Abstract
Objective(s):
Parkinson’s disease (PD) is known for motor impairments. But often, there are non-motor symptoms such as cognitive deficiency and pain misperception, owing to possible role of nigrostriatal pathway. Antioxidants have protective effect on free radical-induced neuronal damage in PD. To further address, we examined the effects of ellagic acid (EA) in a rat model of PD induced by 6-hydroxidopamine (6-OHDA).
Materials and Methods:
Right medial forebrain bundle (MFB) was lesioned by injecting 6-OHDA (16 µg/2 µl), in PD–animals. Sham operated animals received vehicle instead of 6-OHDA. PD was approved by apomorphine-induced contralateral rotation. EA (50 mg/kg/2 ml, PO, for 10 days) was administered to PD-EA group. Some PD-animals received pramipexole (PPX; 2 mg/kg/2 ml, PO) as a positive control group. Analgesia was measured by tail-flick and hot-plate tests. Passive avoidance task was measured by shuttle box apparatus to record the initial and step-through latency. Spatial cognition task was evaluated by Morris water maze test, measuring the escape latency time, path length, swimming speed and time spent in target quadrant.
Results:
MFB-lesioned rats showed hyperalgesic responses to the stimulus in tail-flick and hot-plate tests. Also they showed memory and learning deficit in cognitive tests. These effects reversed by EA treatment.
Conclusion:
6-OHDA can induce oxidative stress and can disrupt the neural mechanisms underlying proper integration of painful stimuli and cognitive processes in MFB-lesioned rats. Consequently, nigrostriatal pathway can play possible role in nociception and cognition. EA, a natural antioxidant, has neuroprotective effect on this pathway and can ameliorate this defect and be considered in PD management.
Keywords: 6-OHDA, Cognition, Ellagic acid, Pain, Parkinson’s disease, Rat
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects more than 1% of people over 65 years old (1). However, only 3% are less than 50 years old (2). Clinically, PD is recognized by resting tremor, rigidity, bradykinesia and postural instability (3). Although these motor symptoms are the most important criteria for diagnosis, non-motor symptoms such as sleep complaints, constipation, damaged olfaction and various neuropsychiatric appearances can become noticeable both before and during PD beginning and progress (4). Moreover cognitive deficiency is also common in PD. So as to, between 15% and 20% of PD patients suffer from dementia (5). Cognitive deficiencies in PD are considered ranging from minor disturbances in learning and memory to various intellectual functions and dementia. In this regard, memory is recognized as one of the basic cognitive functions (6). Also PD patients complain of painful sensations which have been described in five different types: musculoskeletal pain (because of parkinsonian rigidity, rheumatological illness or skeletal abnormality), radicular-neuropathic pain (owing to a root lesion, focal or peripheral neuropathy), dystonic pain (associated with antiparkinsonian medication), central neuropathic pain (related to antiparkinsonian medication) and akathisia (under off period or drug induced) (7). Among sensory symptoms in PD, pain is observed in nearly 30-50% of PD patients; nevertheless, the incidence can increase to 68-85% when all types of pain are taken into account (8).
Genetic and environmental elements are involved in the pathogenesis of PD. Usually, oxidative stress is a hallmark event where the oxidation of dopamine produces reactive oxygen species (ROS) and an unbalanced production of ROS makes neuronal damage, thus leading to neuronal loss. These free radicals react rapidly with membrane lipids and cause lipid peroxidation (LPO) and cell loss (9). Selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNc), projecting to the striatum, causes the reduction of dopamine levels in the striatum leading to motor and cognition impairments (10). Also, oxidative stress and reduction of hippocampal neurons may be involved in learning and memory impairments (11).
Until now, there is no effective therapy that can stop or at least slow down the neurodegeneration in this disease, so it is important to have a relevant animal model, in which new pharmacological agents and treatment strategies can be assessed before clinical trials are initiated (12). A rising attention has been involved to the complementary and alternative medicines including the plant-based medications, in recent years. Plants, similar to conventional medicines, are able to synthesize a wide variety of biologically active compounds which exert their effects through the interaction with biological pathways (13). Therefore, there has been abundant effort to develop beneficial agents from medicinal plants to achieve neuroprotection, and consideration has been paid on a wide variety of natural antioxidants that can scavenge free radicals and protect cells from oxidative damage (14). However, several experiments exhibited that many plant extracts have neuroprotective effect against 6-hydroxidopamine (6-OHDA) induced toxicity in PD animal models through anti-oxidative and anti-apoptotic activities (15).
In this regard, pomegranate (Punica granatum L.), is a polyphenolic fruit, which has been widely used in traditional medicine. Pomegranate juice extracts (PJE) protects neurons against the neurotoxic effects of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The likely mechanisms of this effect could be related to their phenolic constituents and antioxidant properties. Pomegranates enriched by phytochemicals for example polyphenols (comprising phenolic acids and flavonoids), have shown antioxidant activities and can inhibit inflammation and other damaging processes involved in neurodegenerative diseases. Furthermore, pericarp of pomegranate is much enriched with tannins such as gallic acid and ellagic acid, other potent antioxidants (16).
Ellagic acid (2,3,7,8-tetrahydroxybenzopyrano [5,4,3-cde]benzopyran-5-10-dione) is an excretion product of many plant species, particularly fruits and nuts (17). It is found in strawberries, cranberries, walnuts, pecans, and red raspberry seeds (18). Animals with lesions of the dopaminergic system may be valued for studying of the pain and cognition properties as an animal model of Parkinson’s disease. Therefore, according to the role of nigrostriatal pathway in the pain perception and cognition beyond the anti-oxidative properties of EA, the aim of this study was to investigate the effects of EA on pain perception and the learning and memory in 6-OHDA induced rat model of Parkinson’s disease.
Materials and Methods
Drugs
6-hydroxidopamine (6-OHDA), desipramine, apomorphine, ellagic acid (EA) and pramipexole (PPX) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ketamine/xylazine were obtained from Alfasan Co. (Woerden, Holland).
Animals
Adult male Wistar rats (250-300 g) obtained from Ahvaz Jundishapur University of Medical Sciences (AJUMS) central animal Lab (Ahvaz, Iran) were used in this study. Rats were kept in the same room under a constant temperature (22±2 ºc) and humidity (55-60%) on a 12:12-hr light-dark cycle with food pellets and water, ad libitum.
Experimental design
All experiments were conducted during the light phase of the cycle (between 8:00 am and 5:00 pm) and were directed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with approval from the AJUMS Animal Care and Use Committee (AJACUC). We tried to minimize animals stress and reduce the number of rats used in this study. All animals were handled for 5 days (daily 5 min) before beginning of behavioral tests. The animals were divided randomly into five groups (n=8):
-
1)
Sham group, received vehicle of 6-OHDA (2 µl normal saline containing 0.01% ascorbic acid) into right MFB.
-
2)
Sham-EA group, sham operated animals, received EA.
-
3)
PD+veh group, MFB-lesioned rats, received 6-OHDA into right MFB + vehicle of EA (normal saline containing DMSO)
-
4)
PD-PPX group (Positive control), MFB-lesioned rats, received PPX (2 mg/kg/2 ml, PO, for 10 consecutive days).
-
5)
PD-EA group, MFB-lesioned rats, received EA.
In groups 2 and 5, EA (50 mg/kg, PO, for 10 consecutive days) was administered 14 days after stereotaxic surgery (19).
Medial forebrain bundle lesion
Right MFB was lesioned in a rat model of PD by Tadaiesky’s (2008) method with some modifications (20). First, rats were deeply anesthetized with combination of ketamine/xylazine (90/10 mg/kg, IP). Then, rats were placed in a stereotaxic apparatus (Narishige, Japan). 6-OHDA (16 µg/2 µl normal saline containing 0.01% ascorbic acid) or its vehicle (in sham groups) was infused into right MFB using a 5 µl Hamilton syringe according to the coordinates in Paxinos and Watson atlas: AP: -4.4 mm, ML: +1.3 mm from bregma and DV: -8.4 mm from skull surface (21). Following injection, the cannula was left in place for 5 min before being retracted to allow complete diffusion of the drug. All animals were treated with injection of desipramine (25 mg/kg IP), 30 min before surgery, in order to protect noradrenergic terminals depletion by 6-OHDA. Sham-operated rats underwent the same protocol but vehicle was injected instead of 6-OHDA (22, 23).
Apomorphine induced circling behavior
All the animals were tested for rotational behavior 2 week after MFB lesioning (before treatment) and 4 week after lesioning (after treatment). Contralateral rotations of each animal were recorded after subcutaneously injection of apomorphine (0.5 mg/kg in normal saline containing 0.01% ascorbic acid) to confirm the dopamine depletion in nigrostriatal system (24, 25). The rotational behavior was counted over a period of 60 min. The results were expressed in rotations/30 min.
Tail-flick test
The tail-flick (tail withdrawal) latency was detected by standard tail-flick test (26). Rats were located on the analgesia meter apparatus (M.T 9500, Borj Sanat Co. Tehran-Iran), equipped by a radiant infrared heat source focused on 3 different parts of the tail 4 to 7 cm distal end (with 3 min intervals) and mean was selected as latency. When the animal flicked its tail, a photocell was activated and the time between activation of the heat source and tail flick latency was recorded as a pain sensitivity index. The stimulus intensity was adjusted to a baseline tail flick latency of 3 to 4 sec; a cut-off time of 10 sec was used to avoid tissue injury. One day before the test, animals were allowed to acclimatize the apparatus, for two min (27).
Hot-plate test
The hot-plate apparatus (MH 9500, Borj Sanat Co. Tehran, Iran), involved a 25 × 25 cm Plexiglas cage fitted over the hot plate and a foot switch timer. Pain thresholds were measured by latency time to nociceptive responses (hind paw licking or jumping out of the beaker), expressed as hot plate analgesic index. One day before experiment, animals were allowed to acclimatize the apparatus, for two minutes. Rats were individually placed on the center of a metal hot plate, heated to a mean temperature of 52 ± 0.2°C and the reaction time was recorded. The cut-off time was 40 sec to avoid tissue damage (27). After each trial the plate was cleaned to minimize lingering olfactory cues. Feces or urine were first removed and the central platform was cleaned with ethanol and the apparatus was further allowed to air dry for about two min before another test.
Passive avoidance task
The two-way shuttle box instrument (ST-5500, Borj Sanat Co. Tehran-Iran), was used for this test, which was composed of two adjacent Plexiglas boxes of identical sizes (27 cm × 14.5 cm × 14 cm) with plexus floors. The floor of the two compartments has been covered with stainless steel bars (2 mm diameter) spaced 1 cm apart. The bright compartment was lightened by a 5 W lamp attached on its wall just under a mobile transparent Plexiglas ceiling. Tamburella’s method with little modification was used for the passive avoidance memory test (28).
This procedure was performed in three days, at the first day rats were adapted to the acquisition compartment. Each rat was allowed a 10 min adaptation period with free access to either the light or dark compartment of the avoidance training box after being placed in a shuttle box (to make familiar with tools). At the second day, rats were placed into the illuminated compartment and 10 sec later the descending door was elevated, and latency of step-down was recorded as learning phase (initial latency). After entrance the dark compartment, the door was closed and when all four paws touched the grid, a low level electric shock (0.3 mA, 3 sec.) was applied. After 3 min, the rat was removed from the dark compartment and placed into the home cage. On day 3, in order to test short-term memory, the rats were placed in the illuminated chamber again, and 10 sec later the sliding door was raised, and latency of entering the dark compartment was recorded again, as step-through latency (STL). The cut-off time was 300 sec and no shock was delivered in this day (29).
Morris water maze (MWM) test
This test was performed to evaluate the spatial memory, and apparatus (Maze Router V3.1, Techniq Azma Co, Tabriz-Iran) consisted of a dark circular pool (120 cm diameter ×60 cm height) filled with tap water (27±2°C) with a depth 60 cm that was not opaque was used. The pool divided geographically into four equal size quadrants and release points designed in each quadrant as north (N), east (E), south (S) and west (W). A hidden circular escape platform (12cm in diameter) was emerged 2 cm under the water surface and was placed in the center of the northeast quadrant. Some fixed visual cues such as computer, desk, posters and illumination lights placed on the walls around the pool. A camera located above the center of the pool that connected to a computer to record the rat movements. An automated monitoring system (Radiab ver. 2, Tehran, Iran) used to measure the escape latency, swimming distance and speed (30).
One day before the beginning of training procedure, rats were adapted to the pool by allowing them to perform a 60 sec swimming without the platform. Training procedure involved 4 trials daily for four consecutive days. In each trial, the rats released facing the side wall at one of four quadrants and were allowed 60 sec to find the platform; and release positions were randomly arranged. If they did not find the platform within 60 sec, they were gently guided to the platform. They were allowed to remain there for 30 sec. After the completion of a trial, animals were returned to a holding cage for an inter-trial interval of 60 sec. The time to reach the platform (latency), speed and the length of swim path were recorded by a video tracking system. At 5th day as a probe trial, platform was removed and the rats were released from southwest (consisted of a 60 sec free swimming time) and the time spent in the target quadrant was recorded (30).
Statistics
The results were expressed as mean±SEM. The statistical analysis was performed by SPSS version 22, and one-way ANOVA followed by Tukey’s post hoc test for inter groups comparisons. Repeated measures ANOVA was used for analyzing the data of Morris water maze test at days 1 to 4, followed by Tukey’s post hoc test. A P-value less than 0.05 was considered as a significant difference.
Results
Apomorphine induced circling behavior
As shown in Figure 1, two weeks after surgery (before beginning of treatments), apomorphine induced contralateral turns were increased significantly in 6-OHDA lesioned groups (PD+veh, PD+PPX and PD+EA groups) when compared to sham group (***P<0.001). Four weeks after surgery (after treatments), the number of contralateral turns observed in treated groups (PD+PPX and PD+EA groups) decreased significantly as compared with PD+veh group (+++P<0.001).
Figure 1.
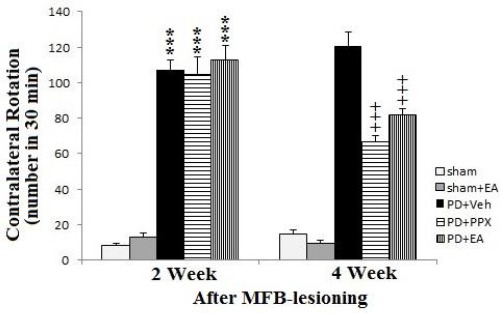
Apomorphine induced circling behavior. The results were expressed as mean ± SEM (n=8). Data analyzed by one-way ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. ***P<0.001 vs. sham group after 2 weeks post-surgery, +++P<0.001 vs. PD+veh group after 4 weeks post-surgery
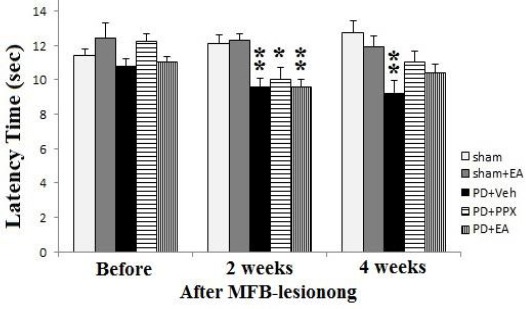
Hot-plate test
As shown in Figure 2, before MFB-lesioning, there was no significant difference between groups. But two weeks after lesion, there was a significant decrease in withdrawal latency of PD+veh group as compared with sham group (**P<0.01). Also, there was a significant decrease in withdrawal latency of PD+EA and PD+PPX groups versus sham group (**P<0.01 and * P<0.05, respectively). Whereas, four weeks after lesion, animals underwent treatment returned to before lesion values, in addition lesioned rats which were treated with EA and PPX had no significant difference with sham-operated animals, and only lesioned rats received vehicle significantly differ from sham group (**P<0.01).
Figure 2.
Hot-plate test. The results were expressed as mean ± SEM (n=8). Data analyzed by one-way ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. *P<0.05 and **P<0.01 vs. sham group after 2 and 4 weeks post-surgery (respectively)
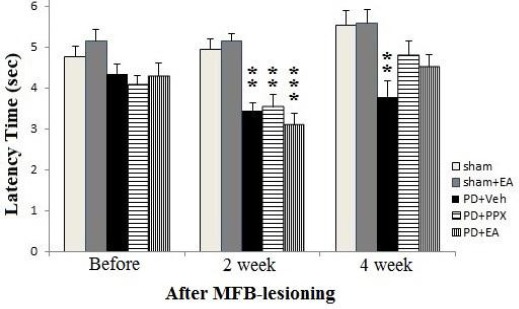
Tail-flick test
According to Figure 3, before MFB lesioning there was no significant difference between groups. But two weeks after lesion, there was a significant decrease in withdrawal latency of PD+veh group versus sham group (**P<0.01). Also, there was a significant decrease in withdrawal latency of PD+EA and PD+PPX groups versus sham group (***P<0.001 and ** P<0.01, respectively). Whereas, four weeks after lesion, treated animals returned to before lesion values, in addition lesioned rats which received EA and PPX had no significant difference with sham-operated animals and only lesioned rats received vehicle significantly differ from sham group (**P<0.01).
Figure 3.
Tail-flick test. The results were expressed as mean ± SEM (n=8). Data analyzed by one-way ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. **P<0.01 vs. sham group after 2 and 4 weeks post-surgery
Passive avoidance test
As shown in Figure 4, in PD+veh group, the initial latency that takes rats going away from light to dark compartment of shuttle box (before exposing to electrical shock) was increased significantly compared sham group (*P<0.05). Treatment with EA and PPX closed initial latency to the sham values, so there was no significant difference between treated and sham groups. Step-through latency was decreased significantly in PD+veh group versus sham operated rats (*P<0.05). Treatment of MFB-lesioned groups with EA and PPX closed the step-through latency to the sham values, and there was no significant difference between treated and sham groups. So, STL in treated groups has increased, but it is lower than control levels.
Figure 4.
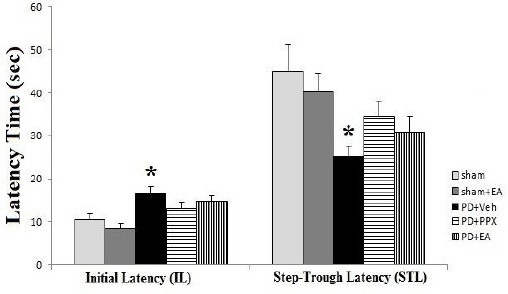
Passive avoidance test. The results were expressed as mean ± SEM (n=8). Data analyzed by one-way ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. *P<0.05 vs. sham group
The spatial cognition test (MWM test)
Escape latency time; as shown in Figure 5, the latency time to find and locate on hidden platform between the non lesioned groups (sham and sham+EA) do not indicate any differences during 4 days in water maze test (P>0.05). On the other hand, there was significant difference (*P<0.05) between the sham and lesioned groups during all of the 4 training days. Over the time, this difference was enhanced and latency time of sham group was showed more decrease, compared to PD+veh group. At 1st to 3rd days, there was no significant difference between PD+PPX and PD+EA groups versus PD+veh group, but at 4th day, the latency time in PD+PPX and PD+EA groups was decreased significantly compared to PD+veh group (respectively P<0.001, P<0.01).
Figure 5.
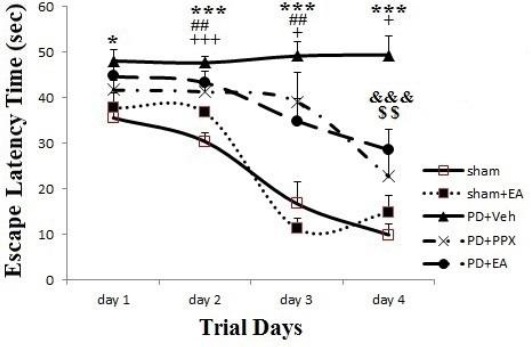
Escape latency time. The results were expressed as mean±SEM (n=8). Data analyzed by repeated measure ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. The difference between PD+veh, PD+PPX and PD+EA groups versus sham group showed by *, #, + symbols (respectively). The difference between PD+PPX and PD+EA groups versus PD+veh group showed by &, $ symbols (respectively)
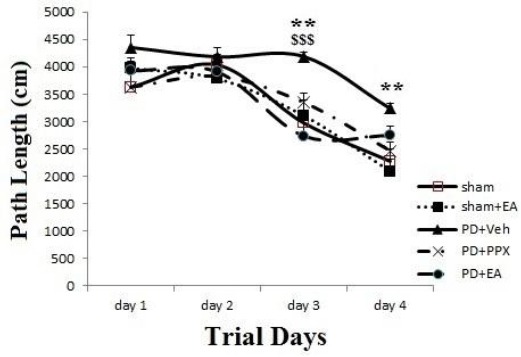
Path length; as shown in Figure 6, path length to find the platform was decreased across days in all groups, but there was differences between groups; and at 3rd and 4th days, path length in PD+veh group was significantly greater than sham group (**P<0.01). While, at 3rd day it was decreased in PD+EA group significantly versus PD+veh group ($$$P<0.001).
Figure 6.
Path length. The results were expressed as mean±SEM (n=8). Data analyzed by repeated measure ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. The difference between PD+veh group versus sham group showed by *symbol. The difference between PD+PPX and PD+EA groups versus PD+veh group showed by &, $ symbols (respectively)
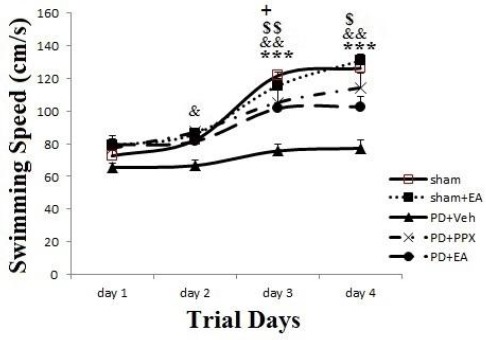
Swimming speed; as presented in Figure 7, the swimming speed to find and locate on hidden platform between the sham and sham+EA groups indicated no significant differences during 4 days (P>0.05). Also, there were no significant differences between groups at 1st day. On the other hand, at 3rd and 4th days, results showed a significant increase in sham group swimming speed compare to PD+veh group (***P<0.001); moreover, in PD+PPX group it was increased significantly versus PD+veh group (&&P<0.01). Similarly at 3rd and 4th days, in PD+EA group it was increased significantly compare to PD+veh group (respectively $$ P<0.01, $ P<0.05).
Figure 7.
Swimming speed. The results were expressed as mean±SEM (n=8). Data analyzed by repeated measure ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. The difference between PD+veh group versus sham group showed by *symbol. The difference between PD+PPX and PD+EA groups versus PD+veh group showed by &, $ symbols (respectively)
Time percent spent in target quadrant (probe trial); as displayed in Figure 8, at 5th day during probe trial while escape platform removed, the percent of time spent in goal quarter evaluated. There was no significant difference between sham, sham+EA, PD+PPX, and PD+EA groups, but it decreased significantly in PD+veh group compared to sham operated animals (**P<0.01). Also it was increased significantly in PD+PPX group in comparison with PD+veh group (&P<0.05).
Figure 8.
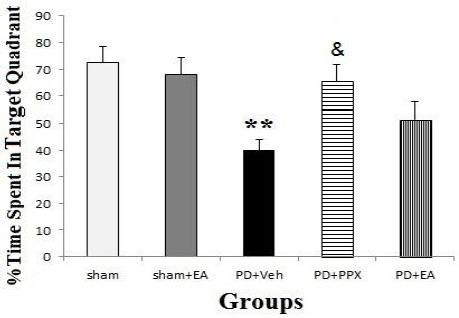
Time percent spent in target quadrant. The results were expressed as mean±SEM (n=8). Data analyzed by one-way ANOVA followed by Tukey’s post hoc test for among multiple groups comparison. **P <0.01 in PD+veh group vs. sham groups, &P <0.05 in PD+PPX group vs. PD+veh group
Discussion
Our results showed that unilateral injection of 6-OHDA to right MFB significantly increased apomorphine induced contralateral rotations. Whereas, after treatments, these contralateral turns decreased significantly to sham values (Figure 1). Therefore, it can be concluded that 6-OHDA has impaired the dopaminergic system and an animal model of PD was induced. Because apomorphine induced rotational behavior is used to confirm the occurrence of PD (24, 25). Moreover, administration of the ellagic acid to lesioned rats has diminished the 6-OHDA induced impairment of the dopaminergic system that can be considered as an improving effect.
In addition, we showed that unilateral lesion to right MFB significantly reduced the pain sensation threshold in both hot plate (Figure 2) and tail flick (Figure 3) tests and induced hyperalgesia which corresponds with other’s studies. For example; bilateral 6-OHDA lesions decreased the latency to the hind paw lick in the hot plate test (31) and enhanced sensitivity to a wide range of thermal and mechanical painful stimuli (32). Hence, despite the major roles of basal ganglia in controlling voluntary movements, neurophysiological, clinical and behavioral data designate that it is involved in central pain processing (33). Although pain is one of the major clinical signs of PD, it is often neglected. Thus, changes in the experience of pain as a result of cognitive impairment are addressed by this study. We treated unilateral 6-OHDA lesioned rats with ellagic acid to evaluate the possible effect of ellagic acid on pain misperception in animal model of PD. As shown in results, ellagic acid improved 6-OHDA induced hyperalgesia such a way that enhanced the pain sensation threshold in both hot plate (Figure 2) and tail flick (Figure 3) tests, compared to PD+veh group. Whereas, these evidences indicate that dopamine depletion in the mesostriatal system has an influence on withdrawal response to the mechanical stimulus, and dopamine may be involved in pain sensation. The neural bases underlying pain misperception in PD are incompletely understood.
Beside the motor disturbances and pain misperception, the cognitive impairments such as learning and memory deficits are common in PD and the idea that a degenerated nigrostriatal dopaminergic system plays a role in cognitive deficiencies in PD has been further confirmed by studies from other researches using animal models of PD. These patients complain executive dysfunction and deficiencies of working memory which resemble that created by frontal lobe injury (34). In this regard, our results showed that unilateral 6-OHDA lesioned rats experienced deficits in memory and learning that significantly compensated by ellagic acid treating.
In passive avoidance test (Figure 4), the initial latency (as a learning criterion) in PD+veh group was increased significantly compared to sham groups that EA and PPX returned it to a level in control. Means, despite the fact that rats are darkness interested animals, PD rats delayed entering to dark chamber of shuttle box; consequently it may be due to impaired learning that improved by EA and PPX administration. Moreover, step-through latency (as a short term memory criterion) was decreased significantly in PD+veh group compared to sham groups, while expected because of shock memory they delay to entering the dark chamber. Indicating, PD animals lost their memory. Mutually, treatment improved the memory, as well there was no significant difference between treated and sham groups.
In MWM test, the escape latency time (Figure 5) showed progressive decrease in sham groups compared to PD+veh group, during all of the days. On the other hand, the latency time in PD+PPX and PD+EA groups were decreased significantly compared to PD+veh group at 3rd and 4th days. Thus, MFB lesioning prevented from reducing of latency over the time, suggesting that there is a learning and memory deficiency in PD rats, which is in accordance with previous evidences. For example, injection of 6-OHDA to SNc produced alterations in cued and spatial memory versions of water maze, reflecting memory deficits similar to PD’s early phase (35). Also, ellagic acid closed latency time in MFB-lesioned animals to control values that can be considered as a learning and memory improvement criterion.
Path length (Figure 6) in PD+veh group was significantly greater than sham group, at 3rd and 4th days. But, there was no difference between treated and sham operated rats. Also at 3rd day in PD+EA group, it was decreased significantly compared to PD+veh group. Hence, MFB-lesioning has caused a significant increase in distance traveling to reach the platform, and EA has closed it to control values. So, it can be suggested that there is learning and memory deficiency in MFB-lesioned rats that improved by ellagic acid.
Swimming speed (Figure 7) at 3rd and 4th days significantly increased in sham, PD+PPX and PD+EA groups compared to PD+veh group. Taken together, PD animals had learning impairments that ellagic acid treatment improved it.
In MWM test, during probe trial (Figure 8) at 5th day, time percent spent in target quadrant not significantly differ between sham, sham+EA, PD+PPX, and PD+EA groups, but it decreased significantly in PD+veh group compared to sham groups indicating that 6-OHDA induced MFB lesion, caused memory deficit that improved by ellagic acid administration. Similarly, studies have shown both procedural and working memory impairments in PD rats, proposing as well the involvement of the prefrontal cortex–basal ganglia loop in the observed failures (36). Since both structural and functional anomalies of this area had been observed in PD patients, the hippocampus is involved in memory insufficiencies observed in PD (37).
On the other hand, maybe there is a relationship between pain misperception and cognition deficiencies in PD. So that, several studies attempted to better reveal the association amongst chronic pain and cognition. These studies provided evidence for lower cognitive performance in chronic pain patients on tests of memory, attention, speed and executive functions (38). Moreover, many studies reported the inverse relationship between pain strength and cognitive performance. Therefore, the presence of pain contests with the attentional resources causes decreased cognitive performance as the level of pain increases (39).
Several studies suggesting that the reaction of 6-OHDA with oxygen leads to the production of superoxide anion radical, H2O2 and hydroxyl radical, which can generate ROS, and eventually oxidative stress damages dopaminergic neurons and induces cell degeneration through apoptosis (40). Because of possible susceptibility of Substantia Nigra neurons to oxidants, the oxidative metabolism of DA can generate cytotoxic free radicals. So, 6-OHDA can imitate human pathological condition of PD and induce striatal DA depletion (41). Polyphenolic compounds such as EA have antioxidant properties and can prevent damaging processes involved in neurodegenerative diseases (16). Therefore, the results of this study suggested that EA can be considered as a new approach in management of cognitive and nociceptive deficiencies in PD and also it is valuable to further investigations.
Conclusion
This study showed that, 6-OHDA can impair the nigrostriatal dopaminergic pathway and cause animal model of Parkinson’s disease that accompanied with pain misperception and cognitive deficiencies. Ellagic acid can significantly improve this pain misperception and cognitive deficiencies in 6-OHDA induced rat model of Parkinson’s disease. Maybe, ellagic acid as a natural antioxidant can play neuroprotective role and improve the noxious oxidative effects of 6-OHDA in nigrostriatal pathway and recover the pain misperception and learning and memory parameters. Consequently, perhaps ellagic acid can be considered as a valuable natural component in Parkinson’s disease management.
Acknowledgment
This work was financially supported by grant: PRC-134, from Vice-Chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences (Iran). The results described in this paper were part of PhD thesis for Mojtaba Dolatshahi.
References
- 1.Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. 2012;8:435–442. doi: 10.1038/nrneurol.2012.126. [DOI] [PubMed] [Google Scholar]
- 2.Hawkes CH. Parkinson's disease and aging: same or different process? Mov Disord. 2008;23:47–53. doi: 10.1002/mds.21766. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson's disease. CNS Drugs. 2007;21:677–692. doi: 10.2165/00023210-200721080-00005. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 5.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgiev D, Danieli A, Ocepek L, Novak D, ZupancicKriznar N, Trost M, et al. Othello syndrome in patients with Parkinson's disease. Psychiatr Danub. 2010;22:94–98. [PubMed] [Google Scholar]
- 7.Ford B. Pain in Parkinson's disease. Clin Neurosci. 1998;5:63–72. [PubMed] [Google Scholar]
- 8.Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: prevalence and characteristics. Pain. 2009;141:173e7. doi: 10.1016/j.pain.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Ag. 2002;23:719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 10.Lu Z, Nie G, Belton PS, Tang H, Zhao B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int. 2006;48:263–274. doi: 10.1016/j.neuint.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi Sh, Khosravi V, Rahman B, et al. Crocin Improved Learning and Memory Impairments in Streptozotocin-Induced Diabetic Rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- 12.Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- 13.Hassanzadeh P, Arbabi E, Rostami F. The ameliorative effects of sesamol against seizures, cognitive impairment and oxidative stress in the experimental model of epilepsy. Iran J Basic Med Sci. 2014;17:100–107. [PMC free article] [PubMed] [Google Scholar]
- 14.Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol againstb-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang DS, Kim HG, Kwon HJ, Cho JH, Lee CH, Lee JM, et al. Dangguijakyak-san, a medicinal herbal formula, protects dopaminergic neurons from 6-hydroxydopamine-induced neurotoxicity. J Ethnopharmacol. 2011;133:934–939. doi: 10.1016/j.jep.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Braidy N, Selvaraju S, Essa MM, Vaishnav R, Al-Adawi S, Al-Asmi A, et al. Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid Med Cell Longev. 2013;2013:1–12. doi: 10.1155/2013/685909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada L, Ou B. Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem. 2002;50:3495–3500. doi: 10.1021/jf011405l. [DOI] [PubMed] [Google Scholar]
- 18.Whitley AC, Stoner GD, Darby MV, Walle T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid-extensive binding to protein and DNA. Biochem Pharmacol. 2003;66:907–915. doi: 10.1016/s0006-2952(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 19.Uzar E, Alp H, Ugur Cevik M, Fırat U, Evliyaoglu O, Tufek A, et al. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 2012;33:567–574. doi: 10.1007/s10072-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 20.Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da Cunha C, Takahashi RN. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson's disease. Neuroscience. 2008;156:830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. Australia: Academic Press; 2006. The rat brain in stereotaxic coordinates. [Google Scholar]
- 22.Sarkaki A, Norooz Zare F, Farbood Y, Pileverian AA. Impaired movements in 6-OHDA induced Parkinson's rat model improves by pomegranate seed hydroalcoholic extract. Health Med. 2013;7(2):348–358. [Google Scholar]
- 23.Sharifi H, Mohajjel Nayebi A, Farajnia S. 8-OH-DPAT (5-HT1A agonist) attenuates 6-hydroxydopamine-induced catalepsy and modulates inflammatory cytokines in rats. Iran J Basic Med Sci. 2013;16:1270–1275. [PMC free article] [PubMed] [Google Scholar]
- 24.Rizelio V, Szawka RE, Xavier LL, Achaval M, Rigon P, Saur L, et al. Lesion of the subthalamic nucleus reverses motor deficits but not death of nigrostriatal dopaminergic neurons in a rat 6-hydroxydopamine-lesion model of Parkinson's disease. Braz J Med Biol Res. 2010;43:85–95. doi: 10.1590/s0100-879x2009007500020. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler MG, Szechtman H. Relation between motor asymmetry and direction of rotational behaviour under amphetamine and apomorphine in rats with unilateral degeneration of the nigrostriatal dopamine system. Behav Brain Res. 1990;39:123–133. doi: 10.1016/0166-4328(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 26.D’Amour FE, Smith DL. A method of determining loss of pain sensation. J Pharmacol Exp Ther. 1941;27:74–79. [Google Scholar]
- 27.Dolatshahi-somehsofla M, Esmaeili-Mahani S, Motamedi F, Haeri A, Ahmadian A. Adrenalectomy potentiates the antinociceptive effects of calcium channel blockers. Pharmacol Biochem Behav. 2009;92:327–334. doi: 10.1016/j.pbb.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 29.Mansouri MT, Farbood Y, Sameri MJ, Sarkaki A, Naghizadeh B, Rafeirad M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013;138:1028–1033. doi: 10.1016/j.foodchem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Doulah AH, Rohani AH, Khaksari Haddad M, Motamedi F, Farbood Y, Badavi M, et al. The effect of peripheral administration of growth hormone on AD-like cognitive deficiency in NBM-lesioned rats. Neurosci Lett. 2009;466:47–51. doi: 10.1016/j.neulet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Lin MT, Wu JJ, Chandra A, Tsay BL. Activation of striatal dopamine receptors induces pain inhibition in rats. J Neural Transm. 1981;51(3-4):213–22. doi: 10.1007/BF01248953. [DOI] [PubMed] [Google Scholar]
- 32.Takeda R, Ikeda T, Tsuda F, Abe H, Hashiguchi H, Ishida Y, et al. Unilateral lesions of mesostriatal dopaminergic pathway alter the withdrawal response of the rat hindpaw to mechanical stimulation. Neurosci Res. 2005;52:31–36. doi: 10.1016/j.neures.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson's disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 35.Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDAand MPTP-lesioned rats as models of the early phase of Parkinson's disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods. 2005;148:78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Reksidler AB, Lima MM, Zanata SM, Machado HB, da Cunha C, Andreatini R, et al. The COX-2 inhibitor parecoxib produces neuroprotective effects in MPTP-lesioned rats. Eur J Pharmacol. 2007;560:163–175. doi: 10.1016/j.ejphar.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Joelving FC, Billeskov R, Christensen JR, West M, Pakkenberg B. Hippocampal neuron and glial cell numbers in Parkinson's disease: a stereological study. Hippocampus. 2006;16:826–833. doi: 10.1002/hipo.20212. [DOI] [PubMed] [Google Scholar]
- 38.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol. 2011;l93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Grisart JM, Plaghki LH. Impaired selective attention in chronic pain patients. Eur J Pain. 1999;3:325–333. doi: 10.1053/eujp.1999.0138. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka K, Ogawa N, Asanumab M. Molecular basis of 6-hydroxydopamine-induced caspase activations due to increases in oxidative stress in the mouse striatum. Neurosci Lett. 2006;410:85–89. doi: 10.1016/j.neulet.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Metz GA, Tse A, Ballermann M, Smith LK, Fouad K. The unilateral 6-OHDA rat model of Parkinson's disease revisited: an electromyographic and behavioural analysis. Eur J Neurosci. 2005;22:735–744. doi: 10.1111/j.1460-9568.2005.04238.x. [DOI] [PubMed] [Google Scholar]


