Abstract
Objective(s):
Estrogen (E2) has neuroprotective effects on blood-brain-barrier (BBB) after traumatic brain injury (TBI). In order to investigate the roles of estrogen receptors (ERs) in these effects, ER-α antagonist (MPP) and, ER-β antagonist (PHTPP), or non-selective estrogen receptors antagonist (ICI 182780) were administered.
Materials and Methods:
Ovariectomized rats were divided into 10 groups, as follows: Sham, TBI, E2, oil, MPP+E2, PHTPP+E2, MPP+PHTPP+E2, ICI+E2, MPP, and DMSO. E2 (33.3 µg/Kg) or oil were administered 30 min after TBI. 1 dose (150 µg/Kg) of each of MPP, PHTPP, and (4 mg/kg) ICI182780 was injected two times, 24 hr apart, before TBI and estrogen treatment. BBB disruption (Evans blue content) and brain edema (brain water content) evaluated 5 hr and 24 hr after the TBI were evaluated, respectively.
Results:
The results showed that E2 reduced brain edema after TBI compared to vehicle (P<0.01). The brain edema in the MPP+E2 and PHTPP+E2 groups decreased compared to the vehicle (P<0.001). There was no significant difference in MPP+PHTPP+E2 and ICI+E2 compared to TBI. This parameter in MPP was similar to vehicle. Evans blue content in E2 group was lower than vehicle (P<0.05). The inhibitory effect of E2 on Evans blue was not reduced by MPP+E2 and PHTPP+E2 groups, but decreased by treatment with MPP+PHTPP or ICI. MPP had no effect on Evans blue content.
Conclusion:
A combined administration of MPP and PHTPP or ICI inhibited the E2-induced decrease in brain edema and BBB disruption; this may suggest that these effects were mediated via both receptors.
Keywords: Blood-brain-barrier, Brain edema, ERα antagonist, ERβ antagonist, ICI182780, Traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a complex neurodegenerative disease, involving many cellular and molecular pathways, including inflammation (1). Paradoxically, the inflammatory response can lead to both aggravation and amelioration of the resulting tissue damage, depending on the tissue involved and the type of injury evoked (2). After traumatic brain injury (TBI), disruption of blood-brain-barrier (BBB) will led to the release of neutrophils, lymphocytes and monocytes from blood to central nervous system and cause inflammatory responses (3).
Estrogen (E2) is a cholesterol-derived hormone that is often known as a reproduction regulator and can play a protective role in different kinds of brain injuries; it has been revealed that the treatment by estradiol following ischemia, will decrease cortical injuries (4). E2 regulates the selective permeability (5) of the BBB in several models of injury, including systemic inflammation, neurotrauma, and ischemia (6). Previous study in our laboratory showed that the administration of estrogen and progesterone, alone and in combination after TBI decreases brain edema, blood-brain-barrier permeability, and intracranial pressure (7).
Many of E2 functions are carried via the classic receptors, estrogen receptor alpha and beta. Estrogen receptors (ERs) are involved in signaling pathways of classical and non-classical pathways (4). Both receptors express throughout the CNS, including microglia, astrocytes, and neurons, and also in many immune system cells such as macrophages/monocytes, T and B cells and dendrites are expressed (8). It has been reported that ERα expression in ischemic brain after stroke greatly increases (9). Beta receptor agonist in animal models of arthritis and inflammatory bowel disease, has anti-inflammatory effects and suppress gene expression of pro-inflammation. Both receptors ERα and ERβ are involved in cell proliferation induced by estradiol after ischemia (10). After brain ischemia, the expression of ERα in injured cortex increases rapidly (11). Which of these two receptors alpha and beta-estradiol-mediated control inflammation, depends on the inflamed tissue (12).
In agreement with previous studies, it was shown that estrogen reduces brain edema and BBB permeability after trauma (7, 13), although the roles of estrogen receptors ERα and ERβ in the neuroprotective actions of estrogen on TBI remain poorly understood. The goal of the present study was to use these ER-selective and non-selective antagonists (MPP, PHTPP and ICI 182780) to evaluate the relative necessity of ERα- and ERβ-activation in mediating the neuroprotective effect of estrogen following diffuse TBI in OVX rats.
Materials and Methods
Animals
This study was conducted in accordance with the guidelines for the animal experimental protocols of Kerman University of Medical Sciences. The protocol was approved by the ethics committee (no. KNRC/90–40) of this University, in accordance with the internationally accepted principles for laboratory animal use and care, as found in the European Community guidelines (EEC Directive of 1986; 86/609/EEC) or US guidelines (NIH publication #85–23, revised in 1985). Adult female Albino N Mary rats (weighing 200–250 g) were housed in an air-conditioned room at 22–25°C, with a 12 hr light: 12 hr dark cycle and free access to food and water.
Ovariectomy surgury
In the first step, animals were anesthetized by injection of 60 mg/kg thiopental (IP), sub abdominal area was shaved and an incision of 1 cm was made. Afterwards, skin, fascia, and abdominal muscles were opened. Both fallopian tubes and vascular base of the ovaries were legated in the proximal parts and then cut from the distal parts. Abdominal cavity was irrigated by 2 ml of normal saline. The incision was closed with a 2–0 silk surgical suture. To avoid interference due to the estrus cycle, all experimental animals were ovariectomized (OVX) 2 weeks before the experiments (14).
Experimental protocols
Before induction of TBI, ovariectomized rats were randomly divided into 10 groups, as follows: sham (animals that underwent ovariectomy 2 weeks before the start of the experiment and underwent false brain trauma under anesthesia but did not receive hormones or vehicle), TBI (brain injury was induced 2 weeks after ovariectomy), E2, MPP+E2, PHTPP+E2, MPP+PHTPP+E2, MPP, ICI+E2, oil (sesame oil, which was used as estrogen solvent) and DMSO (dimethyl sulfoxide, which was used as antagonists solvent). (n=6 in each group). E2(33.3 µg/Kg) or oil were administered (IP) 30 min after TBI (15, 16).
MPP(1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole hydrochloride), ERα antagonist, and PHTPP(4-[2-Phenyl-5, 7-bis (trifluoromethyl) pyrazolo [1,5-a] pyrimidine-3- yl] phenol), ERβ antagonist, or DMSO were used as follows:1 dose (150 µg/Kg) of each of MPP and, PHTPP was injected two times, 24 hr apart, before TBI and oestrogen treatment (17). Then treated with E2 (33.3 µg/Kg) 30 min after TBI (13). One group received only an injection of MPP(150 µg/Kg) 30 min after TBI (17). 4 mg/kg ICI182780 was injected similar to the other two antagonists (18).
Induction of diffuse traumatic brain injury (TBI)
All animals were intubated before TBI. The TBI was induced by the Marmarou method (16), using a TBI induction device made by the Department of Physiology, Kerman University of Medical Sciences. The protocol was as follows: a weight of 300 g was dropped from a 2 m height onto the head of the anesthetized rat while a metal disc (stainless steel, 10 mm in diameter, 3 mm thick) was attached to the animal’s skull. After induction of the trauma, the rats were immediately connected to a respiratory pump untill spontaneous breathing had been restored.
Determination of brain edema
The brain edema of each animal was assessed by measuring brain water content 24 hr after TBI. Anesthetized animals were sacrificed by cervical dislocation; the brain was removed, and brain samples were placed in pre-weighed glass vials and weighed (wet weight). The lids were removed and the vials placed in an incubator (Memmert, Germany) at 60°C for 72 hr, and then reweighed (dry weight). The percentage of water in each sample was then calculated using a formula published previously (16, 19): (100 * [(wet weight–dry weight)/wet weight]).
Determination of blood–brain barrier (BBB) disruption
The degree of BBB disruption was assessed by measuring Evans blue dye leakage (16). Briefly, Evans blue dye was dissolved in 0.01 mol/l PBS at a concentration of 2%, then the dye (2 ml/kg IV) was injected into the tail vein 4 hr after TBI, as a BBB permeability tracer. The rats were then deeply anesthetized and transcardiac perfused with 200 ml heparinized saline through the left ventricle to remove the intravascular dye. The brains were removed, then dissected, weighed, and stored at –80°C for quantitative measurement. Brain samples were homogenized in 1 ml of 0.1 mol/l PBS, and 0.7 ml of 100% (w/v) trichloroacetic acid was added, and centrifuged. After centrifugation for 30 min at 1000 g, the absorbance of Evans blue in supernatant was measured at 610 nm using a spectrophotometer (UV/VIS, Spectrometer, UK). The amount of extravasated Evans blue dye was quantified as µg/g brain tissue.
Statistical analysis
Quantitative data were expressed as mean ± SEM. The data were analyzed by parametric analysis of variance (ANOVA) or independent t test. Fisher’s LSD was employed for the post-hoc analysis. The criterion for statistical significance was sign at P < 0.05.
Results
Brain edema
Changes in the brain water content of the ovariectomized rats are shown in Figure 1. Figure 1a shows that the brain water content in the TBI (78.6±0.33%) group was significantly higher than in sham (75.65±0.001%, P<0.001) group and brain water content in the group treated with E2 (76.76±0.17%) was significantly lower than those in the oil (78.32±0.15%) and DMSO (78.4±0.22%) groups, (P<0.01). Figure 1b shows the effects of administration of estrogen receptors antagonists. In the presence of MPP and PHTPP, brain water content decreased and was 74.86±0.54% in MPP+E2 group and 75.41±0.88% in PHTPP+E2 group. There was no significant difference in MPP+PHTPP+E2 and ICI+E2 compared to TBI. Figure 1c shows that the brain water content in the group treated with MPP (78.06±0.32%) has no significant difference with the water content in DMSO group.
Figure 1.
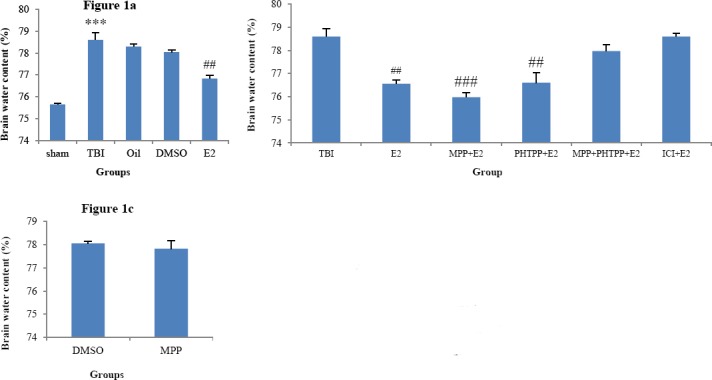
(a) Comparison of brain water content (%) after traumatic brain injury in ovariectomized (OVX) rats (n =6 in each group). The data are represented as mean±SEM. ***: P< 0.001, TBI vs. sham. ##: P<0.01, E2 vs. TBI and PHTPP+E2vs TBI., Oil and DMSO groups. (b) Comparison of brain water content in OVX rats treated with ERs antagonist. ###: P<0.001 MPP+E2. There were no significant differences in MPP+E2 and PHTPP+E2 compared to E2 (c) Brain water content (%) after traumatic brain injury in OVX rats treated with MPP and DMSO. MPP (ER-α antagonist), PHTPP(ER-β antagonist), ICI182780 (non selective estrogen receptors antagonist), DMSO (dimethyl sulfoxide, antagonists solvent), oil (sesame oil, estrogen solvent)
Extravasation of Evans blue content
The effects of estrogen and ERs antagonists on BBB disruption after TBI are shown in Figure 2. Figure 2a shows that the Evans blue content in the TBI (37.18±0.4 μg/g tissue) group was significantly higher than in the sham (33.25±0.05 μg/g tissue, P<0.05) group and the Evans blue content in E2 (33.5±0.65 μg/g tissue) was lower than oil (37.67±0.44 μg/g tissue), TBI, and DMSO (37.34±0.22 μg/g tissue) groups. P<0.05. Figure 2b shows the effect of administration of estrogen receptors antagonist. In the presence of MPP and PHTPP, Evans blue content decreased and was 30.78±0.31 μg/g tissue in MPP+E2and 31.4±0.38 μg/g tissue in PHTPP+E2 groups (P<0.001). There were no significant differences in MPP+PHTPP+E2 & ICI+E2 compared to TBI. Figure 2c shows that the Evans blue content in the groups treated with MPP (36.46±0.68) has no significant difference with the Evans blue in the E2 group.
Figure 2.
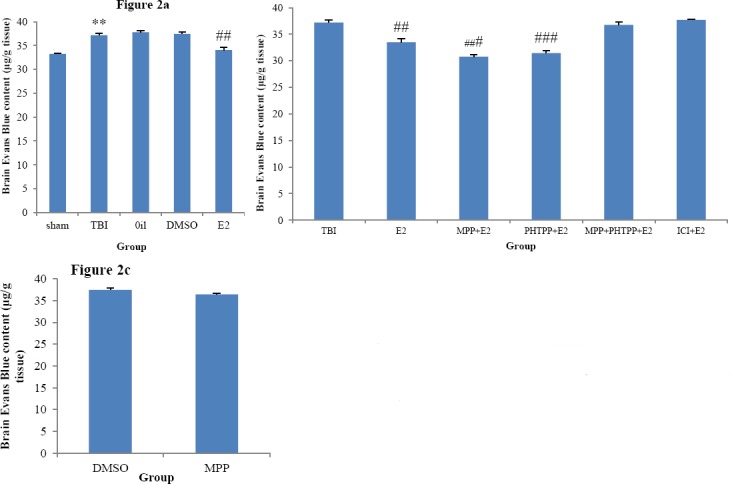
(a) Comparison of Evans blue content (μg/g tissue) after traumatic brain injury in ovariectomized (OVX) rats (n =6 in each group). The data are represented as mean±SEM. **: P< 0.01, TBI vs. sham. ##: P< 0.01, E2 vs. TBI, oil and DMSO groups. (b) Comparison of brain water content in OVX rats treated with ERs antagonist. ###: P< 0.001 MPP+E2 and PHTPP+E2 vs TBI. There were no significant differences in MPP+E2 and PHTPP+E2 compared to E2 (c) Evans blue content (μg/g tissue) after traumatic brain injury in OVX rats treated with MPP and DMSO. MPP(ER-α antagonist), PHTPP(ER-β antagonist), ICI182780 (non selective estrogen receptors antagonist). DMSO (dimethyl sulfoxide, antagonists solvent), oil (sesame oil, estrogen solvent)
Discussion
Previous studies have reported, the neuroprotective effects of estrogen on brain edema and blood-brain barrier permeability (7, 13), In the present study, the roles of alpha and beta estrogen receptors in the development of these effects were investigated. The main findings of this research are as follows: 1-estrogen exerts its anti-edema effects through both receptors, so that any differences between the results of combined use of selective antagonists and a non-selective antagonist do not exist. 2 -BBB protection after TBI by estrogen is mediated by both receptors, which is similar to the results of the anti-edema effect of estrogen. 3- The estrogen receptor alpha antagonist (MPP) has no agonistic effect on its receptor.
TBI caused an increase in brain water content and BBB permeability, these increases were inhibited by estrogen compared to vehicle (oil). There have been a number of reports supporting a neuroprotective role for estrogen after various insults to the CNS. Ovariectomy increases ischemic brain damage in females, and estradiol exerts neuroprotective and anti-inflammatory action against the ischemic brain when administered immediately upon ovariectomy (20). E2 regulates the selective permeability of the BBB in several models of injury (5). The BBB permeability decreased in E2-treated mice (4). Recently, our research showed that estrogen loss facilitates, whereas administration of a different dose of estradiol could prevent edema formation (7) and destruction of the BBB after TBI (13). A significant reduction in the brain water content after TBI was induced by estrogen; there is no difference between estrogen and solo administration of antagonist groups. This suggested that neither selective ERα antagonist (MPP) nor ERβ antagonist (PHTPP) can abolish these effects of estrogen.
On the other hand, administration of the two antagonists together (MPP + PHTPP) or the use of non-selective estrogen receptor antagonist (ICI182780) eliminates the inhibitory effect of estrogen on brain edema and BBB permeability, this means that, the effects of estrogen were not inhibited by the administration of antagonists alone, but when these antagonists were administered in combination, they have an inhibitory effect. It is suggested that, probably both receptors are involved in the development of neuroprotective effects of estrogen, We found that more than one receptor is activated by estrogen at the same time, perhaps activation of both receptors at the same time has reciprocal modulatory effects. Whenever the effect of estrogen was observed during receptor inhibition, it was caused by activation of the other receptor at the same time. To the best of our knowledge, the roles of neither ERα antagonist nor ERβ antagonist in TBI have been reported thus far. Because this study is the first to use a selective ERα and ERβ antagonist to investigate the effects on TBI. However, the effects of these receptors or antagonists in other non-TBI studies, and other estrogen-mediated effects have been demonstrated, including: The receptors ERα and ERβ are essential for E2-mediated regulation of BBB permeability, anti-inflammatory properties, and regulation of neutrophil recruitment into the brain (4). Both ER receptors are involved in the control of inflammation by estradiol (21), neuromodulation, and neuroprotection processes after brain injury (22). The affinity of MPP to ERα is only 11% of that of E2 (23). ER antagonists (MPP and ERβ antagonist (R,R)-THC) treatments alone did not significantly change NPY release levels (24). MPP failed to attenuate the anorexigenic effects of estradiol and an ERα agonist in OVX rats (25). MPP did attenuate the estrous-related decrease in food intake in cycling rats (25). MPP shows no activation of either receptor subtype (23). 17ßestradiol protects dopamine neurons from injury, an effect that was blocked by ICI 182,780 (26). ICI 182,780 after ischemia abolished estrogen protection (27). Estrogen neuroprotection was related to microglial activation of estrogen receptors (ERs), this protective effect was overridden by pretreatment with ICI 182,780, the specific ERa, only partially blocked the effects of estrogen (6).
An alternate mechanism which could explain the differential effects of ICI and MPP, is an involvement of non-genomic/non-classical signaling mechanisms activated by anti-estrogen-occupied ER. Indeed, ICI-mediated Erk1/2(extracellular signal-regulated kinases) activation appears to be critical for modulation in immature cerebellar neurons (28). Some studies suggest that MPP acts as an ERα antagonist following in vitro applications, but exerts mixed ERα agonist/antagonist actions following in vivo applications (29). It is possible that the MPP concentration in this experiment was too low to completely compete with E2 for binding to ER α, because it is reported that MPP is needed in high excess to fully antagonize E2 (30). There are several probable mechanisms by which ERα and ERβ attenuate BBB permeability and brain edema after TBI that result from the inflammatory response; up regulation of defense-related genes, IL-6, MMP-9, and some of phagocytic receptors (31), protection of cortical cells against oxidative glutamate toxicity (32), neuroprotection of estrogen against activated microglia (33) and inhibition of the cytoplasmic transport of NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) (34).
There is, however, some controversy. MPP reduced the E2 effect on protein vitellogenin (35). MPP and PTHPP inhibited DNA synthesis, oxygen species (ROS) formation induced by estrogens according to their receptors affinity (36). Experiments with PHTPP, indicated that E2-mediated neuroprotection against NMDA toxicity was mediated via ERβ receptor (37). Many studies have reported a primary role for ERα as a regulator of the anti-inflammatory properties of estradiol, including the anti-inflammatory and neuroprotective activity of estradiol (38), neuroprotection after ischemia and inhibition of microglial activation (39). Studies involving transgenic mice with targeted deletion of either ERα or Erβ indicate that ERα, but not ERβ, is required for the E2-mediated suppression of BBB permeability (8). In addition, a growing number of studies have reported anti-inflammatory roles for ERβ, such as neuroprotection after ischemia and, inhibition of microglia activity (40). In mammals, the actions of MPP vary, depending on the species, physiological conditions, dosing method, and tissue (17, 41-43).
Since currently, there is no evidence that PHTPP, exerts the mixed agonist/antagonist properties that are often seen for MPP (29), in another part of the present study, MPP was used to replace estrogen after TBI, it was observed this compound did not reduce brain edema, and BBB permeability. In other words, MPP has no agonist effect on ERα. This finding is consistent with other studies that have reported that MPP counteracted the effects of PPT(estrogen receptor agonist) but had little effect on its own (44). On the other hand, it has also been reported that estrogen receptor/(ERα), even in absence of estrogen (E2), plays a critical role in lactotroph homeostasis (45). In vivo tests of MPP’s effects on food intake revealed estrogenic activity, suggesting that MPP functions as a SERM(Selective estrogen receptor modulator), rather than as an ERα antagonist (46).
Conclusion
The present study showed that the administration of PPT or PHTPP alone will not lead to reduced brain edema, and prevention of BBB disruption following TBI, but a combined administration of MPP and PHTPP or ICI inhibited the E2-induced decrease in brain edema and BBB disruption, this may suggest that estrogen neuroprotection was mediated via both ERα and ERβ. Both of them can play roles in classic and non-classic pathways. Further study is needed and intracellular mechanism activation in the presence of E2 should be evaluated. These data, makes them exciting therapeutic targets for further study, in addition, perhaps it is essential that non-genomic mechanisms are also investigated.
Acknowledgment
The present study was financially supported by the Neuroscience Research Center and Physiology Research Center, Kerman University of Medical Sciences, Kerman, Iran. The results reported in this paper were part of a student thesis.
References
- 1.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain pathology. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cederberg D, Siesjö P. What has inflammation to do with traumatic brain injury? Childs Nerv Syst. 2010;26:221–226. doi: 10.1007/s00381-009-1029-x. [DOI] [PubMed] [Google Scholar]
- 3.Venero JL, Machado A, Cano J. Importance of aquaporins in the physiopathology of brain edema. Curr Pharm Des. 2004;10:2153–2161. doi: 10.2174/1381612043384150. [DOI] [PubMed] [Google Scholar]
- 4.Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation. novel roles for estrogen receptors α and β. Endocrinology. 2010;151:4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, et al. Estrogen and Bcl-2 gene induction and effect of transgene in experimental stroke. J Neurosci. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, et al. 17β-Estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia–reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Shahrokhi N, Khaksari M, Soltani Z, Mahmoodi M, Nakhaee N. Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can J Physiol Pharmacol. 2010;88:414–421. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- 8.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzucco C, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris H, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 13.Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can J Physiol Pharmacol. 2010;89:31–40. doi: 10.1139/y10-103. [DOI] [PubMed] [Google Scholar]
- 14.Keshavarzi Z, Khaksari Hadad M, Zahedi MJ, Bahrami A. The effects of female sex steroids on gastric secretory responses of rat following traumatic brain injury. Iran J Basic Med Scie. 2011;14:231–239. [Google Scholar]
- 15.Khaksari M, Mahmmodi R, Shahrokhi N, Shabani M, Joukar S, Aqapour M. The effects of shilajit on brain edema, intracranial pressure and neurologic outcomes following the traumatic brain injury in rat. Iran J Basic Med Sci. 2013;16:858–864. [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Santollo J, et al. Activation of ERα is necessary for estradiol's anorexigenic effect in female rats. Hormones and behavior. 2010;58:872–877. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis AM, Ellersieck MR, Grimm KM, Rosenfeld CS. The effects of the selective estrogen receptor modulators, methyl-piperidino-pyrazole (MPP), and raloxifene in normal and cancerous endometrial cell lines and in the murine uterus. Mol Reprod Dev. 2006;73:1034–1044. doi: 10.1002/mrd.20520. [DOI] [PubMed] [Google Scholar]
- 19.Cotroneo MS, Fritz WA, Lamartiniere CA. Dynamic profiling of estrogen receptor and epidermal growth factor signaling in the uteri of genistein-and estrogen-treated rats. Food Chem Toxicol. 2005;43:637–645. doi: 10.1016/j.fct.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training. Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, et al. Increased estrogen receptor βexpression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- 22.Evans MJ, Harris HA, Miller CP, Karathanasis SK, Adelman SJ. Estrogen receptors α and β have similar activities in multiple endothelial cell pathways. Endocrinology. 2002;143:3785–3795. doi: 10.1210/en.2002-220356. [DOI] [PubMed] [Google Scholar]
- 23.Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor α. Endocrinology. 2002;143:941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 25.Dhillon S, Belsham D. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-αin clonal, immortalized hypothalamic neurons. Int J Obes (Lond) 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- 26.Santollo J, Eckel LA. Effect of a putative ERα antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav. 2009;97:193–198. doi: 10.1016/j.physbeh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bains M, Cousins JC, Roberts JL. Neuroprotection by estrogen against MPP-induced dopamine neuron death is mediated by ERα in primary cultures of mouse mesencephalon. Exp Neurol. 2007;204:767–776. doi: 10.1016/j.expneurol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor α and β to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- 29.Wong JK, Le HH, Zsarnovszky A, Belcher SM. Estrogens and ICI182, 780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J Neurosci. 2003;23:4984–4995. doi: 10.1523/JNEUROSCI.23-12-04984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERα is necessary for estradiol's anorexigenic effect in female rats. Horm Behav. 2010;58:872–877. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 32.Sárvári M, Hrabovszky E, Kalló I, Solymosi N, Tóth K, Likó I, et al. Estrogens regulate neuroinflammatory genes via estrogen receptors a and b in the frontal cortex of middle-aged female rats. J Neuroinflammation. 2011;8:82–89. doi: 10.1186/1742-2094-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- 34.Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor β. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 35.Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis L, Katsu Y, Iguchi T, Lerner DT, Hirano T, Grau EG. Transcriptional activity and biological effects of mammalian estrogen receptor ligands on three hepatic estrogen receptors in Mozambique tilapia. J Steroid Biochem Mol Biol. 2010;122:272–278. doi: 10.1016/j.jsbmb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Somjen D, Katzburg S, Sharon O, Grafi-Cohen M, Knoll E, Stern N. The effects of estrogen receptors α-and β-specific agonists and antagonists on cell proliferation and energy metabolism in human bone cell line. J Cell Biochem. 2011;112:625–632. doi: 10.1002/jcb.22959. [DOI] [PubMed] [Google Scholar]
- 38.Aguirre C, Jayaraman A, Pike C, Baudry M. Proges-terone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β. J Neurochem. 2010;115:1277–1287. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada K, Kitazato KT, Kinouchi T, Yagi K, Tada Y, Satomi J, et al. Activation of estrogen receptor-αand of angiotensin-converting enzyme 2 suppresses ischemic brain damage in oophorectomized rats. Hypertension. 2011;57:1161–1166. doi: 10.1161/HYPERTENSIONAHA.110.167650. [DOI] [PubMed] [Google Scholar]
- 40.Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, et al. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J Cereb Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- 41.Bliedtner A, Zierau O, Albrecht S, Liebhaber S, Vollmer G. Effects of genistein and estrogen receptor subtype-specific agonists in ArKO mice following different administration routes. Mol Cell Endocrinol. 2010;314:41–52. doi: 10.1016/j.mce.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 42.Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav. 2009;55:319–328. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierens JE, Scobie GA, Wilson J, Saunders PT. Cloning of oestrogen receptor beta from Old and New World primates: identification of splice variants and functional analysis. J Mol Endocrinol. 2004;32:703–718. doi: 10.1677/jme.0.0320703. [DOI] [PubMed] [Google Scholar]
- 44.Mattsson A, Olsson JA, Brunström B. Activation of estrogen receptor alpha disrupts differentiation of the reproductive organs in chicken embryos. Gen Comp Endocrinol. 2011;172:251–259. doi: 10.1016/j.ygcen.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Kansra S, Chen S, Bangaru ML, Sneade L, Dunckley JA, Ben-Jonathan N. Selective estrogen receptor down-regulator and selective estrogen receptor modulators differentially regulate lactotroph proliferation. PLoS One. 2010;5:e10060. doi: 10.1371/journal.pone.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


