Abstract
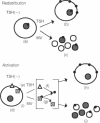
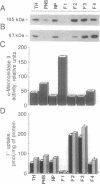
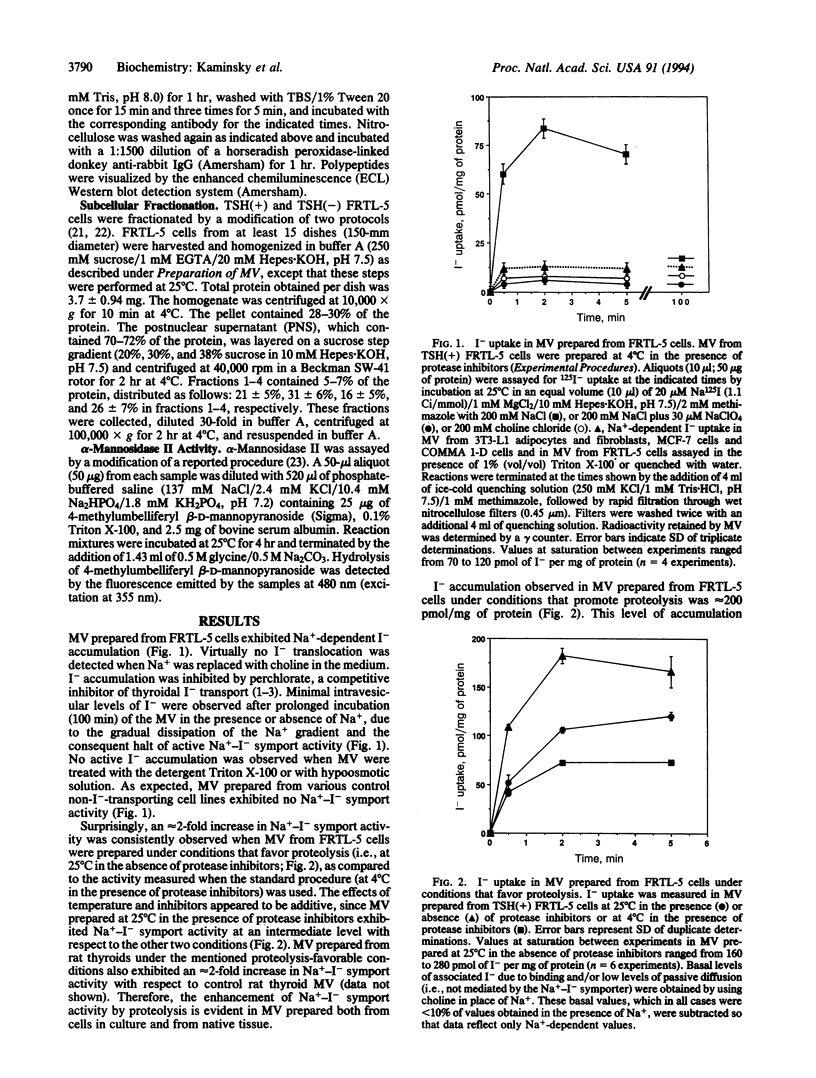
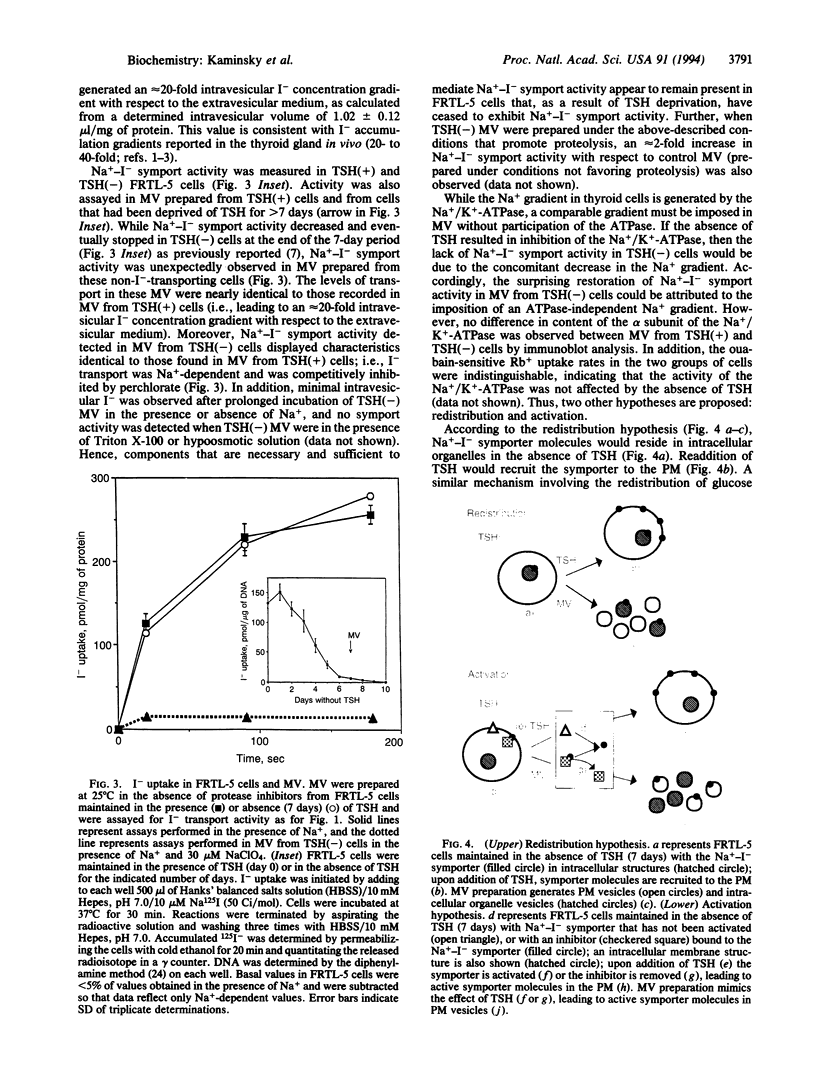
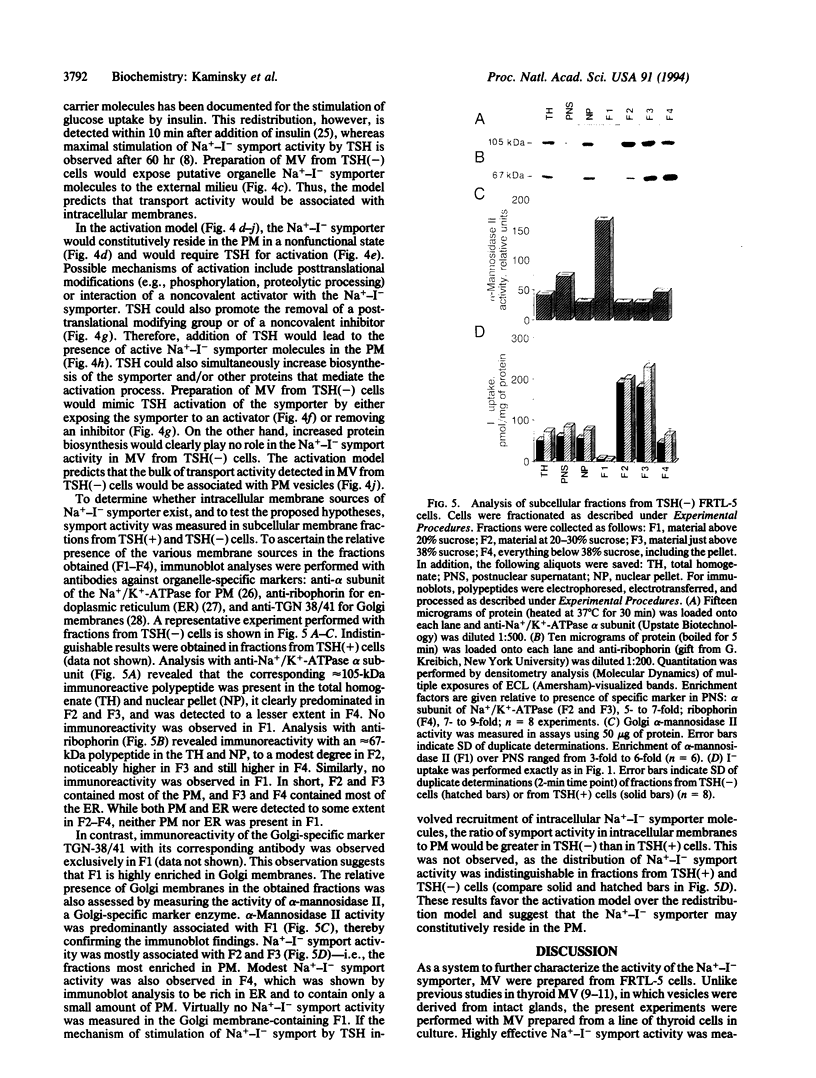
The active accumulation of I- in the thyroid gland is mediated by the Na(+)-I- symporter and driven by the Na+ gradient generated by the Na+/K(+)-ATPase. Thyrotropin (TSH) stimulates thyroidal I- accumulation. Rat thyroid-derived FRTL-5 cells require TSH to accumulate I-. TSH withdrawal for over 7 days results in complete loss of Na(+)-I-symport activity in these cells [Weiss, S. J., Philp, N. J. and Grollman, E. F. (1984) Endocrinology 114, 1090-1098]. Surprisingly, membrane vesicles prepared from FRTL-5 cells maintained in TSH-free medium [TSH(-)cells]accumulate I-, suggesting that the absence of Na(+)-I- symport activity in TSH(-) cells cannot be due solely to a decrease in the biosynthesis of either the symporter or a putative activating factor. This finding indicates that the Na(+)-I- symporter is present, probably in an inactive state, in TSH(-) cells despite their lack of Na(+)-I- symport activity. Na(+)-I- symport activity in thyroid membrane vesicles is enhanced when conditions for vesicle preparation favor proteolysis. Subcellular fractionation studies in both TSH(+) and TSH(-) cells show that Na(+)-I- symport activity is mostly associated with fractions enriched in plasma membrane rather than in intracellular membranes, suggesting that the Na(+)-I- symporter may constitutively reside in the plasma membrane and may be activated by TSH.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi N., Fawcett D. M. Role of sodium ion in active transport of iodide by cultured thyroid cells. Biochim Biophys Acta. 1973 Aug 22;318(2):235–251. doi: 10.1016/0005-2736(73)90117-x. [DOI] [PubMed] [Google Scholar]
- Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993 Jun 8;1154(1):65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- Clancy B. M., Harrison S. A., Buxton J. M., Czech M. P. Protein synthesis inhibitors activate glucose transport without increasing plasma membrane glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1991 Jun 5;266(16):10122–10130. [PubMed] [Google Scholar]
- DeTomaso A. W., Xie Z. J., Liu G., Mercer R. W. Expression, targeting, and assembly of functional Na,K-ATPase polypeptides in baculovirus-infected insect cells. J Biol Chem. 1993 Jan 15;268(2):1470–1478. [PubMed] [Google Scholar]
- Fong A. D., Handlogten M. E., Kilberg M. S. Substrate-dependent adaptive regulation and trans-inhibition of System A-mediated amino acid transport. Studies using rat hepatoma plasma membrane vesicles. Biochim Biophys Acta. 1990 Mar;1022(3):325–332. doi: 10.1016/0005-2736(90)90281-r. [DOI] [PubMed] [Google Scholar]
- Golstein P., Abramow M., Dumont J. E., Beauwens R. The iodide channel of the thyroid: a plasma membrane vesicle study. Am J Physiol. 1992 Sep;263(3 Pt 1):C590–C597. doi: 10.1152/ajpcell.1992.263.3.C590. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Clancy B. M., Pessino A., Czech M. P. Activation of cell surface glucose transporters measured by photoaffinity labeling of insulin-sensitive 3T3-L1 adipocytes. J Biol Chem. 1992 Feb 25;267(6):3783–3788. [PubMed] [Google Scholar]
- Hilgemann D. W. Regulation and deregulation of cardiac Na(+)-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 1990 Mar 15;344(6263):242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- James P., Vorherr T., Krebs J., Morelli A., Castello G., McCormick D. J., Penniston J. T., De Flora A., Carafoli E. Modulation of erythrocyte Ca2+-ATPase by selective calpain cleavage of the calmodulin-binding domain. J Biol Chem. 1989 May 15;264(14):8289–8296. [PubMed] [Google Scholar]
- Jones S. M., Crosby J. R., Salamero J., Howell K. E. A cytosolic complex of p62 and rab6 associates with TGN38/41 and is involved in budding of exocytic vesicles from the trans-Golgi network. J Cell Biol. 1993 Aug;122(4):775–788. doi: 10.1083/jcb.122.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kaminsky S. M., Levy O., Garry M. T., Carrasco N. Inhibition of the Na+/I- symporter by harmaline and 3-amino-1-methyl-5H-pyrido(4,3-b)indole acetate in thyroid cells and membrane vesicles. Eur J Biochem. 1991 Aug 15;200(1):203–207. doi: 10.1111/j.1432-1033.1991.tb21068.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcocci C., Cohen J. L., Grollman E. F. Effect of actinomycin D on iodide transport in FRTL-5 thyroid cells. Endocrinology. 1984 Dec;115(6):2123–2132. doi: 10.1210/endo-115-6-2123. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Björkman U., Ekholm R., Ericson L. E. Iodide transport in primary cultured thyroid follicle cells: evidence of a TSH-regulated channel mediating iodide efflux selectively across the apical domain of the plasma membrane. Eur J Cell Biol. 1990 Aug;52(2):270–281. [PubMed] [Google Scholar]
- Nilsson M., Björkman U., Ekholm R., Ericson L. E. Polarized efflux of iodide in porcine thyrocytes occurs via a cAMP-regulated iodide channel in the apical plasma membrane. Acta Endocrinol (Copenh) 1992 Jan;126(1):67–74. doi: 10.1530/acta.0.1260067. [DOI] [PubMed] [Google Scholar]
- O'Neill B., Magnolato D., Semenza G. The electrogenic, Na+-dependent I- transport system in plasma membrane vesicles from thyroid glands. Biochim Biophys Acta. 1987 Jan 26;896(2):263–274. doi: 10.1016/0005-2736(87)90187-8. [DOI] [PubMed] [Google Scholar]
- Rocmans P. A., Penel J. C., Cantraine F. R., Dumont J. E. Kinetic analysis of iodide transport in dog thyroid slices: perchlorate-induced discharge. Am J Physiol. 1977 Mar;232(3):E343–E352. doi: 10.1152/ajpendo.1977.232.3.E343. [DOI] [PubMed] [Google Scholar]
- Saji M., Kohn L. D. Effect of hydrocortisone on the ability of thyrotropin to increase deoxyribonucleic acid synthesis and iodide uptake in FRTL-5 rat thyroid cells: opposite regulation of adenosine 3',5'-monophosphate signal action. Endocrinology. 1990 Oct;127(4):1867–1876. doi: 10.1210/endo-127-4-1867. [DOI] [PubMed] [Google Scholar]
- Saji M., Kohn L. D. Insulin and insulin-like growth factor-I inhibit thyrotropin-increased iodide transport in serum-depleted FRTL-5 rat thyroid cells: modulation of adenosine 3',5'-monophosphate signal action. Endocrinology. 1991 Feb;128(2):1136–1143. doi: 10.1210/endo-128-2-1136. [DOI] [PubMed] [Google Scholar]
- Shyjan A. W., Levenson R. Antisera specific for the alpha 1, alpha 2, alpha 3, and beta subunits of the Na,K-ATPase: differential expression of alpha and beta subunits in rat tissue membranes. Biochemistry. 1989 May 30;28(11):4531–4535. doi: 10.1021/bi00437a002. [DOI] [PubMed] [Google Scholar]
- Siliprandi L., Vanni P., Kessler M., Semenza G. Na+-dependent, electroneutral L-ascorbate transport across brush border membrane vesicles from guinea pig small intestine. Biochim Biophys Acta. 1979 Mar 23;552(1):129–142. doi: 10.1016/0005-2736(79)90252-9. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Storrie B., Madden E. A. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente W. A., Vitti P., Kohn L. D., Brandi M. L., Rotella C. M., Toccafondi R., Tramontano D., Aloj S. M., Ambesi-Impiombato F. S. The relationship of growth and adenylate cyclase activity in cultured thyroid cells: separate bioeffects of thyrotropin. Endocrinology. 1983 Jan;112(1):71–79. doi: 10.1210/endo-112-1-71. [DOI] [PubMed] [Google Scholar]
- Vannucci S. J., Nishimura H., Satoh S., Cushman S. W., Holman G. D., Simpson I. A. Cell surface accessibility of GLUT4 glucose transporters in insulin-stimulated rat adipose cells. Modulation by isoprenaline and adenosine. Biochem J. 1992 Nov 15;288(Pt 1):325–330. doi: 10.1042/bj2880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidugiriene J., Menon A. K. Early lipid intermediates in glycosyl-phosphatidylinositol anchor assembly are synthesized in the ER and located in the cytoplasmic leaflet of the ER membrane bilayer. J Cell Biol. 1993 Jun;121(5):987–996. doi: 10.1083/jcb.121.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilijn F., Carrasco N. Expression of the thyroid sodium/iodide symporter in Xenopus laevis oocytes. J Biol Chem. 1989 Jul 15;264(20):11901–11903. [PubMed] [Google Scholar]
- WOLFF J. TRANSPORT OF IODIDE AND OTHER ANIONS IN THE THYROID GLAND. Physiol Rev. 1964 Jan;44:45–90. doi: 10.1152/physrev.1964.44.1.45. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Philp N. J., Ambesi-Impiombato F. S., Grollman E. F. Thyrotropin-stimulated iodide transport mediated by adenosine 3',5'-monophosphate and dependent on protein synthesis. Endocrinology. 1984 Apr;114(4):1099–1107. doi: 10.1210/endo-114-4-1099. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Philp N. J., Grollman E. F. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology. 1984 Apr;114(4):1090–1098. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- Yu Y. H., Sabatini D. D., Kreibich G. Antiribophorin antibodies inhibit the targeting to the ER membrane of ribosomes containing nascent secretory polypeptides. J Cell Biol. 1990 Oct;111(4):1335–1342. doi: 10.1083/jcb.111.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]