 1996;8:199–206. doi: 10.1002/(SICI)1520-6300(1996)8:2<199::AID-AJHB7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49:968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MD, Bajnarek J, Lee SY, Nielsen S, Koutsari C. Relationship between postabsorptive respiratory exchange ratio and plasma free fatty acid concentrations. J Lipid Res. 2009;50:1863–1869. doi: 10.1194/jlr.M900021-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 11.Jensen MD, Kanaley JA, Roust LR, O’Brien PC, Braun JS, Dunn WL, Wahner HW. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–873. doi: 10.1016/s0025-6196(12)60695-8. [DOI] [PubMed] [Google Scholar]
- 12.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol. 2005;288:E547–E555. doi: 10.1152/ajpendo.00340.2004. [DOI] [PubMed] [Google Scholar]
- 13.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 14.Morgan JB, York DA. Thermic effect of feeding in relation to energy balance in elderly men. Ann Nutr Metab. 1983;27:71–77. doi: 10.1159/000176625. [DOI] [PubMed] [Google Scholar]
- 15.Visser M, Deurenberg P, van Staveren WA, Hautvast JG. Resting metabolic rate and diet-induced thermogenesis in young and elderly subjects: relationship with body composition, fat distribution, and physical activity level. Am J Clin Nutr. 1995;61:772–778. doi: 10.1093/ajcn/61.4.772. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53:821–825. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- 17.Bloesch D, Schutz Y, Breitenstein E, Jequier E, Felber JP. Thermogenic response to an oral glucose load in man: comparison between young and elderly subjects. J Am Coll Nutr. 1988;7:471–483. doi: 10.1080/07315724.1988.10720263. [DOI] [PubMed] [Google Scholar]
- 18.Weststrate JA. Resting metabolic rate and diet-induced thermogenesis: A methodological reappraisal. Am J Clin Nutr. 1993;58:592–601. doi: 10.1093/ajcn/58.5.592. [DOI] [PubMed] [Google Scholar]
- 19.Segal KR, Edano A, Tomas MB. Thermic effect of a meal over 3 and 6 hours in lean and obese men. Metabolism. 1990;39:985–992. doi: 10.1016/0026-0495(90)90312-z. [DOI] [PubMed] [Google Scholar]
- 20.D’Alessio DA, Kavle EC, Mozzoli MA, Smalley KJ, Polansky M, Kendrick ZV, Owen LR, Bushman MC, Boden G, Owen OE. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai MM, Castillo P, Pi-Sunyer FX. Meal size and frequency: effect on the thermic effect of food. Am J Clin Nutr. 1991;54:783–787. doi: 10.1093/ajcn/54.5.783. [DOI] [PubMed] [Google Scholar]
- 22.Hill JO, Heymsfield SB, McMannus C, 3rd, DiGirolamo M. Meal size and thermic response to food in male subjects as a function of maximum aerobic capacity. Metabolism. 1984;33:743–749. doi: 10.1016/0026-0495(84)90216-6. [DOI] [PubMed] [Google Scholar]
- 23.Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40:542–552. doi: 10.1093/ajcn/40.3.542. [DOI] [PubMed] [Google Scholar]
- 24.Leene R, van der Kooy K, Deurenberg P, Seidell JC, Weststrate JA, Schouten FJ, Hautvast JG. Visceral fat accumulation in obese subjects: relation to energy expenditure and response to weight loss. Am J Physiol. 1992;263:E913–E919. doi: 10.1152/ajpendo.1992.263.5.E913. [DOI] [PubMed] [Google Scholar]
- 25.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron Artery Dis. 1998;9:503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kunz I, Klaus S, Kallies B, Schorr U, Sharma AM. Kinetic analysis of the thermic effect of food and its relationship to body composition in humans. Metabolism. 2000;49:1340–1345. doi: 10.1053/meta.2000.9533. [DOI] [PubMed] [Google Scholar]