Abstract
Objective(s):
Protective effects of different extracts and essential oil from Pimpinella anisum L. seeds were examined against carbon tetrachloride (CCl4)-induced toxicity. The parameters such as serum transaminases, lactate dehydrogenase activity, hepatic glutathione content, liver lipid peroxidation and histopathological changes of liver were assessed as toxicity markers. In the in vitro model of this study, markers such as cell viability, cellular reduced and oxidized glutathione and lipid peroxidation in HepG2 cells were evaluated.
Materials and Methods:
Human liver cancer cell line HepG2 and male Sprague-Dawley rats were treated with extracts and essential oil, and markers of hepatotoxicity were investigated.
Results:
The data revealed that the n-hexane extract, effectively attenuated CCl4-induced toxicity in both in vitro and in vivo models in current investigation.
Conclusion:
As the oxidative stress markers were ameliorated, it might be concluded that anise seed possesses protective effects probably due to its antioxidant constituents.
Keywords: Anise seed, CCl4, Hepatotoxicity, HepG2 cells, Rat, Oxidative stress
Introduction
Liver is the main organ responsible for xenobiotics metabolism; hence, it is vulnerable to damage induced by different chemicals. Hepatic injury is a major clinical problem associated with different xenobiotics including drugs and industrial chemicals. Hence, many hepatoprotective agents are studied to protect liver from toxic insults (1). Recently, interest in the discovery of natural antioxidants has risen exponentially. Principal candidates in this discovery process are medical plants (2).
Pimpinella anisum (anise) belongs to the Umbelliferae family and grows in the Southern and northwest Iran, and countries such as Egypt, Turkey, and Greece (3). This herb is used in Iranian folk medicine as an analgesic, anti-inflammatory and anticonvulsant (3, 4). Different extractions of P. anisum seed have been studied for their protective properties (5). In some studies, the properties of P. anisum essential oil have been investigated (6). It has been reported that essential oil and extracts of P. anisum have a wide range of biological activities (7). Previously, the phytochemical constituents of P. anisum have been analyzed and detected (8). Some compounds in the P. anisum extracts and essential oil demonstrated to have antioxidant activity (8). El Haliem et al found that P. anisum oil protected rats from aspartame-induced liver histopathological changes (9). Moreover, Cengiz et al showed that diethyl ether extract of anise seed could ameliorate carbon tetrachloride (CCl4)-induced liver injury (10). The current study evaluated the effect of n-Hexane and hydroalcoholic extracts of anise seed in addition to essential oil against CCl4-induced hepatotoxicity.
CCl4 as an industrial agent is now applied prior to treatment as a model toxin for studying hepatotoxicity and hepatoprotective effects of agents. Current investigation attempted to evaluate if different extracts and/or essential oil of P. anisum have any hepatoprotective activity against CCl4-induced toxicity in vivo and also in HepG2 cell line as an in vitro experimental model of hepatotoxicity.
Materials and Methods
Plant material and chemicals
Pimpinella anisum seed were bought from a local market of Shiraz, Fars, Southern Iran, and it was authenticated at the Botany Department, Shiraz University of Medical Sciences, Shiraz, Iran. The voucher specimen with code “PM 151” has been deposited in the Botany Department of the Faculty of Pharmacy of Shiraz University of Medical Sciences.
Human liver cancer cell line HepG2 was obtained from Pasteur Institute (Tehran, Iran). RPMI-1640 and FBS were from GibCo (United States). Trypan blue, methylthiazolydiphenyl-tetrazolium bromide (MTT), Dithio-bis-nitro benzoic acid (DTNB), CCl4 and Na2HPO4 were purchased from Merck (Darmstadt, Germany), and thiobarbituric acid (TBA) was from Sigma Chemical Company (Germany). All other used chemicals were of highest quality available in the market.
Preparation of extracts and essential oil
For extractions, 10 g of fine powder of seeds were subsequently mixed with 100 ml of n-hexane, ethanol 80% (v/v) for 4 hr under soxhlet conditions. Then extractions were orderly desiccated by rotary and speed vacuum for 24 hr. The total solid yields of hydroalcoholic and n-hexane were 55 and 45 g/kg, respectively. For preparation of essential oil, 20 g of seeds powder were added to 100 ml of twice-distilled water at 45°C for 4 hr by Clevenger. The total essential oil yield was 0.15 ml/kg.
Thin layer chromatography (TLC)
In this method, ethyl acetate and toluene solvents in proportions of 7 to 93 v/v were used. After spotting, TLC paper was put into the tank containing mentioned solvents. When the solvents reach the end of paper, the solvents were evaporated by heat gun. For spot detection, UV light in 254 nm and anhydric sulfuric acid was used.
In vivo hepatoprotective activity studies
Male Sprague-Dawley rats (200-250 g) were obtained from the Laboratory Animals Research Center of Shiraz University of Medical Sciences. The rats were maintained under controlled temperature, 12 hr light/12 hr dark conditions for one week before the start of the experiments. They had access to standard laboratory chow and tap water ad libitum. The animals were handled and used, according to the ethical guidelines of Shiraz University of Medical Sciences, Shiraz, Iran. Animals were randomly divided into eight groups, containing six rats in each. The treatments groups were as follow:
-
A
)Control (vehicle-treated, received olive oil, 1.5 ml/kg, IP)
-
B
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days)
-
C
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days) + n-hexane extract (100 mg/kg, IP for three consecutive days)
-
D
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days) + n-hexane extract (200 mg/kg, i.p for three consecutive days)
-
E
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days) + Hydroalcoholic extract (100 mg/kg, IP for three consecutive days)
-
F
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days) + Hydroalcoholic extract (200 mg/kg, IP for three consecutive days)
-
G
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days) + Essential oil (20 mg/kg, IP for three consecutive days)
-
H
)CCl4 (1.5 ml/kg in olive oil, IP, for three consecutive days) + Essential oil (100 mg/kg, IP for three consecutive days)
The extract and/or essential oil doses used in current investigation were gained experimentally, then the non-toxic doses were selected for further investigation. There were no significant differences between extract/essential oil-treated and the control (vehicle-treated) animals in the parameters assessed in this study, when these agents were administered alone. The hepatotoxic dose of CCl4 in rats was selected from previous investigations (11).
Twenty four hr after CCl4 injection, animals were anaesthetized by pentobarbital injection (50 mg/kg), and blood was collected from the vena cava. Serum was separated and used for different enzyme measurements. The rats were then decapitated and the livers were carefully dissected, cleaned of extraneous tissues, and part of the liver tissue was immediately transferred to 10% formalin for histopathological assessments.
Measurement of transaminases level in serum of rats
Biocon standard kits and DAX-48® auto analyzer were used to measure alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities in serum using commercial kits (Pars Azmun®, Tehran, Iran).
Histopathological studies
The livers were removed from the animals and a part of the tissues were fixed in 10% formalin for at least 24 hr. Then, the paraffin sections were prepared (Automatic tissue processor, Auto-technique) and cut into 5 µm thick sections in a rotary microtome. The sections were then stained with haematoxylin-eosin dye (H&E), and studied for histopathological changes (12), i.e. necrosis, fatty changes, ballooning degeneration, and lymphocyte infiltration. Histological damages were scored as follows: 0: absent; 1: mild; 2: moderate; and 3: severe.
Liver glutathione content
The excised livers were analyzed for their glutathione (GSH) content. The GSH contents were assessed by determining non-protein sulphydryl contents with the Ellman reagent (13). Samples of liver (200 mg) were homogenized in 8 ml of 20 mM EDTA. Then, 5 ml of homogenized tissue was mixed with 4 ml distilled water and 1 ml of trichloroacetic acid (TCA) (50 % w/v). The mixture was shaken and centrifuged (15 min, 700 g, 4°C). Then, 2 ml of supernatant was treated with 100 µl of Ellman reagent (DTNB, 0.01M in methanol), and the absorbance of developed color was measured at 412 nm with an Ultrospec 2000® UV spectrophotometer.
Determination of lipid peroxidation in liver tissue
Level of lipid peroxidation was measured in different experimental groups. Briefly, 500 mg of liver tissue gently minced in 4.5 ml of 0.25 M sucrose. The minced tissues gently homogenized and then centrifuged at 2000 rpm for 30 min. Afterwards, 0.1 ml of the supernatant was treated with a buffer containing 0.75 ml of thiobarbituric acid (0.8%, w/v), 0.75 ml of 20% acetic acid (pH = 3.5) and 0.1 ml of sodium dodecyl sulfate (8.1%, w/v). The solution was mixed up with 2 ml of distilled water, and heated in a boiling water bath for 60 min. Samples were centrifuged (3000 g for 5 min) and the absorbance of developed color was read at 532 nm using an Ultrospec 2000 ®UV spectrophotometer (13).
In vitro hepatoprotective activity studies
Human hepatoma cell lines (HepG2) were cultured and maintained in RPMI-1640, pH 7.3, containing 0.37% NaHCO3 supplemented with 10% FBS (fetal bovine serum albumin), 1% penicillin and streptomycin (100 IU/ml penicillin and 100 IU/ml streptomycin) in a humidified 5% CO2-95% air mixture at 37°C. Cells were seeded in 96-well microplates (30000 cells/well/90 µl) and routinely cultured in a humidified incubator for 24 hr. The cells were then treated with different concentrations from 1 µg/ml to 1000 µg/ml of the seed extracts and essential oil (10 µl/well) 1 hr before CCl4 100 mM (CCl4/ethanol; 1/10) exposure. The appropriate, safe and non-toxic dose of extracts and essential oil of anise seed was determined (Table 1) for using in other experiments on HepG2 cell line.
Table 1.
Effect of different concentrations of anise seed essential oil and extracts on HepG2 cells viability, as assessed by Methyl thiazolydiphenyl-tetrazolium test (% viability)
| Incubate | Concentration | 10 µg/ml | 50 µg/ml | 100 µg/ml | 500 µg/ml |
|---|---|---|---|---|---|
| Control | 100±7.6 | 100±8.4 | 100±7.8 | 100±8.9 | |
| E | 97.26±5.6 | 97.3±5.2 | 41.2±6.3 # | 32.5±4.6 # | |
| HE | 96.2±4.5 | 96.5±5.6 | 45.6±4.5 # | 36.58±6.5 # | |
| NE | 96.4±4.3 | 97.5±6.8 | 49.6±6.2 # | 39.5±5.4 # |
Data are given as Mean±SD for three independent experiments
# Indicates significant differences as compared to control cells (P<0.05)
E: Essential oil, HE: Hydroalcoholic extract, NE: n-hexane extract
After 24 hr of incubation, 10 µl MTT solutions were added to each well, and cells were re-incubated for an additional 4 hr. The cell culture media and MTT solution were removed and the cells remained in the bottom of the wells. Then, 100 µl of DMSO was added to each well to dissolve the formazan crystals formed. The absorbance of the converted dye was measured at wavelength of 570 nm. Five wells were used for each concentration of extracts, and three independent experiments were performed for each extract.
Lipid peroxidation in HepG2 cell
As a biomarker for lipid peroxidation, concentration of thiobarbituric acid-reactive (TBARs) agents was measured. HepG2 cells (3×106 cells/flask) were pre-incubated in flasks for 24 hr at 5% CO2-95% air at 37°C. The control cultures were prepared by adding RPMI-1640 without any addition. After incubation with or without extract and essential oil, the culture medium was removed. After rinsing with 0.5 ml free PBS twice, cells were collected by trypsinization. After determining the viability of the detached cells, 250 µl of 70% (w/v) trichloroacetic acid containing 1 ml of 0.8% (w/v) thiobarbituric acid with 750 µl deionized water was added to cells and was shaken with vortex. The suspensions were transferred into glass tubes and boiled for 30 min. After cooling to room tempera- ture and centrifugation for 10 min at 5000 rpm, the absorbance of the supernatant was determined at 532 nm.
Glutathione content in HepG2 cells
HepG2 cells (3×106 cells/flask) were pre-incubated in flasks for 24 hr at 5% CO2-95% air at 37°C. Cells were rinsed with PBS and were collected by trypsinization. After determining the viability of detached cells, 200 µl of 20% trichloroacetic acid with 1800 µl of PBS were added to the cell suspension. After shaking with vortex and centrifuging, supernatant was divided to two even parts (each 1 ml).
For measurement of reduced glutathione (GSH), two ml of Na2HPO4 (0.3 M) and 0.5 ml of DTNB (0.01 M) were added to 1 ml of the supernatant and was shaken with vortex. The absorbance was then measured at 412 nm. For measurement of oxidized glutathione (GSSG), 1 ml of 5% sodium borohydride was added to 1 ml of the supernatant, and was incubated for 1 hr in 45°C, then 0.5 ml of Na2HPO4 (0.3 M) was added to each tube. After neutralization with HCl (2.7 N), 0.5 ml of DTNB (0.01 M) was added and was shaken with vortex. The absorbance was then measured at 412 nm.
Statistical analysis
All data were presented as mean±SD for at least three separate experiments. Statistically significant differences between control and experimental groups were obtained using one way analysis of variance (ANOVA) and Tukey’s as post hoc test. The Kruskal–Wallis tests followed by Mann Whitney U test were employed for histopathological data comparison. The minimal level of significance chosen was P<0.05.
Results
CCl4 administration to rats caused hepatotoxicity as judged by elevated serum transaminases level (Figures 1 and 2), serum lactate dehydrogenase (LDH) activity (Figure 3), and lipid peroxidation (Table 3). The serum biochemical changes after CCl4 administration was endorsed by liver histopathological changes, which was observed as liver fatty changes, centrilobular necrosis, and inflammatory cells infiltration (Table 4). N-hexane extract administration effectively reduced CCl4-induced hepatotoxicity as revealed by lowering serum transaminase activity (Figures 1 and 2) and LDH level (Figure 3), and attenuated liver histopathological changes (Table 4). Anise seed hydroalcoholic extract and/or essential oil administration showed no significant protective properties against CCl4-induced liver damage in rats (Figures 1, 2, 3 and Table 4). Moreover, CCl4 caused a significant amount of thiobarbituric reactive substances (TBARs) to form in rat liver (Table 3), which was significantly reduced by anise n-hexane extract administration (Table 3). Anise seed essential oil (20 mg/kg) and/or hydroalcoholic extract (100 mg/kg) had no significant effect on lipid peroxidation and/or GSH depletion caused by CCl4 (Table 3). Higher doses of anise seed hydroalcoholic extract (200 mg/kg) and essential oil (100 mg/kg) was also unable to ameliorate lipid peroxidation and glutathione depletion in animals’ liver (Data not shown).
Figure 1.
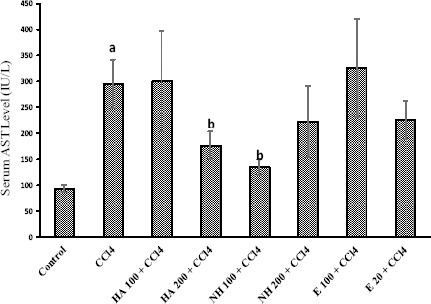
Liver aspartate aminotransferase (AST) levels in rats after CCl4 administration. The role of different extract and essential oil of anise seed is shown
Data are represented as Mean±SD for six animals
a Significantly higher than control animals (P<0.05)
b Significantly lower than CCl4-treated animals (P<0.05)
E: Essential oil, HA: Hydroalcoholic extract, NH: n-hexane extract
Figure 2.
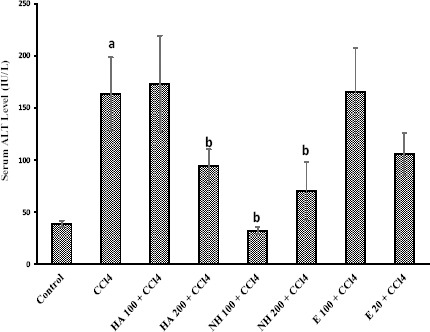
The role of anise seed extracts and essential oil on serum alanine amino transferase (ALT) levels in CCl4-treated rats
Data are given as Mean±SD for six animals
a Significantly higher than control group (P<0.05)
b Significantly lower than CCl4-treated animals (P<0.05)
E: Essential oil, HA: Hydroalcoholic extract, NH: n-hexane extract
Figure 3.
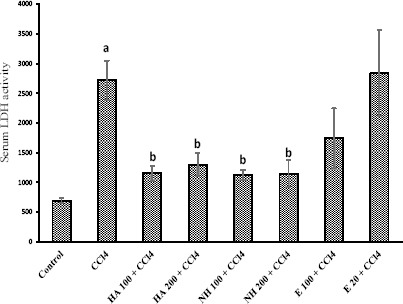
Effects of anise seed extracts and essential oil on lactate dehydrogenase (LDH) activity in CCl4-intoxicated rats
Data are shown as Mean±SD for six rats
a Significantly higher than control group (P<0.05)
b Significantly lower than CCl4-treated group (P<0.05)
E: Essential oil, HA: Hydroalcoholic extract, NH: n-hexane extract
Table 3.
Effect of anise seed essential oil and extracts on lipid peroxidation (TBARs) and hepatic glutathione (GSH) content in rat
| Treatment | TBARs (nM/mg liver tissue) | GSH (µM/mg liver tissue) |
|---|---|---|
| Control | 0.09±0.010 | 0.28±0.10 |
| + CCl4 | 0.15±0.010 a | 0.18±0.01 |
| + E (20 mg/kg) | 0.17±0.10 | 0.4±0.10 |
| + HA (100 mg/kg) | 0.23±0.020 | 0.37±0.15 |
| + NE (100 mg/kg) | 0.10±0.024 b | 0.33±0.12 |
Data are shown as Mean±SD for six independent experiments
a Indicates significantly higher than control group (P<0.05)
b Indicates significantly lower than CCl4-treated group (P<0.05)
E: Essential oil, HA: Hydroalcoholic extract, NE: n-hexane extract
Table 4.
Histopathological changes of rat liver after CCl4 and anise seed extracts and essential oil administration
| Groups | Changes | Centrilobular necrosis | Fatty changes | Inflammatory cells infiltration |
|---|---|---|---|---|
| Control | 0 (0,0) | 0 (1,0) | 0 (0,1) | |
| + CCl4 | 1 (1,1) a | 1 (2,1) a | 1 (2,2) a | |
| + NE (100 mg/kg) | 0 (1,0) b | 1 (0,1) b | 0 (1,0) b | |
| +HE (200 mg/kg) | 1 (1,0) | 1 (2,2) | 1 (1,1) | |
| + E (100 mg/kg) | 1 (1,1) | 1 (2,1) | 1 (1,1) | |
0=absent; 1=mild; 2=moderate; and 3=severe histopathological changes
Data are presented as median and quartiles for five random pictures per group
a Indicates significant difference as compared with control group (P<0.05)
b Indicates significant difference as compared with CCl4-treated group (P<0.05)
E: Essential oil, HE: Hydroalcoholic extract, NE: n-hexane extract
In another part of this investigation on HepG2 cells, different concentrations of essential oil and extracts were tested on cells to obtain a non-toxic appropriate dose for further experiments (Table 1). CCl4 was toxic towards HepG2 cells in a dose-dependent manner and caused an acceptable loss of cell viability for 24 hr of about 100 mM. The results presented in Table 2, revealed that pre-incubation of HepG2 cells with 10 to 50 µg/ml of n-hexane extract of anise seed 1 hr before CCl4 (100 mM), resulted in reduction of cytotoxicity. But the essential oil (Table 2) and hydroalcoholic extract (Table 2) with all concentration did not have protective effects on CCl4-induced cytotoxicity.
Table 2.
Effect of anisum seed extracts and essential oil on cytotoxicity, glutathione (GSH) content and lipid peroxidation (TBARs) in HepG2 cell
| Incubate | GSH (µM/3×103 cell) | GSSG (µM/3×103 cell) | TBARs (ng/5×105 cell) | % Cytotoxicity (MTT assay) |
|---|---|---|---|---|
| Control | 25.16± 2. 66 | 3. 83±. 103 | 0. 8890± 0. 043 | 100±8.8 |
| + CCl4 (100 mM) | 11.52±3. 38 a | 19. 02±3. 47 a | 1. 970±0. 132 a | 65.2±7.3 a |
| + NE (10 µg/ml) | 20.101± 3. 81 b | 11. 17±4. 71 b | 0. 738±0. 047 b | 77±4.9 b |
| + NE (20 µg/ml) | 20.65±2. 52 b | 11. 26± 1. 79 b | 0. 703±0. 064 b | 84±4 b |
| + NE (50 µg/ml) | 24.28±2. 24 b | 10. 99±2. 74 b | 0. 641±0. 535 b | 86±3.2 b |
| + HA (50 µg/ml) | 13.32±3. 12 | 17. 99±3. 1 | 1. 4990±0.175 | 70±8.3 |
| + E (50 µg/ml) | 11.25±1.24 | 17.45±2.6 | 1.690000±6.4 | 71±5.2 |
Data are given as Mean±SD for at least three independent experiments
a Significant difference as compared to control cells (P<0.05)
b Significant difference as compared to CCl4-treated hepatocytes (P<0.05)
E: Essential oil, HA: Hydroalcoholic extract, NE: n-hexane extract
Incubation of the cells with 100 mM CCl4 for 24 hr decreased the GSH and increased GSSG content and TBARs level of the cells significantly (Table 2). Pre-incubation of the cells with n-hexane extracts also significantly affected TBARs level and GSH content of the cells (Table 2), but all of the extracts of seed did not have significant effects on TBARs level and GSH content of the cells (Table 2).
The outcome of thin layer chromatography (TLC) test (Figure 4) showed that n-hexane extract and essential oil have similar pattern of spotting, but there is a series of bands in n-hexane that were not observed in essential oil (Figure 4).
Figure 4.
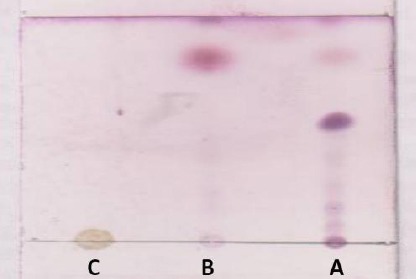
TLC analysis of pimpinella anisum essential oil and extracts
A: n-hexane extract, B: Essential oil, C: hydroalcoholic extract
n-hexane extract possess a series of nonvolatile and high lipophilicity compounds that there are not present in essential oil
Discussion
N-hexane extract of anise seed has shown protective properties against CCl4 both in vitro and in vivo as revealed by decreasing cell death, serum transaminase levels, LDH activity, and liver histopathological changes induced by CCl4. Furthermore, HepG2 cells and liver of rats treated by n-hexane extract of anise seed showed higher levels of glutathione (GSH) and lower levels of TBARs. Hydroalcoholic extract and essential oil of anise seed showed no significant protective properties against CCl4-induced injury in this study.
To study the hepatoprotective effects of drugs or medicinal plants, extract, CCl4-induced hepatic injury is usually used as an experimental method. CCl4 is believed to be metabolized by microsomal CYP450 in the liver to a highly reactive trichloromethyl free radical (•CCl3), which can start a chain of reactive free radical formation resulting in peroxidation of lipids and damage to proteins and other components of the cell, which finally can result in cell lyses. Induction of oxidative stress and deactivation of cellular defense mechanisms are also believed to be involved in CCl4-induced hepatotoxicity. Oxidative stress affects a vast range of intracellular targets, including lipids, proteins and DNA and cellular defense mechanisms, namely glutathione reservoirs. Lipid peroxidation is a common consequence of reactive oxygen species (ROS) formation and oxidative stress in liver.
The n-hexane extract of anise seed decreased the lipid peroxidation induced by CCl4 (Table 2, 3). N-hexane extract administration also prevented cellular GSH consumption (Table 2), and formation of oxidized glutathione (GSSG) (Table 2). The role of anise seed extract in attenuating the lipid peroxidation and its effects on cellular glutathione reservoirs may have been due to its antioxidant effects and attenuating oxidative stress.
It has been found that anise seed possess a potent antioxidant activity (14). Many polyphenol compounds have been detected in extracts from different anise species (15). Polyphenolic compounds are good examples of antioxidant agents with different characteristics in biological systems, which they exert their protective properties through them. Hence, protective properties of anise seed extract (n-hexane extract in this study) could be attributed to the polyphenolic compounds in this fraction.
Thin layer chromatography (TLC) test represented that n-hexane extract possess a series of nonvolatile and high lipophilic compounds that are not found in essential oil. These compounds probably caused hepatoprotective effect. Its cytoprotective and hepatoprotective effects against CCl4 could be mediated by one or several mechanisms such as: inhibition of cytochrome P450 responsible for metabolism of CCl4 to reactive free radicals; antioxidant effects; scavenging free radicals responsible for cell damage; or induction or regeneration of the liver cells. However, the lack of an appropriate positive control might serve as a limitation for current investigation.
Conclusion
In conclusion, n-hexane extract of anise seed possesses protective effects both in vitro and in vivo against CCl4-induced hepatotoxicity probably due to its antioxidant constituents. However, more future studies on this extract are needed to clarify the exact component(s) responsible for hepatoprotection. Furthermore, using n-hexane extract of anise seed against xenobiotics induced hepatotoxicity could be the subject of further investigations.
Acknowledgment
This study is a part of thesis written by Mojtaba Razmjou and Forouzan Karimi students of Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran. The research was supported financially (Grant No: 90-5612/5613), from Vice Chancellor of Research, Shiraz University of Medical Sciences, Shiraz Iran. Anise seed was kindly provided by Dr Pouya Faridi (Traditional Pharmacy and Pharmacognosy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz Iran). Authors thank Dr Faridi for providing the mentioned material.
References
- 1.Yilmaz O, Ersan Y, Ozsahin AD, Ozturk AI, Ozkan Y. Consequences of the combined α-tocopherol, ascorbic acid and α-lipoic acid on the glutathione, cholesterol and fatty acid composition in muscle and liver of diabetic rats. Iran J Basic Med Sci. 2013;16:165–172. [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma V, Singh M. Attenuation of N-nitro- sodimethylamine induced hepatotoxicity by Operculina turpethum in Swiss Albino mice. Iran J Basic Med Sci. 2014;17:73–80. [PMC free article] [PubMed] [Google Scholar]
- 3.Pourgholami MH, Majzoob S, Javadi M, Kamalinejad M, Fanaee GHR, Sayyah M. The fruit essential oil of Pimpinella anisum exerts anticonvulsant effects in mice. J Ethnopharmacol. 1999;66:211–215. doi: 10.1016/s0378-8741(98)00161-5. [DOI] [PubMed] [Google Scholar]
- 4.Tabanca N, Ma G, Pasco DS, Bedir E, Kirimer N, Baser K, et al. Effect of essential oils and isolated compounds from Pimpinella species on NF-κB: a target for antiinflammatory therapy. Phytother Res. 2007;21:741–745. doi: 10.1002/ptr.2154. [DOI] [PubMed] [Google Scholar]
- 5.Conforti F, Tundis R, Marrelli M, Menichini F, Statti GA, De Cindio B, et al. Protective effect of Pimpinella anisoides ethanolic extract and its constituents on oxidative damage and its inhibition of nitric oxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Med Food. 2010;13:137–141. doi: 10.1089/jmf.2008.0154. [DOI] [PubMed] [Google Scholar]
- 6.Orav A, Raal A, Arak E. Essential oil composition of Pimpinella anisum L. fruits from various European countries. Nat Product Res. 2008;22:227–232. doi: 10.1080/14786410701424667. [DOI] [PubMed] [Google Scholar]
- 7.Alhaider AA, Al-Moqh IA, Mossa JS, Al-Soohaibani MO, Rafatullah S. Aqueous suspension of anise “Pimpinella an/sum” protects rats against chemically induced gastric ulcers. World J Gastroenterol. 2007;13:1112–1118. doi: 10.3748/wjg.v13.i7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andarwulan N, Shetty K. Phenolic content in differentiated tissue cultures of untransformed and Agrobacterium-transformed roots of anise (Pimpinella anisum L.) J Agricult Food Chem. 1999;47:1776–1780. doi: 10.1021/jf981214r. [DOI] [PubMed] [Google Scholar]
- 9.El Haliem NGA, Mohamed DS. The effect of aspartame on the histological structure of the liver and renal cortex of adult male albino rat and the possible protective effect of Pimpinella anisum oil. Egyp J Histol. 2011;34:715–726. [Google Scholar]
- 10.Cengiz N, Ozbek H, Him A. Hepatoprotective effects of Pimpinella anisum seed extract in rats. Pharmacologyonline. 2008;3:870–874. [Google Scholar]
- 11.Janbaz KH, Saeed SA, Gilani AH. Protective effect of rutin on paracetamol-and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557–563. doi: 10.1016/s0367-326x(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 12.Moezi L, Heidari R, Amirghofran Z, Nekooeian AA, Monabati A, Dehpour AR. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: The role of nitric oxide and IL-1b. Pharmacol Rep. 2013;65:134–143. doi: 10.1016/s1734-1140(13)70971-x. [DOI] [PubMed] [Google Scholar]
- 13.Heidari R, Babaei H, Roshangar L, Eghbal MA. Effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Advanc Pharm Bull. 2013 doi: 10.5681/apb.2014.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammad Al-Ismail K, Aburjai T. Antioxidant activity of water and alcohol extracts of chamomile flowers, anise seeds and dill seeds. J Sci Food Agric. 2004;84:173–178. [Google Scholar]
- 15.Liu H, Qiu N, Ding H, Yao R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res Int. 2008;41:363–370. [Google Scholar]


