Abstract
Objective(s):
The aim of this study was to investigate the effects of mild hypothermia therapy on oxidative stress injury of rabbit brain tissue after cardiopulmonary resuscitation (CPR).
Materials and Methods:
Rabbit models of cardiac arrest were established. After the restoration of spontaneous circulation, 50 rabbits were randomly divided into normothermia and hypothermia groups. The following five time points were selected: before CPR, immediately after CPR, 2 hr after CPR (hypothermia group reached the target temperature), 14 hr after CPR (hypothermia group before rewarming), and 24 hr after CPR (hypothermia group recovered to normal temperature). Glutathione (GSH) concentrations in both the blood and cerebrospinal fluid of the normothermia and hypothermia groups were measured.
Results:
At 2, 14, and 24 hr after CPR, the GSH concentrations in both the blood and cerebrospinal fluid were significantly higher in the hypothermia group than in the nomorthermia group.
Conclusion:
Mild hypothermia therapy may increase GSH concentrations in rabbit blood and cerebrospinal fluid after CPR as well as promote the recovery of cerebral function.
Keywords: Cardiopulmonary resuscitation (CPR), Mild hypothermia therapy Glutathione (GSH)
Introduction
With the popularity of cardiopulmonary resuscitation (CPR) techniques and the improvement of emergency systems, increasingly more patients are receiving early relief after cardiac arrest. However, 80% of survivors after CPR enter a comatose state (either long- or short-term), 40% enter a persistent vegetative state, and 80% die within 1 year (1). Therefore, cerebral resuscitation is the key to successful CPR.
Oxidative stress is one of the main mechanisms of secondary brain injury after CPR. In the cerebral ischemia period, especially the ischemia–reperfusion period, the formation of oxygen radical increases while the activity of the tissue antioxidant enzyme system that scavenges free radicals decreases; therefore, the levels of antioxidants, represented by glutathione (GSH), reduced. This results in the disruption of the interactions between the oxygen free radicals and antioxidants, which further worsens the cerebral injury (2). Studies indicated that mild hypothermia therapy could inhibit the release of free radicals, reduce reperfusion injury, and decrease neuronal apoptosis (3). In this study, GSH levels in rabbit blood and cerebrospinal fluid of hypothermia and normothermia groups after CPR were compared to study the effect of mild hypothermia on oxidative stress and brain protection mechanisms (4-6).
Materials and Methods
Experimental animals and groups
Ordinary adult male New Zealand rabbits with an average weight of 2 kg were purchased from the Experimental Animal Center of Medical Sciences, Zhejiang Province (the license number of experimental animals was SYXK [Zhejiang] 2010-0149). After the restoration of spontaneous circulation (ROSC) from cardiac arrest, 50 rabbits were randomly divided into the normothermia and hypothermia groups according to odd and even numbers. Rabbits after ROSC from cardiac arrest in the hypothermia group were first treated with mild hypothermia therapy, while those in the normothermia group were not. After that point, both groups received the same treatment. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of XXX.
Rabbit models of cardiac arrest
As published elsewhere (7, 8), the rabbits were kept fast for 18 hr before surgery, with only water consumption. The rabbits were anesthetized via an ear vein injection of sodium pentobarbital (Guangdong Bangmin Pharmaceutical Co., Ltd., Jiangmin, China) (30 mg/kg), fixed, and subjected to electrocardiography (ECG), tracheotomy, and tracheostomy for the insertion of an indwelling tube (Teleflex Medical, Research Triangle Park, US) to maintain indoor spontaneous breathing. The right femoral artery was catheterized and heparinized, and an invasive blood pressure monitoring tube (Argon Critical Care Systems Singapore Pte Ltd. Singapore) and a pressure transducer (Argon Critical Care Systems Singapore Pte Ltd) were used to determine the mean arterial pressure (MAP) and arterial blood gas. The catheter in the right internal jugular vein went in the opposite direction to reach the retrograde venous bulb for measuring the jugular bulb oxygen saturation (SjvO2). The catheter in the left femoral vein was used for intravenous infusion and administration. The cerebrospinal fluid was collected via cisterna magna puncturing, and 0.3 ml of cerebrospinal fluid was collected from each rat, diluted by 10 times the volume, and then centrifuged to measure the GSH concentration.
We observed and recorded the heart rate and respiration over 5 min, noted the MAP and ECG findings before the experiment, and detected SjvO2. An arterial blood gas analysis was performed to detect normal blood and cerebrospinal fluid GSH concentrations. Tracheal tube occlusion was performed to induce oxygen-deficient cardiac arrest in the end-expiratory phase (cardiac arrest indicators: 0 for MAP; ventricular fibrillation, electromechanical dissociation, and a straight line on an ECG report). The clip was then released on the closed trachea 5 min after cardiac arrest, and the rabbits were immediately resuscitated, placed on a ventilator (The Medical Instrument of Zhejiang University, Hangzhou, China), administered epinephrine (Yokon, Beijing, China) intravenously, and given chest compressions—to reach the ROSC standard aortic systolic pressure of ≥6.67 kPa—that was continued for 1 min (9). The successful recovery models were included in the study groups, while the unsuccessful recovery models were excluded.
The 50 rabbits after the ROSC from cardiac arrest were randomly divided into the normothermia and hypothermia groups according to odd and even numbers. Continuous treatment was given using intravenous propofol (AstraZeneca, London, UK) as a sedative and vecuronium bromide (Ojianong, Nanjing, China) as a muscle relaxant. After ROSC from cardiac arrest, the rabbits in the hypothermia group were treated with mild hypothermia therapy (10) consisting of saline at 4°C, cooling blankets, ice caps, and ice packs placed on the head and large blood vessels in the shallow areas for cooling to reach the target temperature (controlled rectal temperature at 32 to 4°C) within 2 hr. We then maintained the target temperature for 12 hr by rewarming at increasing temperatures of 0.3 to 0.5°C per hr. When the rabbit was back to normal body temperature (39 to 39.5°C) after about 10 hr, we stopped using the muscle relaxant to prevent the occurrence of reactive fever after rewarming (11,12). The normothermia group was given the same treatment without the hypothermia component.
Index detection
We continuously performed ECG monitoring and MAP and recorded the ECG changes before and after endotracheal tube clipping, the cardiac arrest time, ROSC situation, and the time when the target temperature was reached in the hypothermia group.
Monitoring indicators before CPR were taken as normal control values. Another four time points were set: 0, 2, 14, and 24 hr after CPR. SjvO2 and arterial blood gas analysis in the normothermia and hypothermia groups were observed. An enzyme-linked immunosorbent assay was used to detect GSH in the blood and cerebrospinal fluid. The measurements were carried out in accordance to the GSH kit instructions (lot E90294Ge; USCN Life Science Inc., Wuhan, China).
At 72 hr after CPR, the rabbits were divided into the following five groups according to cerebral nerve function criteria: 1 point, normal daily activities and sensitive environmental stimuli response; 2 points, mild impairment of daily activities, unresponsive to environmental stimuli; 3 points, severe impairment of daily activities, conscious but unresponsive to environmental stimuli; 4 points, coma; and 5 points, death. In short, 1 to 3 points indicated a good prognosis, while 4 and 5 points indicated a poor prognosis (13).
Statistical analysis
SPSS13.0 software was used for the data analysis. Normally distributed data were expressed as mean ± standard deviation, while a t-test was used to identify differences between the groups, and the chi-square test was used to assess prognosis. Values of P < 0.05 were considered to statistically significant.
Results
Physiological indicators
The physiological indicators including MAP, SjvO2, PaO2, PaCO2, and pH of the rabbits blood before cardiac arrest in the two groups were not significantly different. The indicators at the other four time points (0, 2, 14, and 24 hr after CPR) in the two groups are shown in Table 1.
Table 1.
Rabbit physiological indicators before and after cardiopulmonary resuscitation
| Normothermia group | |||||
|---|---|---|---|---|---|
| Before CPR | 0 hr after CPR | 2 hr after CPR | 14 hr after CPR | 24hr after CPR | |
| Map (mmHg) | 69.75±3.24 | 71.05±2.02 | 63.83±1.66 | 64.03±0.85 | 67.61±1.64 |
| Heart rate (bpm) | 224.00±5.20 | 240.68±4.36 | 208.64±3.20 | 202.13±3.77 | 228.04±3.92 |
| PaO2 (mmHg) | 88.87±1.77 | 78.7±2.66 | 90.03±3.00 | 90.39±4.60 | 85.5±3.17 |
| SjvO2 (mmHg) | 62.88±2.10 | 57.48±3.32 | 58.88±3.62 | 61.54±3.48 | 62.06±3.41 |
| PaCO2 (mmHg) | 37.31±1.10 | 52.20±1.63 | 40.63±1.07 | 38.40±8.66. | 39.42±1.87 |
| pH | 7.21±0.021 | 7.10±0.021 | 7.13±0.050 | 7.17±0.11 | 7.20±0.56 |
| Lactic acid (mmol/l) | 3.15±0.43 | 12.26±2.02 | 8.09±1.39 | 6.37±0.95 | 5.31±1.08 |
| Mild hypothermia group | |||||
| Before CPR | 0 hr after CPR | 2 hr after CPR | 14 hr after CPR (Target value of mild hypothermia) | 24 hr after CPR (After rewarming) | |
| Map (mmHg) | 69.37±1.79 | 68.41±2.03 | 63.07±3.39 | 63.59±1.52 | 68.14±2.28 |
| Heart rate (bpm) | 224.56±2.89 | 243.36±4.98 | 186.40±5.41 | 183.34±5.62 | 224.22±5.18 |
| PaO2 (mmHg) | 89.3±1.95 | 79.68±1.93 | 90.77±2.18 | 93.48±1.88 | 84.04±4.38 |
| SjvO2 (mmHg) | 60.87±11.72 | 59.33±3.67 | 61.62±3.86 | 66.74±3.71 | 62.39±1.48 |
| PaCO2 (mmHg) | 37.38±0.82 | 52.13±1.53 | 40.53±3.39 | 35.24±1.34 | 38.70±1.90 |
| pH | 7.22±0.19 | 7.11±0.012 | 7.14±0.033 | 7.18±0.65 | 7.20±1.32 |
| Lactic acid (mmol/l) | 3.23±0.21 | 11.76±1.26 | 7.18±2.11 | 5.33±0.92 | 4.53±1.08 |
Blood GSH levels
The blood GSH levels were significantly higher in the hypothermia group than in the normothermia group at 2, 14, and 24 hr after CPR (P < 0.05, Figure 1).
Figure 1.
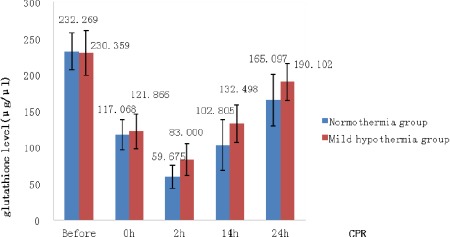
Comparison of rabbit blood glutathione levels at different time points after cardiopulmonary resuscitation
* P < 0.05 indicates significant differences
Cerebrospinal fluid GSH levels
The cerebrospinal GSH levels were significantly higher in the hypothermia group than in the normothermia group at 2, 14, and 24 hr after CPR (P<0.05, Figure 2).
Figure 2.
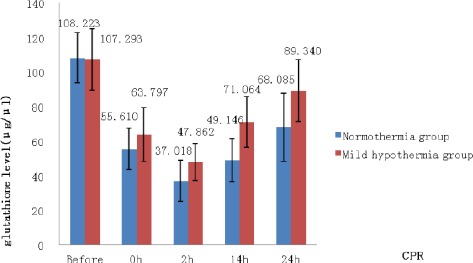
Comparison of rabbit CSF glutathione levels at different time points after cardiopulmonary resuscitation
* P <0.05 indicates significant differences
Prognostic classification of cerebral function
At 72 hr after CPR, the prognostic classification of rabbit cerebral function in the hypothermia group was significantly better than that in the normothermia group (P <0.05), as determined by using the chi-square test (Table 2).
Table 2.
Prognostic classification of rabbit brain function at 72 hr after cardiopulmonary resuscitation
| 1 class | 2 class | 3 class | 4 class | 5 classes | |
|---|---|---|---|---|---|
| Normothermia group | 3 | 4 | 5 | 11 | 2 |
| Mild hypothermia group | 6 | 7 | 6 | 4 | 2 |
Good prognosis: 1, 2, 3 classes. Poor prognosis: 4, 5 classes
X2=4.160, P<0.05
Discussion
After cardiac arrest, the body’s blood circulation almost stops and the body organs and tissues enter an extremely serious condition of ischemia and hypoxia. After effective and timely CPR, systemic blood flow is restored, but the body faces a new challenge i.e. reperfusion injury (14, 15). A large amount of oxygen free radicals are formed and cause tissue macromolecule peroxide damages, resulting in large amounts of methane dicarboxylic aldehyde and other degradation products of peroxidation that damage cell membranes, cause structural and functional changes in the mitochondria and endoplasmic reticulum, and increase cellular energy metabolism, which creates a vicious cycle and ultimately leads to neuronal apoptosis and necrosis (16). The cerebral blood supply becomes rich, oxygen consumption increases, and xanthine oxidase enzyme levels increase; thus, ischemia–reperfusion injury to the brain tissue has an extremely serious impact. Studies have shown that oxidative stress is one of the main mechanisms of secondary brain injury after CPR (17, 18).
The intracellular antioxidant barrier consists of the following two systems: the antioxidant enzyme systems represented by superoxide dismutase and the cell small molecule antioxidants represented by GSH; both these systems are related and coordinated under normal circumstances, and they maintain a stable intracellular environment (19, 20). GSH peroxidase is an important peroxide enzyme present throughout the body. As an important part of GSH, the mercapto group in cysteine combines with radicals to clear peroxide metabolites and breaks the lipid peroxidation chain reaction. It also protects cell membrane structure and function integrity as well as brain cells from damage, making it an important deoxidizer in the body. The protective effects could be divided into early and late stages. Early protection appeared a few minutes after administration, lasted for 2 to 3 hr, and was mainly related to the active substance and the endogenous active substances. The late stage occurred 24 hr after administration, lasted for several days, and was mainly related with gene expression enhancement and inhibition of proteins such as heat shock protein, brain-derived neurotrophic factor, cytokines, and antioxidant enzymes (21). Under normal circumstances, in the cerebral ischemia period after CPR, particularly the ischemic–reperfusion period, oxygen radical formation increased, while the activity of the tissue antioxidant enzyme system, which can scavenge the free radicals, decreased in order to reduce antioxidants (represented by GSH levels), then disrupted the intergroup balance, resulting in further worsening of brain damage (22, 23). However, as the disease improved, its content rebounded. The study found that GSH levels in the blood and cerebrospinal fluid in the ischemia–reperfusion period after CPR significantly decreased, which confirmed the findings of the reports mentioned above.
Mild hypothermia therapy has already been widely used in clinical studies, but its use after CPR has been seldom reported. After cardiac arrest, free radicals and a large variety of inflammatory factors are produced, calcium overload occurs, excitatory amino acids are released, and persistent and severe inflammation is induced, followed by cell damage and death (24). Studies have indicated that mild hypothermia therapy can inhibit the release of free radicals, decrease the release of inflammatory cytokines (25-27) and calcium ions, improve the ion pump function, and decrease reperfusion injury (28, 29). Hypothermia could also decrease the brain metabolic rate (30-32), maintain the integrity of blood–brain barrier, inhibit the expression of aquaporin-4, and ease cerebral edema by reducing vascular permeability (33, 34). Another study reported that hypothermia can significantly decrease the release of glutamate and dopamine and inhibit protein kinase C and nitric oxide synthase, inhibit the release of excitatory amino acids, decrease the degree of brain damage and neuronal death (35), promote the production of the brain anti-apoptotic B-cell lymphoma-2 protein after cardiac arrest, inhibit the expression of pro-apoptotic factor Bax (36), and prevent apoptosis in the early stages of brain injury. This study found that GSH levels in rabbit blood and cerebrospinal fluid after CPR were significantly higher in the hypothermia group than in the normothermia group, and the prognosis was significantly better in the former group, suggesting that hypothermia can reduce oxidative stress injury and neuronal apoptosis.
Conclusion
After CPR, GSH levels in rabbit blood and cerebrospinal fluid were significantly higher in the hypothermia group than in the normothermia group. In addition, the prognosis of the rabbits was significantly better in the former group. These findings show that mild hypothermia therapy can inhibit the release of oxygen free radicals and relieve reperfusion injury and neuronal death. This work provides a theoretical foundation for the use of mild hypothermia in the prevention of secondary brain injury after CPR.
Acknowledgment
The work was supported by Science and Technology Department of Zhejiang province, China, decided as No. 2012C33024 project.
References
- 1.Madl C, Kramer L, Domanovits H, Woolard RH, Gervais H, Gendo A, et al. Improved outcome prediction in unconscious cardiac arrest survivors with sensory evoked potentials compared with clinical assessment. Crit Care Med. 2000;28:721–726. doi: 10.1097/00003246-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Zakaria MM, Hajipour B, Khodadadi A, Afshari F. Ameliorating effects of dexpanthenol in cerebral ischaemia reperfusion induced injury in rat brain. J Pak Med Assoc. 2011;61:889–892. [PubMed] [Google Scholar]
- 3.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Zhang JJ, Mei YW, Sun SG, Tong ET. Effects of immediate and delayed mild hypothermia on endogenous antioxidant enzymes and energy metabolites following global cerebral ischemia. Chin Med J (Engl) 2011;124:2764–2766. [PubMed] [Google Scholar]
- 5.Kern KB. Optimal treatment of patients surviving out-of-hospital cardiac arrest. JACC Cardiovasc Interv. 2012;5:597–605. doi: 10.1016/j.jcin.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med. 2004;30:556–575. doi: 10.1007/s00134-003-2152-x. [DOI] [PubMed] [Google Scholar]
- 7.He FM, Zhang YJ, Li LD, Liang ZR, Xu WC, Wei H, et al. Rabbit model of heart arrest by tracheal occlusion asphyxia. Chin J Exp Surg. 2004;21:375–376. [Google Scholar]
- 8.Yapca OE, Turan MI, Borekci B, Akcay F, Suleyman H. Bilateral ovarian ischemia/reperfusion injury and treatment options in rats with an induced model of diabetes. Iran J Basic Med Sci. 2014;17:294–302. [PMC free article] [PubMed] [Google Scholar]
- 9.Najafi M. Effects of postconditioning, preconditioning and perfusion of L-carnitine during whole period of ischemia/reperfusion on cardiac hemodynamic functions and myocardial infarction size in isolated rat heart. Iran J Basic Med Sci. 2013;16:648–655. [PMC free article] [PubMed] [Google Scholar]
- 10.Comments on the 2010 guidelines on cardiopulmonary resuscitation of the European Resuscitation Council. Anaesthesist. 2010;59:1105–1123. doi: 10.1007/s00101-010-1820-9. [DOI] [PubMed] [Google Scholar]
- 11.Scirica BM. Therapeutic Hypothermia after Cardiac Arrest. Circulation. 2013;127:244–250. doi: 10.1161/CIRCULATIONAHA.111.076851. [DOI] [PubMed] [Google Scholar]
- 12.Rittenberger JC, Callaway CW. Temperature management and modern post-cardiac arrest care. N Engl J Med. 2013;369:2262–2263. doi: 10.1056/NEJMe1312700. [DOI] [PubMed] [Google Scholar]
- 13.Movassaghi S, Nadia Sharifi Z, Soleimani M, Joghataii MT, Hashemi M, Shafaroodi H, et al. Effect of pentoxifylline on ischemia- induced brain dfamage and spatial memory impairment in rat. Iran J Basic Med Sci. 2012;15:1083–1090. [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider A, Böttiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg. 2009;108:971–979. doi: 10.1213/ane.0b013e318193ca99. [DOI] [PubMed] [Google Scholar]
- 15.Yi ZH, Zhi XZ, Wang YJ, Li JY, Huang JF, Guo QL, et al. Effects of selective head mild hypothermia on endogenous neuroprotection in brain following global cerebral ischemia/reperfusion injury: experiment with dogs. Zhonghua Yi Xue Za Zhi. 2007;87:1318–1321. [PubMed] [Google Scholar]
- 16.Krep H, Brinker G, Pillekamp F, Hossmann KA. Treatment with an endothelin type A receptor-antagonist after cardiac arrest and resuscitation improves cerebral hemodynamic and functional recovery in rats. Crit Care Med. 2000;28:2866–2872. doi: 10.1097/00003246-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Busu C, Circu ML, Aw TY. Glutathione in cerebral microvascular endothelial biology and pathobiology: implications for brain homeostasis. Int J Cell Biol 2012. 2012 doi: 10.1155/2012/434971. 434971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiklund L, Martijn C, Miclescu A, Semenas E, Rubertsson S, Sharma HS. Central nervous tissue damage after hypoxia and reperfusion in conjunction with cardiac arrest andcardiopulmonary resuscitation: mechanisms of action and possibilities for mitigation. Int Rev Neurobiol. 2012;102:173–187. doi: 10.1016/B978-0-12-386986-9.00007-7. [DOI] [PubMed] [Google Scholar]
- 19.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 20.Dringen R, Hirrlinger J. Glutachione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 21.Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampalaxidatives stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janata A, Holzer M. Hypothermia after cardiac arrest. Prog Cardiovasc Dis. 2009;52:168–179. doi: 10.1016/j.pcad.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Gulati P, Muthuraman A, Jaggi AS, Singh N. Neuroprotective effect of gadolinium: a stretch-activated calcium channel blocker in mouse model of ischemia-reperfusion injury. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:255–264. doi: 10.1007/s00210-012-0819-y. [DOI] [PubMed] [Google Scholar]
- 24.Walson KH, Tang M, Glunac A, Alexander H, Manole MD, Ma L, et al. Normoxic versus hyperoxic resuscitation in pediatric asphxial cardiac arrest: Effecits on oxidative stress. Crit Care Med. 2011;39:335–343. doi: 10.1097/CCM.0b013e3181ffda0e. [DOI] [PubMed] [Google Scholar]
- 25.Smith TL, Bleck TP. Hypothermia and neurologic outcome in patients following cardiac arrest: Should we be hot to cool off our patients. Critical Care. 2002;6:377–380. doi: 10.1186/cc1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meybohm P, Gruenewald M, Zacharowski KD, Albrecht M, Lucius R, Fösel N, et al. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit Care. 2010;14:R21. doi: 10.1186/cc8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairchild KD, Singh IS, Carter HC, Hester L, Hasday JD. Hypothermia enhances phosphorylation of IkB kinase and prolongs nuclear localization of NF-kB in lipoopolysaccharide-activated macrophages. Am J Physiol Cell Physiol. 2005;289:1114–1121. doi: 10.1152/ajpcell.00152.2005. [DOI] [PubMed] [Google Scholar]
- 28.Lishmanov YB, Naryzhnaya NV, Maslov LN, Prokudina ES, Gorbunov AS, Tsibulnikov SY. Functional state of myocardial mitochondria in ischemia reperfusion of the heart in rats adapted to hypoxia. Bull Exp Biol Med. 2014;156:645–648. doi: 10.1007/s10517-014-2416-1. [DOI] [PubMed] [Google Scholar]
- 29.Fritz HG. Mild therapeutic hypothermia in cardiac arrest. Dtsch Med Wochenschr. 2014;139:141–146. doi: 10.1055/s-0033-1359917. [DOI] [PubMed] [Google Scholar]
- 30.Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006;70:369–380. doi: 10.1016/j.resuscitation.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Nordmark J, Enblad P, Rubertsson S. Cerebral energy failure following experimental cardiac arrest hypothermia treatment reduces secondary lactate/pyruvate- ratio increase. Resuscitation. 2009;80:573–579. doi: 10.1016/j.resuscitation.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Erb JL, Hravnak M, Rittenberger JC. Therapeutic hypothermia after cardiac arrest. Am J Nurs. 2012;112:38–45. doi: 10.1097/01.NAJ.0000415959.85050.1a. [DOI] [PubMed] [Google Scholar]
- 33.Pynnönen L, Falkenbach P, Kämäräinen A, Lönnrot K, Yli-Hankala A, Tenhunen J. Therapeutic hypothermia after cardiac arrest - cerebral perfusion and metabolism during upper and lower threshold normocapnia. Resuscitation. 2011;82:1174–1179. doi: 10.1016/j.resuscitation.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Fujita Y, Yamamoto N, Sobue K, Inagaki M, Ito H, Arima H, et al. Effect of mild hypothermia on the expression of aquaporin family in cultured rats astrocytes under hypoxic condition. Neurosci Res. 2003;47:437–444. doi: 10.1016/j.neures.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Kuo JR, Lo CJ, Chang CP, Lin MT, Chio CC. Attenuation of brain nitrostative and oxidative damage by brain cooling during experimental traumatic brain injury. J Biomed Biotechnol 2011. 2011 doi: 10.1155/2011/145214. 145214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberspacher E, Wemer C, Engelhard K, Pape M, Laacke L, Winner D, et al. Long-term effects of hypothermia on neuronal cell death and the concentration of apoptotic proteins after incomplete cerebral ischemia and reperfusion in rates. Acta Anaesthesiol Scand. 2005;49:477–487. doi: 10.1111/j.1399-6576.2005.00649.x. [DOI] [PubMed] [Google Scholar]


