A 27-year-old woman requesting lip augmentation presented to our clinic (Figs. 1, 2). She had not been treated previously with any dermal filler. She was generally healthy and had no signs of active soft tissue infection, cheilitis, or herpes simplex lesions around her perioral area and face. She was not using any medication or herbal supplements (such as vitamin E, ginseng, ginger, or ginkgo biloba). Overall, she seemed to be an ideal candidate for lip augmentation using fillers. Before injection, written informed consent was obtained from the patient. According to standard procedure, EMLA (AstraZeneca PLC, London, UK) cream and cooling packs were applied to the perioral area ten minutes prior to injection. Mucosal and skin preparation was carried out with chlorhexidine gluconate. An Food and Drug Administration (FDA)-approved filler, consisting of 1 mL (0.5 mL per lip) of 24 mg/mL hyaluronic acid (HA) with 0.3% lidocaine (Juvederm Ultra Plus XC, Allergan, Irvine, CA, USA), was injected into the upper and lower vermilion border and vermilion. Retrograde fashion linear threading and serial puncture methods were used with a 30-gauge needle. The procedure was finished with a gentle massage to blend and smooth the region, and cooling packs were reapplied.
Fig. 1. A twenty-seven-year old woman presented requesting lip augmentation.
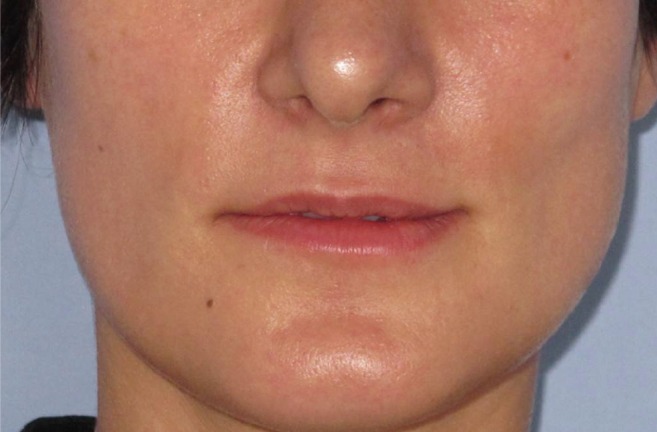
Fig. 2. A progressive lip edema occurred within minutes following injection with hyaluronic acid and lidocaine.
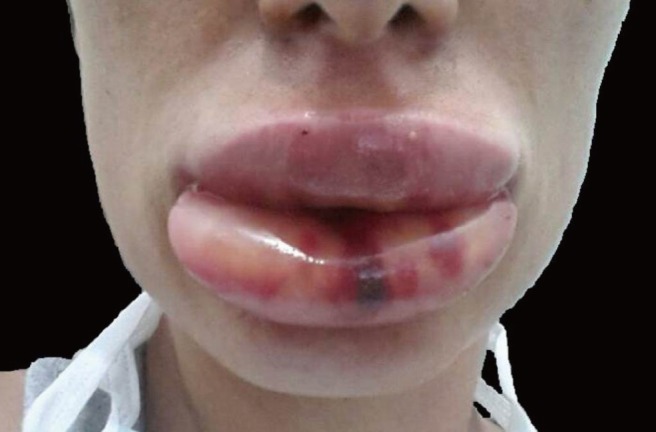
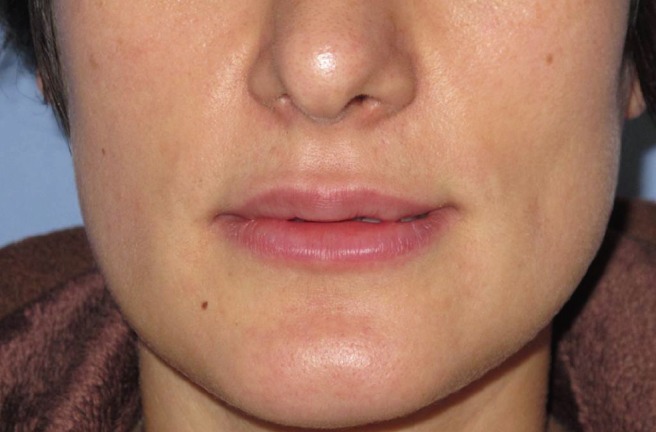
Everything was normal upon the completion of the filler injection, but a progressive edema arose on the injection site within minutes. Lip volume increased 3-4 fold during the first hour, and swelling progressed during the first 12 hours after the injection (Fig. 3). The patient was monitored for approximately 2 hours and treated with intravenous antihistamines (a slow infusion of 2 mL of 45.5 mg/2 mL pheniramine maleate). She was closely followed for any sign of respiratory distress or systemic reaction until the reaction was clearly confined. Systemic findings such as hypotension, changes in consciousness, generalized rash, tongue and pharynx edema, dyspnea, or dysphagia were not observed. The edema did not spread outside the injection area and remained localized to the lips. After 3 hours follow-up, she was sent home with oral antihistamines (5 mg desloratadine 2 time/day). Ointments were applied for lubrication to avoid lip fissures and cold compression was continued. The edema started to resolve 48 hours after the start of the reaction and was completely resolved by the seventh day. The patient was worried about her lips after the initial treatment, but ultimately was satisfied enough that she requested another filler session. Before the first session, we examined the patient carefully and asked about her experiences with any previous lidocaine injections such as those that occur during dental treatments, but she did not mention any kind of allergic reaction. Likewise, she had an uneventful rhinoplasty operation one year before this procedure.
Fig. 3. Edema was almost completely resolved at 7 days post-injection.
HA fillers have a low hypersensitivity profile rate compared to other soft tissue fillers, ranging from 0.6% to 0.8% [1]. Generally, reactions are mild to moderate, self-limited, and continue for less than seven days. However, Leonhardt et al. [2] reported a rare case of severe local hypersensitivity. They observed a sudden swelling of the lips after a HA filler injection. Their patient was treated with steroids and valacyclovir for herpes prophylaxis and reaction was almost completely improved in four days. Recently, FDA-approved fillers containing HA and lidocaine have become available, with the goal of increasing patient comfort. Although there are only a few studies comparing pure HA fillers and fillers containing HA and lidocaine, the available results reveal no significant differences in the safety, efficacy, and longevity between the two treatments, while less procedural pain has been associated with the use of a filler containing HA and lidocaine [3].
The present report is the first case of a severe angioedema-type acute hypersensitivity reaction to a filler containing HA and lidocaine. In the present case, this acute reaction could be secondary to HA, lidocaine, EMLA cream, or simply due to the repeated punctures of the needle. Although no immunological tests were performed because the patient did not consent, the patient's previous medical history indicates that HA hypersensitivity was more probable than other possible causes of the reaction. Although hypersensitivity reactions can often be treated by topical tacrolimus, intralesional steroids, systemic steroids, or antihistamines, the most commonly used drugs are steroids [4]. However, antihistamines are also frequently indicated in cases of hypersensitivity and they lead successful results. We preferred antihistamines in the treatment of this case and a satisfactory result was obtained. In light of our case and the similar cases in the literature, it seems that lips may have a greater tendency to swell and to show more severe reactions compared to other regions of the face. The high regional blood flow found in the lips may be the reason for this tendency. However, previous review articles about filler complications do not mention a higher possibility of hypersensitivity reactions following lip augmentation [5]. Once an allergic reaction has been observed after procedures involving artificial fillers, autologous lip augmentation options such as fat grafting become the first choice for future use in these patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Andre P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA -- Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol. 2004;18:422–425. doi: 10.1111/j.1468-3083.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 2.Leonhardt JM, Lawrence N, Narins RS. Angioedema acute hypersensitivity reaction to injectable hyaluronic acid. Dermatol Surg. 2005;31:577–579. doi: 10.1111/j.1524-4725.2005.31166. [DOI] [PubMed] [Google Scholar]
- 3.Weinkle SH, Bank DE, Boyd CM, et al. A multi-center, double-blind, randomized controlled study of the safety and effectiveness of Juvederm injectable gel with and without lidocaine. J Cosmet Dermatol. 2009;8:205–210. doi: 10.1111/j.1473-2165.2009.00451.x. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31:110–121. doi: 10.1177/1090820X10391083. [DOI] [PubMed] [Google Scholar]
- 5.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–575. doi: 10.1177/1090820X13484492. [DOI] [PubMed] [Google Scholar]


