Abstract
Objective(s):
Some pathologic situations such as diabetes and metabolic syndrome are associated with alternation in nitric oxide level. Incidence of these condition increases with aging. On the other hand, insulin secretion is modulated by nitric oxide, and nitric oxide synthase (NOS) activity is also altered in diabetes. In this study, modification in the enzyme activity associated with aging and also optimized procedure for islet NOS assay was investigated.
Materials and Methods:
Male Wistar rats were randomly divided in two experimental groups: A: adult rats; were 4 month old and B: old rats; were 12 month old. In all groups, plasma glucose, insulin and NOX (nitrite + nitrate = NOX) were measured, and also insulin secretion in isolated pancreatic islet with or without L-NAME was investigated. Furthermore, the inducible NOS activity with L-citrulline measurement in islets was measured.
Results:
L-citrulline was quantified using one step HPLC column. Aging induced hyperglycemia (P<0.05) and excess plasma NOX (17.74 ± 1.664 and 26.25 ± 2.166 μmol/l in A and B groups respectively, P<0.05) with unaltered plasma insulin. Islet insulin secretion was significantly reduced in aging rats. L-NAME induced islet insulin secretion especially in aging rats (P=0.003). Inducible NOS activity in islets of aging rats was significantly higher than adult rats (1.082 ± 0.084 and 6.277 ± 0.475 pmol/min per mg protein in adult and aging rats, respectively, P<0.001).
Conclusion:
These findings show that decreased in islet insulin secretion may be related to increase in iNOS activity in islets, which follows impaired carbohydrate metabolism in aging.
Keywords: Aging, High performance liquid chromatography, Islet, L-Citrulline, Nitric oxide synthase activity, Rat
Introduction
Nitric oxide (NO) is a soluble gaseous molecule with numerous biological activities that has multiple influences on the physiology and pathophysiology of the cells including cytotoxic and cytoprotective effects (1). In human and animals bodies, it is produced by nitric oxide synthase (NOS) enzyme isoforms and by NO donors. Three isoforms of NOS are so far introduced; calcium dependent and membrane associated endothelial NOS, calcium dependent neuronal NOS (nNOS), and calcium independent cytosolic inducible NOS (iNOS). These enzymes oxidize L-arginine to produce NO and L-citrulline; NADPH is electron donor and acts as co-substrate and is strictly required for this enzymatic reaction (2). Pancreatic β-cells constitutively express nNOS, which is required for survival and physiological function (3). Inducible NOS is not usually expressed in these cells, but produces constantly large amount of NO in responses to inflammatory mediators (4). NO participates in regulation of β- cell mass in islets of pancreas (5). In this context, it should be mentioned that relation to its concentration and origin, NO has a dual biological functions; both proliferative (6) and apoptotic (7). Low amount of NO derived from nNOS has beneficial effects, but higher concentration resulting from iNOS activity induces inflammatory process, and is toxic both for host cells and invading biological organisms [d1](8, 9). Exposure to pathogens increases with aging; on the other hand metabolic activities especially carbohydrate metabolism deteriorates during aging processes (10). Also, the prevalence of glucose intolerance and type II diabetes is markedly increased with aging. In this situation, both insulin secretion and plasma insulin levels are reduced (11). Furthermore, iNOS activity in pancreatic islets of type II diabetes noticeably increases (12). According to research based theory, increase in iNOS activity and subsequent production of nitric oxide is associated with aging (13). On the other hand, NO can be considered a true inflammatory mediator (14). In the course of inflammation, the role of iNOS appears to be prominent (15). Consequently increase in iNOS activity has been reported in the wide range of inflammatory condition including; type2 diabetes, metabolic syndrome and Alzheimer’s disease (16). Due to important role of iNOS activity in above mentioned conditions and especially in type 2 diabetes, which increases during aging processes, this necessitates the measurement of iNOS activity in pancreatic islets. The aim of this study was to find out the possible relation between changes in iNOS activity and aging and also establish/modify a rapid and sensitive HPLC method of assay for NOS activity in tissue homogenate.
Materials and Methods
Animals and sample preparation
A total of twelve male Wistar rats (15 weeks old, 200 to 250 g) were supplied from Pasture Institute, Tehran, Iran and then were divided into two experimental groups; adult young group: six rats of four months of age; and six of four months age rats in old groups were kept in animal house for extra 8 months that in final became 12 months of age. The animals were housed under standard conditions (12 hr light–12 hr dark cycle starting at 07:00 AM at 24°C in a controlled humidity) with free access to food and water during one week of adaptation to laboratory situation. This study was approved by the local ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran, and all experiments were conducted in accordance with standard ethical guidelines. Animals either after two weeks of adaptation or 8 months of housing (old group) were anesthetized by intraperitoneal injection of sodium pentobarbital, 60 mg/kg body weight after an overnight fasting (17). Blood samples were obtained from heart for NOX, insulin and glucose measurement, and then followed by median laparotomy for pancreatic islets isolation. For NOS assay, groups of 200 islets were collected in ice-cold lysis buffer (200 μl) containing hydroxyethyl piperazine ethane sulfonic acid (HEPES; 20.0 mM, pH 7.4, Sigma) as a buffer, L-dithiothreitol (L-DTT; 1.0 mM, Sigma) and cocktail of protease inhibitor with EDTA supplement (1tablet dissolved in 10 ml buffer, Cat. No.11836153001, Roche Company Germany) and then aliquots were stored at -80°C (18). Insulin and glucose were measured using glucose oxidase and ELISA procedures, respectively. Insulin and glucose kits were supplied from Zistchimi (Iran) and Mercodia (Sweden) companies, respectively. The intra-assay coefficients of variations for insulin, glucose and NOx were 8.42%, 2.5% and 3.87%, respectively.
Islet isolation
For islet isolation, 10 ml ice-cold Hanks’ balanced salt solution (HBSS) (pH, 7.4; containing NaCl, 136; KCl, 5.36; CaCl2, 1.26; MgSO4 · 7H2O, 0.8; Na2HPO4 · 2H2O, 0.33; KH2PO4, 0.44; NaHCO3, 4.16) all in mM concentration including 0.5 mg/ml of collagenase P (Roche, Cat. Number 1213, Germany) was injected into the pancreatic duct, and then the islets were removed, and transferred to a sterile 50 ml falcon tube and then digested in a 37°C water bath for 15 min (19). Reaction was terminated by adding ice-cold HBSS, and then tube was shaken for 1 min. After 2 washes with cold HBSS, the suspension was filtered through a 500 μm plastic mesh to discard any undigested tissue particles. Followed by one washing, then the islets were hand-picked under a stereomicroscope (Kyowa Optical, SDZ-TR-PL model, Japan).
Glucose stimulated insulin secretion
For evaluation of insulin secretion in vitro, batches of 5 islets (three replicas for each condition from 4 animals) were incubated in tubes containing 1 ml of Krebs – Ringer solution [(pH, 7.4); NaCl 115; KCl 5; MgCl2 6H2O 1; CaCl2 2.5; NaHCO3 24 and HEPES, 16 all in mM] and 5 g/dl bovine serum albumin (BSA; Fluka, USA) (19) with different glucose concentrations (5.6, 8.3, 16.7 mM) for 60 min in 37° C water bath, and gassed with 95% O2 /5% CO2 for 5 min at the beginning. Then the supernatant was stored at -20°C for insulin determination. In some experiment, groups of five islets were incubated for 60 min with various concentration of glucose containing 5 mM of NOS inhibitor L-NG-Nitro arginine Methyl Ester (L-NAME, Sigma) (20).
Measurement of plasma NOX concentration
For indirect measurement of NOS activity, nitrite concentration was measured using Griess protocol. Briefly, serum proteins were precipitated using ZnSO4 (Merck), and then the reduction of nitrate to nitrite was performed by saturated solutions of VCl3 (Vanadium Chloride) (0.8% w/v) in 1 M HCl (Merck) and then the color generating solution containing N-L(naphthyl) ethylenenndiamine dihydrochloride (NEDD; 0.1% w/v, Merck) in H2O and sulfanilamide (2% w/v, Merck) in 5% HCl (Griess reagents) were added to the reaction vessels. After 20 min of incubation at room temperature, the absorbance was read in 540 nm (21) using spectrophotometer.
NOS activity assay
Pancreatic islets were sonicated using three pulses, 10 sec each at 40% intensity. After centrifugation at 35,000 g for 10 min, the supernatant was kept on ice for determination of protein concentrations using Bradford method for protein and enzyme assay (22). For iNOS activity assay, 100 μl of supernatant was added to reaction mixture containing HEPES, 20.0 mmol/l (pH 7.4), 0.2 mM L-arginine (Sigma) and 2 mM NADPH (Sigma) in a total volume of 0.5 ml and was then incubated under constant air bubbling (1 ml/min) at 37°C for 120 min. The blank sample contained all reaction components except NADPH. Inducible nitric oxide synthase activity was calculated from the difference between samples and blank reading.
Chromatographic conditions and analysis of L-citrulline
Chromatographic separation was performed using an Agilent 1100 series high-performance liquid chromatography (HPLC) system. The accompanying Agilent LC Chemstation software program was used for instrument control, data acquisition, and processing. An analytical NUCLEODUR RP- column (100-5 C18 ec, 150×4.6 mm Machery-Nagel, Germany) gave the best results. HPLC analysis was performed by isocratic elution with a flow rate of 1 ml/min. The mobile phase was 10 mM KH2PO4 buffer solution containing acetonitrile-methanol-tetrahydrofuran (76:11.5:11.5:1 all in v/v). The temperature was maintained at 20°C and the excitation and emission wavelengths in the fluorescence detector were set to 338 and 455 nm, respectively. For determination of L-citrulline, first 100 μl of the incubated pancreatic islets extract were derivatized with o-phtaldialdehyde (OPA). Both the extracts and L-citrulline (Sigma) standards were separately injected (injection volume: 100 µl) into the C18-reverse phase column immediately or after 2, 10 and 15 min of derivatization in room temperature or 37°C. L-citrulline quantification was carried out using comparison of retention times of the pancreatic islets extracts with standards of citrulline and also spiking the known concentration of standards to the sample. Each experiment was repeated at least three times and was run in triplicate. Calibration curves were constructed by plotting the measured peak in the range of 10 to 500 pmol of L-citrulline standards. Linearity of the standard curves was assessed by the coefficient of determination (R2), equally 0.995 in this analysis. Accuracy was determined using high purity standard and precision was expressed as the relative standard deviation which was calculated in percentage for each of the replicate concentrations (n = 3). 2-mercaptoethanol (2-ME), OPA and the other minerals were >95% purity from Merck Chemicals (Darmstadt, Germany). All the solvents (HPLC gradient grade) were purchased from Samchun Chemical (Pyeongtaek, Korea). Ultrapure water (from a Direct Q UV-3 Millipore system) was used in all experiments.
Statistics
All analysis were validated by D’Agostino and Pearson (omnibus K2 test performed with Prism version 5) normality test. Statistical study between groups of data was assessed by using unpaired Student’s t-test or, where applicable, one way analysis of variance (ANOVA) followed by Bonferroni post- hoc tests using Graph Pad Prism software (Version 5). Results were expressed as means ± SEM of triplicate experiments. A value of P< 0.05 was considered statistically significant.
Results
Variation in plasma insulin, glucose and NOx level with aging
As shown in Table 1, fasting plasma glucose and NOx levels of aged rats was significantly increased compared to adult rats (P<0.01), but aging had no significant effect on plasma insulin level.
Table 1.
Plasma glucose, insulin and NOx levels in different experimental groups
| Groups | ||
|---|---|---|
| Variable | Adult | Old |
| Fasting plasma glucose (±SEM) (mg/dl) | 97.90 (5.498) | 117.7 (4.116)* |
| Basal plasma insulin (±SEM) (pmol/l) | 147.5 (18.16) | 142.9 (16.61) |
| Plasma NOx (±SEM) (μmol/l) | 17.74 (1.664) | 26.25 (2.166) * |
Statistical comparison between groups was made using an unpaired t test, values are mean ± SEM, n= 6 in adult (4 month old) and old (12 month old). *P<0.05, statistically significant differences between groups.
Pancreatic islets insulin secretion in adult and aged rat
Figure 1 shows that the basal glucose concentration (5.6 mM) has no significant influence on insulin secretion in each condition (adults and aged) of experimental groups. Stimulation of pancreatic islets with 8.3 mM glucose increased insulin secretion in both experimental groups. Furthermore, L-NAME a NOS inhibitor improved insulin secretion, but this improvement was not statistically significant. The results also showed that insulin secretion, especially in supra physiological high concentration of glucose (16.7mM/l) did not increase in senescent rats, in which insulin release was significantly lower compared to adult rats (Figure 2, P<0.05). In contrast, using NOS inhibitor-L-NAME (5 mM for 60 min) concomitantly with glucose (16.7 mM/l) in incubation medium of pancreatic islets, markedly increased islet insulin secretion (Figure 2, P<0.05). This enhancement was considerably prominent in aged rats compared to adults rats; 42.39% ± 4.73% versus 15.27% ± 5.41% in adult rats (P=003).
Figure 1.
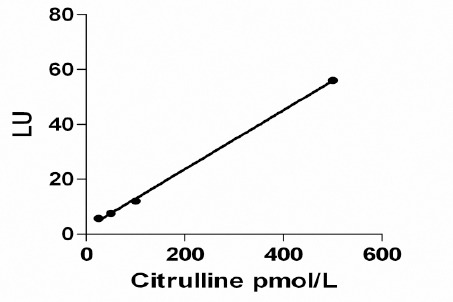
Calibration curve of the OPA derivative of L-citrulline LU: Arbitrary luminescence unit
Figure 2.
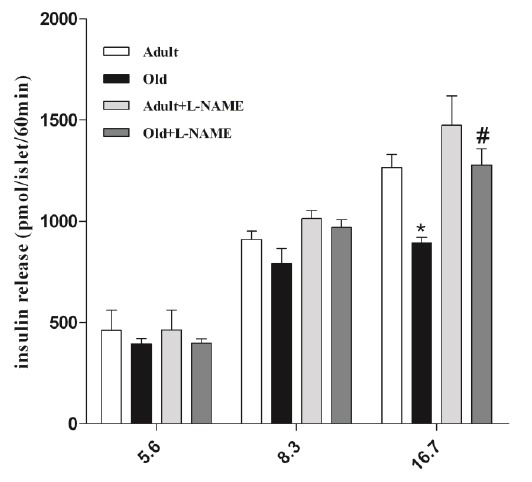
Glucose stimulated insulin secretion on isolated islets of langerhans. Values are mean ± SEM for 8 cups (2 cups each containing 5 islets for each condition from each animal; 4 animals in each group); * P < 0.05 statistically significant differences between adult (4- month- old) and old (12- month- old). #P<0.05, statistically significant differences between inhibitor and glucose 16.7 mM/l
Inducible NOS activity assay
The iNOS activity in intact pancreatic islets of aged rats compared to adult rats was significantly increased (1.082 ± 0.084 and 6.277 ± 0.475 pmol/min per mg protein in adult and aging rats respectively, P<0.001, Figure 3). The specific activity of the enzyme in pancreatic islets of adult rats was too low, which in one of the samples it was not detectable with current assay.
Figure 3.
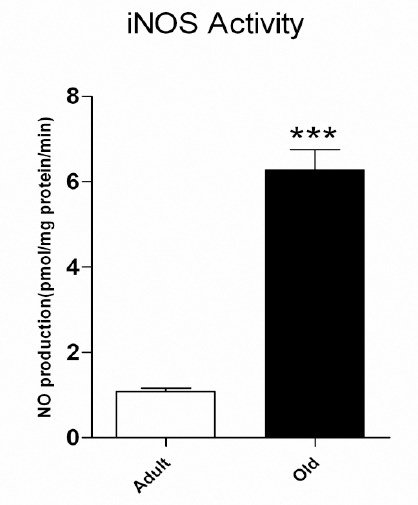
Inducible nitric oxide synthase activity in pancreatic islets of langerhans. Statistical comparison between groups was made using an unpaired student t-test. ***P<0.001, statistically significant differences between groups. n=3 in adult (4- month- old) and n= 4 in old (12- month- old) groups
Optimization of L-citrulline detection by HPLC
Time (immediately or after 2, 5, 10 and 15 min) and temperature (room temperature and 37°C) of incubation had no effect on the fluorescence intensity. Samples were incubated in room temperature for two min for unification. Retention time of citrulline was 8.5 to 8.9 min and samples were run 15 min. Column was cleaned and regenerated by sequentially washing with water and methanol 50% (15 min with each). This procedure had the best results on column cleaning and citrulline detection compared to other solvent such as acetonitrile. Detection limit based on the least detectable limit was 5 pmol. Validation of the chromatographic method was determined by linearity in specified range. Concentrations of 25, 50, 100, 500 pmols of L-citrulline were used to establish a calibration curve (Figure 1). Slop of the linear equation (r2) was 0.995. Precision is the measure of the degree of repeatability of the assay which is performed by using enter and intra assays coefficient of variation (CV %). In our study, intra and inter assay of CV for L-citrulline assay were 2.976% (0.654) and 7.645% (1.325), respectively.
Discussion
In accordance to the documented studies on human (23) and animals (11), hyperglycemia increases with aging. Variation in NOX level in relation with aging has shown controversial results on previous studies. Some of the studies are in favors of the results obtained in this study (24, 25), and some other studies shows different contrast results (26). Controversy in the results might dependent on the kind of studies and or change in other parameters associated with aging such as body weight and fat. In general, plasma insulin level is related to insulin secretion and clearance. Change in plasma insulin level in this study (Table 1) associated with decreased insulin secretion may be followed by decreased insulin clearance as have previously been reported (27, 28). The study also showed decrease in insulin secretion, especially in high concentration of glucose (16.7 mM/l), which was significantly lower in aged rats (Figure 2, P<0.05). The previous studies also confirm the current results (27-30). Pancreatic islet size and beta cell number increase with age (29), which compensate islet insulin secretion in basal state. Despite these structural changes in the endocrine pancreas, islet insulin capacity, insulin content and insulin mRNA levels decrease in aging animals (27, 29). With regard to above explanation, in high concentration of glucose, the islets are not able to secrete adequate insulin. It seems that the reduced insulin secretion of pancreatic islets in this condition is inevitable and NO may be involved in this process. Use of NOS inhibitor increased insulin secretion in both groups, especially in aged rats. According to NO as a negative modulator of islet insulin release (20), iNOS activity is induced and increased in high glucose concentration ≥ 10 mM/l (31). It is suggested that NO production from iNOS is prominent in aged rats, and it is responsible for decrease in insulin secretion, which is compensated by NOS inhibitor L-NAME. In respect to iNOS activity, the previous studies had investigated an increase in iNOS activity and the gene/protein expression on aorta and heart of aged 14- month- old mice, respectively (32, 33). The obtained result supports the oxidative and inflammatory processes associated with aging. Inducible NOS activity is involved in the antiproliferative /apoptotic effect of proinflamatory cytokines that cause islet destruction and inhibit insulin secretion from pancreatic β-cells (6, 34). Based on the oxidation-inflammation theory of aging (35), these conditions are associated with an impairment in the physiological functions, especially in homeostatic systems including the immune system. Therefore, rise in iNOS activity which results in NO production is associated with decreased islet insulin secretion, which is implicated in impaired glucose metabolisms. This presumably increases the prevalence of type II non-insulin dependent diabetes during aging.
In this study for the first time we have established a modification in the procedure of the NOS activity assay in rat islets. In our laboratory, we have employed the L-citrulline assay for measuring NO synthase activity in rat islets with minimal sample manipulation; without stop solution and using a Cation exchange column. Carlberg (18) has also performed NOS activity by L-citrulline measurement. Although in his procedure also L-arginine was used as the substrate, but the enzymatic reaction was stopped by the addition of acidic solution and finally aliquots of incubation medium were passed through the additional steps using an Amprep CBS Cation-exchange column- and then processed samples were used for HPLC analysis. Finally L-Citrullin elated from the Amprep column was determined by HPLC analysis (18). There are various methods for the measurement of nitric oxide. These include both indirect and direct measurement of NO. The direct measurement of NO is complex and difficult because NO is unstable and highly reactive molecule (the half-life of a few tenths of a sec). Nonetheless, several indirect methods have been used to measure NO metabolites including: NOX measurement by Griess reagent, which can be measured by spectrophoto-metric procedure (21), and chemiluminescence procedure in which NO is assayed by measurement of the difference in the absorption spectrum for oxyhemoglobin (36) (NO oxidize oxyhemoglobin to methemoglobin). But these bioassay approaches cannot measure NOS activity and are potentially nonspecific. In addition, NOS activity can be determined with great reliability, sensitivity, and specificity by conversion of L-arginine to L-citrulline. L-citrulline is a stable by-product and is equimolar of NO production from L-arginine. Due to its stability, the measurement of L-citrulline production provides a much more accurate assessment of NOS enzymatic activity. Previous studies have shown that the iNOS activity measurement is more sensitive and superior to protein measurement using western blotting or ELISA methods (31, 37). For HPLC analysis, incubation with OPA is performed in room temperature (temperature of incubation had no effect on the fluorescence intensity). As mentioned previously, OPA required a few min of reaction time (38). Column conditioning before analysis is a critical step to obtain good recovery and better separation (39) of the L--Citrulline. In this study, the column was initially conditioned by methanol solution (50%) for 50 min at a flow rate of 0.5 ml/min followed by 60 min the mobile phase. In chromatography, column washing is a critical and important step to improve reproducibility and precision. Thus, in order to prevent contamination of the column and possibly memory effect, after each sample run (15 min), the columns were washed sequentially with water/methanol 50% (15 min with each). Wu and Meininger (38) used column washing with methanol solution for satisfactory separation of L-arginine and L-citrulline in plasma and biological fluids. Also Schwarz et al (40) for analysis of plasma amino acids by HPLC with fluorescence detection used a mixture of 90% isopropanol and 10% methanol in order to maintain column integrity.
Conclusion
Our results showed that iNOS activation and increase in NO production might be responsible for decreased in insulin secretion in aging. The study emphasizes the link between reduced capacity of carbohydrate metabolism and increase in NO signaling pathway in senescence. Further studies in this field are required to uncover mechanistic reaction pathways. Inducible nitric oxide synthase activity assay using l-citrulline measurement by HPLC method was achieved by minimum sample handling, short time of assay, and maximum precision and accuracy.
Acknowledgment
The results described in this paper were part of student thesis, funded by the Neurosciences Research Center, Baqiyatallah (a.s.) University of Medical Sciences. The author wishes to thank Mrs. Neda Sheijooni Fumani, Lab specialist of INIOAS and Asieh Naderi, PhD student in Neuroscience Research Center of Shahid Beheshti University of Medical Sciences for her kind assistance in HPLC analysis. None of the authors declared a conflict of interest.
References
- 1.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lajoix AD, Reggio H, Chardes T, Peraldi-Roux S, Tribillac F, Roye M, et al. A neuronal isoform of nitric oxide synthase expressed in pancreatic beta-cells controls insulin secretion. Diabetes. 2001;50:1311–1323. doi: 10.2337/diabetes.50.6.1311. [DOI] [PubMed] [Google Scholar]
- 4.Soskic SS, Dobutovic BD, Sudar EM, Obradovic MM, Nikolic DM, Djordjevic JD, et al. Regulation of inducible nitric oxide synthase (inos) and its potential role in insulin resistance, diabetes and heart failure. Open Cardiovasc Med J. 2011;5:153–163. doi: 10.2174/1874192401105010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedoya FJ, Salguero-Aranda C, Cahuana GM, Tapia-Limonchi R, Soria B, Tejedo JR. Regulation of pancreatic beta-cell survival by nitric oxide: clinical relevance. Islets. 2012;4:108–118. doi: 10.4161/isl.19822. [DOI] [PubMed] [Google Scholar]
- 6.Quintana-Lopez L, Blandino-Rosano M, Perez-Arana G, Cebada-Aleu A, Lechuga-Sancho A, Aguilar-Diosdado M, et al. Nitric oxide is a mediator of antiproliferative effects induced by proinflammatory cytokines on pancreatic beta cells. Mediators Inflamm 2013. 2013 doi: 10.1155/2013/905175. 905175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou JL, Fang HS, Peng H, Hu QJ, Liu SQ, Ming JH, et al. PKCa agonists enhance the protective effect of hyaluronic acid on nitric oxide-induced apoptosis of articular chondrocytes in vitro. Iran J Basic Med Sci. 2013;16:1276–1281. [PMC free article] [PubMed] [Google Scholar]
- 8.Ghasemi A, Zahediasl S. Is nitric oxide a hormone? Iran Biomed J. 2011;15:59–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Kazeem A, Olubayo A, Ganiyu A. Plasma nitric oxide and acute phase proteins after moderate and prolonged xercises. Iran J Basic Med Sci. 2012;15:602–607. [PMC free article] [PubMed] [Google Scholar]
- 10.Muller DC, Elahi D, Tobin JD, Andres R. Insulin response during the oral glucose tolerance test: the role of age, sex, body fat and the pattern of fat distribution. Aging (Milano) 1996;8:13–21. doi: 10.1007/BF03340110. [DOI] [PubMed] [Google Scholar]
- 11.Wu D, Yue F, Zou C, Chan P, Alex Zhang Y. Analysis of glucose metabolism in cynomolgus monkeys during aging. Biogerontology. 2012;13:147–155. doi: 10.1007/s10522-011-9364-1. [DOI] [PubMed] [Google Scholar]
- 12.Salehi A, Meidute Abaraviciene S, Jimenez-Feltstrom J, Ostenson CG, Efendic S, Lundquist I. Excessive islet NO generation in type 2 diabetic GK rats coincides with abnormal hormone secretion and is counteracted by GLP-1. PLoS One. 2008;3:e2165. doi: 10.1371/journal.pone.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCann SM, Mastronardi C, de Laurentiis A, Rettori V. The nitric oxide theory of aging revisited. Ann N Y Acad Sci. 2005;1057:64–84. doi: 10.1196/annals.1356.064. [DOI] [PubMed] [Google Scholar]
- 14.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]
- 15.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 16.Shafiee-Nick R, Ghorbani A, Vafaee Bagheri F, Rakhshandeh H. Chronic administration of a combination of six herbs inhibits the progression of hyperglycemia and decreases serum lipids and aspartate amino transferase activity in diabetic rats. Adv Pharmacol Sci 2012. 2012 doi: 10.1155/2012/789796. 789796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindlycke M, Jansson L. Glucose tolerance and pancreatic islet blood flow in rats after intraperitoneal administration of different anesthetic drugs. Ups J Med Sci. 1992;97:27–35. doi: 10.3109/03009739209179279. [DOI] [PubMed] [Google Scholar]
- 18.Carlberg M. Assay of neuronal nitric oxide synthase by HPLC determination of citrulline. J Neurosci Methods. 1994;52:165–167. doi: 10.1016/0165-0270(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 19.Zardooz H, Zahedi Asl S, Gharib Naseri MK, Hedayati M. Effect of chronic restraint stress on carbohydrate metabolism in rat. Physiol Behav. 2006;89:373–378. doi: 10.1016/j.physbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Akesson B, Henningsson R, Salehi A, Lundquist I. Islet constitutive nitric oxide synthase and glucose regulation of insulin release in mice. J Endocrinol. 1999;163:39–48. doi: 10.1677/joe.0.1630039. [DOI] [PubMed] [Google Scholar]
- 21.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 24.Alusik S, Jedlickova V, Paluch Z, Zecova S. Plasma levels of nitrite/nitrate and inflammation markers in elderly individuals. Bratisl Lek Listy. 2008;109:289–292. [PubMed] [Google Scholar]
- 25.Ghasemi A, Zahedi Asl S, Mehrabi Y, Saadat N, Azizi F. Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sci. 2008;83:326–331. doi: 10.1016/j.lfs.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:895–902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 27.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 28.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. J Clin Endocrinol Metab. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- 29.Perfetti R, Rafizadeh CM, Liotta AS, Egan JM. Age-dependent reduction in insulin secretion and insulin mRNA in isolated islets from rats. Am J Physiol. 1995;269:E983–980. doi: 10.1152/ajpendo.1995.269.6.E983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reaven E, Wright D, Mondon CE, Solomon R, Ho H, Reaven GM. Effect of age and diet on insulin secretion and insulin action in the rat. Diabetes. 1983;32:175–180. doi: 10.2337/diab.32.2.175. [DOI] [PubMed] [Google Scholar]
- 31.Henningsson R, Salehi A, Lundquist I. Role of nitric oxide synthase isoforms in glucose-stimulated insulin release. Am J Physiol Cell Physiol. 2002;283:C296–304. doi: 10.1152/ajpcell.00537.2001. [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Larson DF, Watson RR. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004;75:655–667. doi: 10.1016/j.lfs.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 33.Chou TC, Yen MH, Li CY, Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- 34.Broniowska KA, Oleson BJ, Corbett JA. beta-cell responses to nitric oxide. Vitam Horm. 2014;95:299–322. doi: 10.1016/B978-0-12-800174-5.00012-0. [DOI] [PubMed] [Google Scholar]
- 35.Alonso-Fernandez P, De la Fuente M. Role of the immune system in aging and longevity. Curr Aging Sci. 2011;4:78–100. doi: 10.2174/1874609811104020078. [DOI] [PubMed] [Google Scholar]
- 36.Salter M, Knowles RG. Assay of NOS activity by the measurement of conversion of oxyhemoglobin to methemoglobin by NO. Methods Mol Biol. 1998;100:61–65. doi: 10.1385/1-59259-749-1:61. [DOI] [PubMed] [Google Scholar]
- 37.Ekelund M, Qader SS, Jimenez-Feltstrom J, Salehi A. Selective induction of inducible nitric oxide synthase in pancreatic islet of rat after an intravenous glucose or intralipid challenge. Nutrition. 2006;22:652–660. doi: 10.1016/j.nut.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008;440:177–189. doi: 10.1016/S0076-6879(07)00810-5. [DOI] [PubMed] [Google Scholar]
- 39.Mohammadpour AH, Ramezani M, Tavakoli Anaraki N, Malaekeh-Nikouei B, Amel Farzad S, Hosseinzadeh H. Development and Validation of HPLC Method for Determination of Crocetin, a constituent of Saffron, in Human Serum Samples. Iran J Basic Med Sci. 2013;16:47–55. [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz EL, Roberts WL, Pasquali M. Analysis of plasma amino acids by HPLC with photodiode array and fluorescence detection. Clin Chim Acta. 2005;354:83–90. doi: 10.1016/j.cccn.2004.11.016. [DOI] [PubMed] [Google Scholar]


