Abstract
Objective:
Melanocortin-4 receptor (MC4R) and agouti-related peptide (AgRP) are involved in energy homeostasis in the rat. The aim of the present study was to evaluate the expression of MC4R and AgRP mRNAs in arcuate nucleus (ARC) during long term malnutrition of female ovariectomized rats.
Materials and Methods:
Ten female ovariectomized rats were divided into two equal groups (n=6) of normal and restricted diet groups. Using real-time PCR, the relative expressions (compared to controls) of MC4R and AgRP mRNAs were compared between both diet groups.
Results:
The relative expression of MC4R and AgRP mRNA in the ARC of female ovariectomized rats during long term malnutrition was higher than those with normal diet (P<0.05).
Conclusion:
Changes in the relative expression level of MC4R and AgRP mRNAs during long term malnutrition of rat indicated a stimulatory role of MC4R and AgRP in regulating energy balance in ARC of rat hypothalamus.
Keywords: Agouti-related peptide, Hypothalamus, Malnutrition, Melanocortin-4 receptor, Rat
Introduction
Melanocortin-4 receptor (MC4R) is the cognate receptor for α-melanocyte stimulating hormone (α-MSH), a cleavage product of proopiomelanocortin (POMC) which is expressed in hypothalamic arcuate nuclei (ARC) neurons (1). MC4R activation by agonists such as α-MSH inhibits feeding and causes weight loss (2).
Agouti related peptide (AgRP) and a MC4R antagonist neuropeptide, with 132 amino acid are expressed in the ARC of rat (3). Using immunohistochemistry, AgRP-containing cell bodies were found almost exclusively in the ARC, but their projections were distributed widely in the hypothalamus, most conspicuously in paraventricular nucleus (PVN), ARC, dorsomedial nuclei, and the posterior hypothalamic area (4). In some species, AgRP neurons are involved in energy balance (2). Ectopic AgRP expression in hypothalamus causes obesity in the mouse, while AgRP is expressed by Neuropeptide Y (NPY) neurons in ARC (2) and NPY is expressed by neurons that project to important nuclei of appetite-regulating, including PVN (2). Injection of NPY into the PVN is known the most potent central stimulant of appetite (2). The NPY neurons in ARC are stimulated by starvation, probably mediated by decreases in circulating leptin and insulin which both inhibit these neurons and contribute to increased hunger in energy deficit conditions (2). Moreover, excitatory effect of MC4R and inhibitory effect of AgRP in proestrus phase during the preovulatory period on GnRH/LH secretion (5), in ARC of rats control the energy homeostasis.
These results support the hypothesis that the brain MC4R and AgRP is intimately involved in the control of food intake and act homeostatically to correct negative energy balance. Therefore, the aim of the present study was to investigate MC4R and AgRP mRNAs expression in the ARC during long term malnutrition of female ovariectomized rats.
Material and Methods
Fourteen female adult (3-4 months old) Sprague-Dawley rats (Rattus norvegicus) that weighed 200±20 g (mean ± SD) were randomly selected from the Laboratory Animal Center of Shiraz University of Medical Sciences, Shiraz, Iran. They were housed under controlled temperature (22±2°C), humidity (60±6%) and light (12 hr light to 12 hr dark ratio; lights on at 7:30 AM). All experimental procedures were carried out between 12.00-2.00 pm and in compliance with the Animal Care Committee recommendations of the Shiraz University of Medical Sciences. Standard rat feed (before the start of the study) and water were freely available to them. The rats were ovariectomized to remove the effects of reproductive hormones during the estrous cycle. The rats were anesthetized by ketamine (intraperitoneal injection, 100 mg/kg, Woerden, Netherlands) and xylazine (intraperitoneal injection, 7 mg/kg, Alfazyne, Woerden, Netherlands). They were ovariectomized through a ventral midline incision and after a two week recovery period, further procedures were undertaken.
Standard rat diet contained 90% dry matter, 8% ash, 4.1% crude fat, 21.6% crude protein, 70.8% total digestible nutrients (TDN), 0.4% calcium, 0.35% potassium and 0.1% sodium, which were measured based on AOAC, 1995. Two weeks after surgery (day 0), 12 rats were randomly divided into two equal groups and the first group were fed with a complete ration (15 g/kg) of standard rat diet (normal diet group, n=5) and the other group with a half ration (7.5 g/kg) of the first group (restricted diet group, n=5) for 14 days and then were anesthetized with ether and were ethically killed. Four rats of control group were ethically killed two weeks after surgery (day 0).
The rats were decapitated, brains immediately dissected out, and the diencephalon was dissected out by an anterior coronal section, anterior to the optic chiasm, and a posterior coronal cut at the posterior border of the mammillary bodies. To separate ARC from other nuclei, a third coronal cut was made through the middle of the optic tract, just rostral to infundibulum. The specimens consisted of ARC were stored in liquid nitrogen and then stored at -80°C. RNA extraction, DNase treatment, cDNA synthesis and relative real-time PCR procedure were performed. Primers were designed with Allele ID 7 software for the reference gene, AgRP (NM_033650.1) and MC4R (NM_013099.2). The rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (M32599) was used as reference gene for data normalization (Table 1).
Table 1.
Sequences of real-time PCR primers used to evaluate relative expression of AgRP and MC4R genes in the rat
| Primer | Primer sequence | Amplicon length (bp) |
|---|---|---|
| MC4R-F MC4R-R |
5` TGGGTGTCATAAGCCTGTTGG 3` 5` GCGTCCGTGTCCGTACTG 3` |
181 |
| AgRP-F AgRP-R |
5` TGAAGAAGACAGCAGCAGACC 3` 5` TGAAGAAGCGGCAGTAGCAC 3` |
189 |
| GAPDH-F GAPDH-R |
5` AAGAAGGTGGTGAAGCAGGCATC 3` 5` CGAAGGTGGAAGAGTGGGAGTTG 3` |
112 |
Relative expressions of AgRP and MC4R based on the threshold cycle (CT) method were calculated for quantitative real-time PCR data. Using Line-gene K software, CT for each sample was calculated. Fold expression of the target mRNAs over reference values was calculated by the equation 2-ΔΔCT, where ΔCT is determined by subtracting the internal control (corresponding GAPDH CT value) from the specific CT of the AgRP or MC4R. ΔΔCT was obtained by subtracting the ΔCT of each experimental sample from that of the control ovariectomized rats.
Normality of the data on the relative expression of AgRP and MC4R mRNAs were evaluated by Kolmogorov-Smirnov test using SPSS version 11.5 (SPSS Inc, Chicago, Illinois). Expression of AgRP and MC4R mRNAs were compared between groups using independent sample t-test. Correlation coefficient of their expression was analyzed by Pearson correlation test. We considered P<0.05 as significant.
Results
Expression of AgRP (P=0.04, Figure 1A) and MC4R (P=0.02, Figure 1B) mRNAs in the ARC of restricted diet group of female ovariectomized rats was more than normal diet group. Food-restricted rats showed a 6.2 fold increase in the expression of AgRP mRNA in the ARC of the hypothalamus. Moreover, the expression of MC4R mRNA increased 6.7 times in long term food-restricted in comparison with normal feeding female rats. A positive correlation was observed between expression of AgRP and MC4R mRNAs in the ARC of female ovariectomized rats of both diet groups (r=0.99, P<0.001, Figure 1C).
Figure 1.
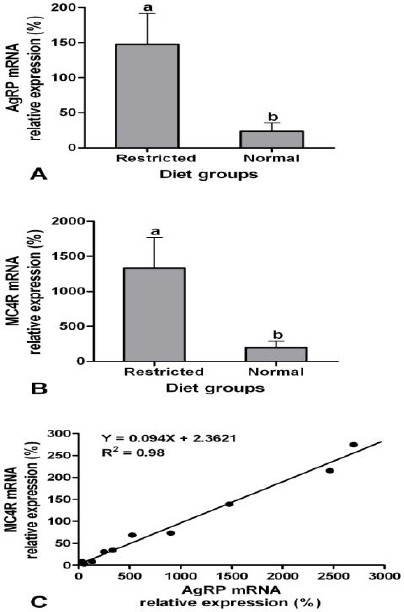
Mean (± standard error) of the relative expression of A) agouti-related peptide (AgRP), and B) melanocortin-4 receptor (MC4R) mRNAs in the arcuate nucleus (n=5) during long term malnutrition in female ovariectomized rats. Different letters indicate significant difference (P<0.05). C) Correlation of the relative expression of AgRP and MC4R mRNAs in the arcuate nucleus in female ovariectomized rats
Discussion
Results of the present study showed that long term malnutrition increased simultaneously expression of AgRP and MC4R mRNAs in the ARC of rats. The ARC contains the orexigenic NPY and AgRP neurons, and the POMC neurons from which the satiety factor α-MSH is derived (6). NPY and AgRP neurons are responsive to leptin and are involved in integration of the leptin signals with the neural circuits involved in energy homeostasis (6). Therefore, the ARC of hypothalamus during long term malnutrition can control energy homeostasis by alterations of AgRP and MC4R mRNAs in rats.
Consistent with our findings, fasting increased AgRP expression in rats (7). A 24 hr fast resulted in a 2.2-fold increased AgRP mRNA expression in the infundibular nucleus in Japanese quail in comparison with the ad-libitum-fed state (8). A 4-fold increase in the number of AgRP-ir cells in the hypothalamus tuberal region was reported in food-deprived doves and the amount of food consumed by food-deprived birds that were allowed to re-feed attenuated after 5 ng of the MC3R and MC4R agonist MTII (9). Moreover, the AgRP expression level in unfed fish was increased at 3 and 4 hr post-fasting than in fed fish but did not affect AgRP mRNA expression after 14 days fasting (10). Adrenalectomy decreased AgRP, neuropeptide gene expression in the medial basal of the hypothalamus despite of the drop in plasma leptin and insulin concentrations which in other situations would increase these neuropeptides (7). Furthermore, glucocorticoids are not required for fasting-induced upregulation of AgRP and NPY expression (7). During starvation, activated autophagy in neurons of hypothalamus mobilized neuron-intrinsic lipids to generate free fatty acids that increased AgRP levels (11). Mice lacking the expression of MC4R developed hyperphagia resulting in early-onset obesity (12). Food-restricted rats in the present study showed 6.2 fold increase of AgRP mRNA expression and together with previous study results showed the role of AgRP increase during short and long term malnutrition.
This increase of AGRP and MC4R mRNA expression during food restriction in female rat can be more important when it determines that the relationship between these two factors with reproductive hormones. Upregulated AMPK activity increases the AgRP expression in response to fasting and decreases POMC peptides in intrauterine fetal growth restricted newborn rats (13). Endogenous AgRP may be involved in luteinizing hormone (LH) and prolactin surges during starvation, showing further evidence that the melanocortin system is important for the surges of these hormonal in female rats (14). In addition, increase in expression of AgRP during diestrus in rat was reported, a stage of the cycle when estrogen levels are basal (5). Our previous finding, increase in mRNA expression of gonadotropin inhibitory hormone during long term malnutrition in female rats (15), along with the findings of this study and previous mentioned reports suggest that reproductive cycle cessation during malnutrition condition in females can be expected by increase in expression of AgRP and MC4R mRNA in rat.
Conclusion
Changes in the relative expression level of MC4R and AgRP mRNAs during long term malnutrition of rat indicated a stimulatory role of MC4R and AgRP in regulating energy balance in ARC of female ovariectomized rat hypothalamus.
Acknowledgment
This research was financially supported by the Infertility Research Center and Vice-Chancellor for Research, Shiraz University of Medical Sciences, Shiraz, Iran with the code number of 93-01-50-7698.
Footnotes
Financial disclosure
There is no financial interest.
References
- 1.Martin WJ, McGowan E, Cashen DE, Gantert LT, Drisko JE, Hom GJ, Nargund R, et al. Activation of melanocortin MC4 receptors increases erectile activity in rats ex copula. Eur J Pharmacol. 2002;454:71–79. doi: 10.1016/s0014-2999(02)02479-2. [DOI] [PubMed] [Google Scholar]
- 2.Williams G, Harrold JA, Cutler DJ. Proceedings-Nutrition Society of London: Cambridge Univ Press; 2000. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. [DOI] [PubMed] [Google Scholar]
- 3.Stofkova A, Skurlova M, Kiss A, Zelezna B, Zorad S, Jurcovicova J. Activation of hypothalamic NPY, AgRP, MC4R, AND IL-6 mRNA levels in young Lewis rats with early-life diet-induced obesity. Endocr Regul. 2009;43:99–106. [PubMed] [Google Scholar]
- 4.Légrádi G, Lechan RM. Agouti-related protein containing nerve terminals innervate thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 1999;140:3643–3652. doi: 10.1210/endo.140.8.6935. [DOI] [PubMed] [Google Scholar]
- 5.Zandi MR, Jafarzadeh Shirazi MR, Tamadon A, Akhlaghi A, Salehi MS, Niazi A, Moghadam A. Hypothalamic expression of melanocortin-4 receptor and agouti-related peptide mRNAs during the estrous cycle of rats. Int J Mol Cell Med. 2014;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbæk C, Flier JS, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 7.Savontaus E, Conwell IM, Wardlaw SL. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–138. doi: 10.1016/s0006-8993(02)03674-0. [DOI] [PubMed] [Google Scholar]
- 8.Phillips-Singh D, Li Q, Takeuchi S, Ohkubo T, Sharp PJ, Boswell T. Fasting differentially regulates expression of agouti-related peptide, pro-opiomelanocortin, prepro-orexin, and vasoactive intestinal polypeptide mRNAs in the hypothalamus of Japanese quail. Cell Tissue Res. 2003;313:217–225. doi: 10.1007/s00441-003-0755-8. [DOI] [PubMed] [Google Scholar]
- 9.Strader AD, Schiöth HB, Buntin JD. The role of the melanocortin system and the melanocortin-4 receptor in ring dove (Streptopelia risoria) feeding behavior. Brain Res. 2003;960:112–121. doi: 10.1016/s0006-8993(02)03799-x. [DOI] [PubMed] [Google Scholar]
- 10.Wei R, Yuan D, Wang T, Zhou C, Lin F, Chen H, Wu H, et al. Characterization, tissue distribution and regulation of agouti-related protein (AgRP) in a cyprinid fish (Schizothorax prenanti) Gene. 2013;527:193–200. doi: 10.1016/j.gene.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Singh R. Autophagy in the control of food intake. Adipocyte. 2012;1:75–79. doi: 10.4161/adip.18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui H, Lutter M. The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine. Genes Brain Behav. 2013;12:658–665. doi: 10.1111/gbb.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukami T, Sun X, Li T, Desai M, Ross MG. Mechanism of programmed obesity in intrauterine fetal growth restricted offspring: paradoxically enhanced appetite stimulation in fed and fasting states. Reprod Sci. 2012;19:423–430. doi: 10.1177/1933719111424448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schioth HB, Kakizaki Y, Kohsaka A, Suda T, Watanobe H. Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport. 2001;12:687–690. doi: 10.1097/00001756-200103260-00014. [DOI] [PubMed] [Google Scholar]
- 15.Jahanara M, Tamadon A, Jafarzadeh Shirazi MR, Rahmanifar F, Sabet Sarvestani F, Tanideh N, Moghadam A, et al. Long term malnutrition and mRNAs expression of RFRP-3 and KiSS-1 in hypothalamus of female ovariectomized rats. Physiol Pharmacol. 2013;17:370–378. [Google Scholar]


