Abstract
Objective(s):
Fumonisins are a group of toxic and carcinogenic mycotoxins, which contaminate the grains and their products. The aim of this study was to examine the apoptotic and proliferative activity of mouse gastric mucosa following administration of fumonisin B1 (FB1).
Materials and Methods:
Twenty-nine female mice divided into treatment (n=15) and control (n=14) groups. The treatment group received FB1 (150 mg/kg diet) for 16 weeks. The gastric atrophy was allocated using grading criteria modeled on the updated Sydney System. Immunohistochemistry studies were performed for evaluation of apoptosis and proliferative activity in gastric mucosa.
Results:
Mild to moderate gastric atrophy were observed in microscopic findings of the gastric mucosa in treated animals (P<0.05). Number of parietal cells significantly decreased in the treatment group in comparison with the control (P<0.05). Treatment with FB1 for 16 weeks significantly reduced both gastric mucosa height and mitotic index in the gastric glands (P<0.05). TUNEL- and Bax-labeled positive cell numbers significantly increased in the FB1-treated group compared to the control (P<0.05). In addition, proliferative activity of gastric glands in the treated group was significantly lower than the control (P<0.05).
Conclusion:
Oral administration of FB1 caused atrophy in gastric mucosa both via increasing of apoptosis and suppressing the mitotic activity of these cells.
Keywords: Apoptosis, Fumonisin B1, Gastric atrophy, Mice, Proliferative activity
Introduction
Fumonisin which belongs to the mycotoxins family was initially derived from the culture medium of Fusarium verticillioides in South Africa in 1988 (1). Fumonisin B1 (FB1), B2 and B3 are the most abundant types of fumonisins among which the B1 type can be found in maize, rice, sorghum, wheat and soybean (2). Fumonisins are absorbed through digestive system and affect organs at cellular level (3). The biological effects of fumonisins have been studied in the past 20 years and have shown their toxic and carcinogenic side effects (4, 5). The mechanism(s) of fumonisin toxicity is complex. Their most important role is contribution to sphingolipid synthesis, sphinganine and sphingosine aggregation and thus causing defective membrane duties (3). They have also other cellular effects such as cellular growth regulation, differentiation, apoptosis and cell morphology changes (3, 6). On the other hand, repeated accumulation of free cellular sphingoid bases acts as cancer promoter and mutative factor (3). A correlation between fumonisin contaminated maize (>100 mg/kg diet) and the esophageal cancer has been found (7). In vitro, fumonisins (1 to 10 μg) may activate apoptosis, inhibit proliferation and increase inflammatory cytokine production (8, 9). In vivo studies have also shown that fumonisins can cause intestinal villi atrophy and absorption disorder (10). Administration of FB1 in a dose of 8 to 10 mg/kg in pig’s diet, changes cytokine profiles and reduces special antibody responses (10, 11). In addition, long-term consumption of fumonisin may also change intestinal cell morphology such as goblet cell reduction, cause atrophy and decrease mucin secretion (12).
Previous studies have shown a strong correlation between gastric atrophy, and incidence of the gastric and esophageal cancers (13-16). Epidemiologic studies in some areas of South Africa, Japan, China and Iran have shown the high incidence of the esophageal cancer with the FB1 contaminated maize high consumption compared to low risk regions of esophageal cancer that had a lower intake of the FB1 contaminated maize (13, 17-20). Previous ecological studies similarly suggested positive correlation between fumonisin contamination of cereals and the risk of cancers (17, 21). Some epidemiological studies have also shown a correlation between consumption of FB1 contaminated foods and the risk of esophageal cancer (7, 18). Due to high incidence of esophageal cancer in developing countries including Iran, and the existence of toxic fumonisin in their grains (20), this hypothesis supports that fumonisin can act as a carcinogenic factor in high incidence rate of gastric and esophageal cancer in developing countries. Thus, the objective of the present study is to detect apoptotic and proliferative activity of mouse gastric mucosa following administration of FB1 through histo-pathological and immunohistochemical methods.
Materials and Methods
Food preparation
Fumonisin B1 (a gift by prof Khosravi AR from Tehran University, Iran) was mixed with rat chow purchased from Pars Animal Feed Co Iran. First, the animal food was completely ground (SK 100 comfort Gubelisen, Retsch, Germany). FB1 was mixed with ground pellet for 30 min and then tap water was added to the mixture. The prepared dough was then shaped into strips and remade into pellet in a special pellet-making device. The pellets were finally put for 24 to 72 hr into an oven set at 75°C to make it dry and ready to use.
Animals
Female mice (25 to 30 g) were maintained in animal quarters under standardized conditions with a 12 hr light/dark cycle, 20 to 22°C ambient temperature and 40 to 50% humidity with free access to rat chow and water. All experimental procedures were performed according to the guidelines of the Animal and Human Ethical Committee of Tehran University of Medical Sciences.
The study design
Twenty-nine female mice divided into the treatment (N=15) and control (N=14) groups. Animals in the treatment group received FB1 (150 mg/kg diet) for 16 weeks (22). Mice were weekly weighed and euthanized at the end of 16th weeks. They were dissected and their stomachs were removed. In all animals, the body of stomach was sampled, fixed in 10% formaldehyde, passaged and embedded in paraffin. Apoptotic changes were evaluated by immunohistochemistry using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), Bcl–2 and Bax staining. For evaluation of proliferative activity of gastric mucosa Ki-67 staining were used.
Histopathological study
Gastric atrophy was allocated using grading criteria modeled on the updated Sydney System (23, 24) as follows: (0) No parietal cell loss; (I) Mild parietal cell loss, up to 25%; (II) Moderate parietal cell loss, 25 to 75% and (III) Severe parietal cell loss, more than 75%. Gastric glandular and mucosal heights were also measured using graticule linear method as previously described (25).
Immunohistochemical assay
Briefly, the sections were treated with 3% (v/v) H2O2 at room temperature, blocked with 10% (v/v) goat or rabbit serum (Nichirei, Tokyo, Japan), and incubated with a rabbit monoclonal antibody against Ki-67 (Thermo Fisher Scientific Inc., San Jose, CA) diluted 1:200. Then, the sections were incubated with biotinylated goat anti-rabbit IgG (Nichirei). The sections were incubated with a solution of streptavidin-conjugated horseradish peroxidase (Nichirei) according to the manufacturer’s recommen-dations. The sections were counterstained with hematoxylin before dehydration and mounting (26). In each animal, mitotic indexes were measured in 10 to 15 gastric glands in transverse sections. At first, the Ki-67 positive cells were counted and divided into Ki-67 positive and non-positive cells. The obtained figure was then multiplied by 100 and reported.
In situ nick end-labeling assay
In situ detection of cells with DNA strand breaks was performed in formalin-fixed, paraffin-embedded tissue sections by TUNEL according to the manufacturer’s instructions (Roche, Germany). Paraffin sections of 5 μm thick were deparaffinized, and endogenous peroxidase was blocked by immersing the sections in phosphate-buffered saline (PBS) containing 0.5% H2O2 for 15 min. For proteins nuclei stripping, tissue sections were incubated with proteinase K (Roche, Germany) (20 μg/ml) for 10 min. The sections were incubated with a mixture of terminal deoxynucleotidyl transferase (TdT) and reaction buffer containing digoxigenin-labeled dUTP. Hematoxylin staining was performed followed by incubation with anti-digoxigenin antibody conjugated with peroxidase. Positive cells containing the fragmented nuclear chromatin which is a characteristic of apoptosis have brown-stained nuclei. In each animal, 2 to 3 slides and in each slide, 5 microscopic fields were studied. TUNEL-positive cell number in each field was divided by apoptotic + non apoptotic cells and expressed as percentage (27).
Bcl-2 and bax detection
Immunohistochemistry was carried out on 5 μm tissue sections from formalin-fixed paraffin blocks using the avidin-biotin immunoperoxidase method. Sections were stained by monoclonal mouse/Rabbit Bcl-2, Bax oncoprotein (DAKO Corporation, USA) using a K0673 LSAB2 detection system (DAKO Corporation, USA) according to the manufacturer’s instructions. Briefly, the paraffin sections were deparaffinized with xylene and rehydrated through a series of descending graded ethanol solutions. Endogenous peroxidase activity was blocked by incubation for 15 min in 0.3% H2O2 buffer. To unmask the epitopes of Bcl-2 and Bax, a microwave processing pretreatment was carried out in a citrate buffer, pH = 6.0 for 10 min. A biotinylated secondary antibody and an avidin-biotin complex with horseradish peroxidase were applied, followed by addition of the chromogen. Finally, slides were counterstained with hematoxylin and observed with a light microscope. In each animal, 2 to 3 slides and in each slide, 5 microscopic fields were studied. Bcl2 and Bax positive cell number in each field were divided by apoptotic+non apoptotic cells and expressed as percentage (28).
Statistics
Student’s t-test was used to find the differences between TUNEL, Bax-and-Bcl-2 labeled positive cell number and atrophy. Data were expressed as mean ± SEM. P<0.05 considered statistically significant. Statistical analysis was performed using SPSS statistical software version 16.
Results
General observations
Out of 30 mice, one died due to unknown reason three weeks after starting nutrition in control group. Both in treated and control groups, the weight has been increased but not significantly. Body weight rose steadily to reach a plateau after about 8 weeks in all mice, but showed a slight fall in the treated group in the last few weeks. There were no significantly differences in food intake (g/day) in the control and treatment groups. Fumonisin B1 fed animals demonstrated no evidence of weight loss, and no gross organ changes were observed at necropsy. No behavioral changes were observed in the mice during the course of administration, or in the ensuing follow-up period.
Histopathological parameters observations
Microscopic findings of the gastric mucosa in the treated animals revealed a mild to moderate gastric tissue atrophy (P<0.05). Parietal cells significantly decreased in the treatment group (12±0.3) compared with the control (24±1.3) (P<0.05) (Figures 1 and 2). In addition, treatment with FB1 for 16 weeks, significantly reduced both gastric mucosa height (345.00±68.04 μ vs. 512.85±86.93 μ) and mitotic index in gastric glands (43.22±16.67 % vs. 72.67±10.50 %) (P<0.05) (Table 1). In both groups, the mitotic cells observing in basal part of gastric glands, indicated stem cells activity (Figure 2).
Figure 1.
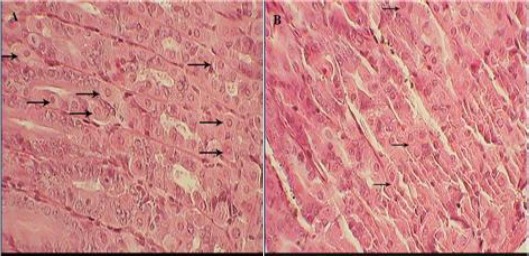
Light photomicrograph of mice gastric gland (A) in control group, normal parietal cells present in the mucosa and (B) in treatment group; a few parietal cells present in the final microscopy (H & E, 400×)
Figure 2.
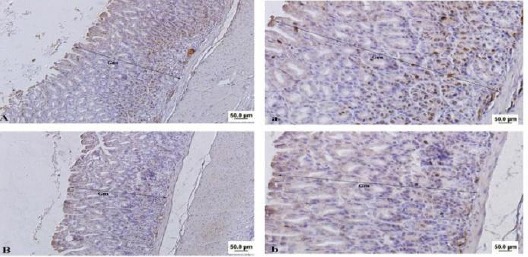
Light photomicrograph from mice gastric mucosa. Brown cells indicate Ki-67 positive cells. Note to reduce mitotic index and height in gastric mucosa (Gm) in fumonisin treated animal (B & b) in compared to control (A & a). Bar: 50 µ. Magnifications in A & B: 100 × and in a & b: 400 ×
Table 1.
Effects of fumonisin B1 on apoptosis and proliferative activity of cells in mouse gastric mucosa
| Groups | N | Apoptotic index (%) | Bcl-2 (%) | Bax (%) | Bcl-2/Bax | Gastric mucosa height (μ) | Gastric glands mitotic index (%) |
|---|---|---|---|---|---|---|---|
| Control | 14 | 0.56 ± 0.11 | 8.90 ± 0.33 | 1.74 ± 0.91 | 6.33 ± 4.00 | 513 ± 87 | 73 ± 11 |
| FB1 | 15 | *1.56 ± 0.21 | *1.39±0.34 | *16.21±1.01 | *0.081±0.01 | *345 ± 68 | *43 ± 17 |
Data are presented as mean ± SEM. *P < 0.05 vs. control group. FB1: fumonisin B1
Scatter TUNEL positive cells were observed in controls among the gastric glands (Figure 3). The percentage of TUNEL-positive cells in FB1 treated group were 1.56±0.21 vs. 0.56±0.11, and it was significantly higher than controls (Table 1).
Figure 3.
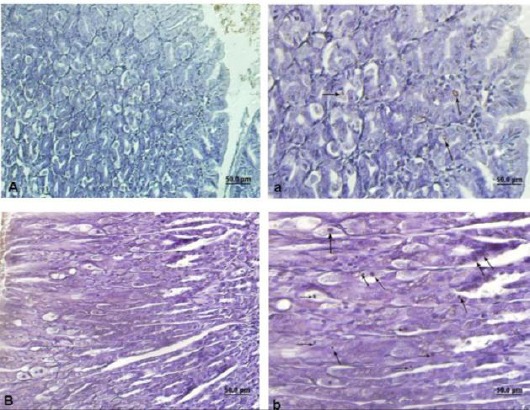
Light photomicrograph from mice gastric mucosa. Arrows show brown nuclear’s cells indicating TUNEL positive cells. Control (A & a), Treated animals (B & b). Bar: 50 µ. Magnifications in A & B: 200 × and in a & b: 400 ×
In control animals, the Bcl-2 positive cells were mostly observed in surface and neck mucosal cells of the gastric glands. Immunoreactivity with Bcl-2 in control group was 8.90±0.33, which was higher (P<0.05) than the same cells in treated group (Figure 4 and Table 1). However, Bax immunoreactivity in gastric mucosa was statistically higher in the FB1 group than the control (P<0.05) (Figure 5). Treatment with FB1 caused a decrease in Bcl-2/Bax ratio compared to the control group (P<0.05) (Table 1).
Figure 4.
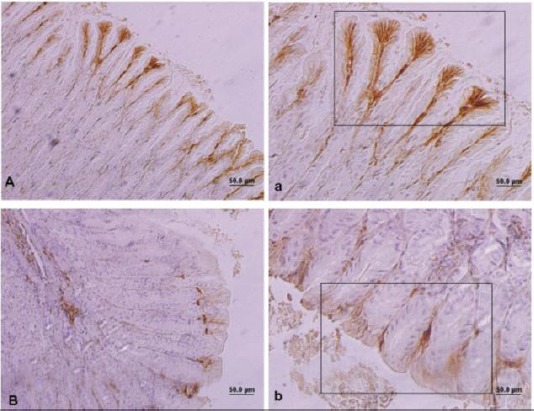
Light photomicrograph from mice gastric mucosa. The quadrate shows brown cells indicating Bcl-2 positive cells. Control (A & a), Treated animals (B & b). Bar: 50 µ. Magnifications in A & B: 200 × and in a & b: 400 ×
Figure 5.
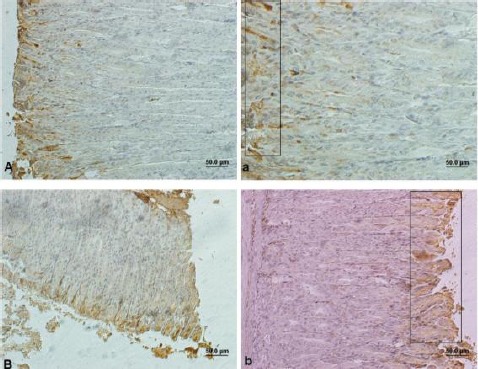
Light photomicrograph from mice gastric mucosa. The quadrate shows brown cells indicating Bax positive cells. Control (A & a), Treated animals (B & b). Bar: 50 µ. Magnifications in A & B: 200 × and in a & b: 400 ×
Discussion
In this study, FB1 significantly reduced parietal cell number together with gastric body glandular cell height and caused pronounced atrophy. This study showed that administration of fumonisin B1 for 16 consecutive weeks in mouse induces atrophy in gastric mucosa partly through increasing apoptosis and decreasing proliferative activity of the cells.
Previous studies have shown that apoptosis is the early effect of fumonisin on target organs (29, 30). FB1 may be considered as the first clear example of a non-genotoxic substance that can give rise to a tumor through different ways including apoptotic necrosis and atrophy (30). There are two possibilities; one is that the dead cells are not replaced and atrophy occurs, and the other is alteration in cell regeneration or proliferative activity of gastric basal cells. In previous study, we showed the changes of cell viability in a dose dependent manner of FB1. This toxin caused a decrease in the viability of gastric and intestinal cells (31, 32). In the present study, we showed that orally administration of FB1 for 16 weeks can induce apoptotic markers including increasing of BAX and fragmentation of DNA mouse gastric mucosa/, and can also alter balance between Bcl-2 and Bax proteins in gastric epithelial cells. Previous studies have suggested a correlation between gastric atrophy, apoptosis and alterations in the balance of Bcl-2 and Bax expression (33, 34). Similarly, an increased Bax expression and apoptosis but decreased Bcl-2 expression is shown in chronic atrophic gastritis (33, 34). Bcl-2 is an antiapoptotic gene family member; while Bax is a member of proapoptotic genes (35). Thus, these results suggest that an altered Bcl-2/Bax ratio may account for the atrophy-associated apoptosis in these mice models. On the other hand, FB1 has been identified as an inhibitor of ceramide enzyme, leading to significant alterations in sphingolipid metabolism, which is a key enzyme in sphingolipid metabolism, and various sphingolipids production involved in apoptosis (6, 36). Conceivably, chain of events linking FB1-induced ceramide synthase inhibition, disrupted sphingolipid metabolism and apoptosis induction can be theoretically drawn, raising the issue of apoptosis role in gastric atrophy.
On the other hand, previous studies have shown a strong relationship between the gastric atrophy and the onset of gastric and esophagus cancers (13-16). In addition, there is strong board of evidence proving that nitrosamines are esophageal cancer initiators, and mycotoxins such as FB1 can play a key role in the development of esophageal cancer (37). Acute gastric atrophy provides a proper environment for bacterial nitrosamines production (38). Nitrosamines from stomach origin can reach the mucus of esophagus through the gastric-esophageal blood vessel networks. Then, they can be converted to different active carcinogenic substances by P450 cytochrome isoforms existing in the mucus of esophagus (39). Therefore, endogenous nitrosamines produced by mycotoxins may etiologically play a role in esophageal and gastric cancer occurrence. However, further studies are required to understand the possible roles of FB1 in the signaling pathways.
Conclusion
This study showed that the administration of FB1 for 16 consecutive weeks in mice can induce gastric mucosal atrophy partly through increasing apoptosis and decreasing proliferative activity of the cells.
Acknowledgment
This study was supported by grant of Tehran University of Medical Sciences, Tehran, Iran.
Footnotes
Conflict of interest
The Author(s) declare(s) that they have no conflict of interest to disclose.
References
- 1.Gelderblom WC, Jaskiewicz K, Marasas WF, Thiel PG, Horak RM, Vleggaar R, et al. Fumonisins-novel mycotoxins with cancer-promoting activity produced by fusarium moniliforme. Appl Environ Microbiol. 1998;54:1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat RV, Shetty PH, Amruth RP, Sudershan RV. A food borne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. Clin Toxicol. 1997;35:249–255. doi: 10.3109/15563659709001208. [DOI] [PubMed] [Google Scholar]
- 3.Merrill AHJ, Liotta DC, Riley RT. Fumonisins: fungal toxins that shed light on sphingolipid function. Trends Cell Biol. 1996;6:218–223. doi: 10.1016/0962-8924(96)10021-0. [DOI] [PubMed] [Google Scholar]
- 4.Gelderblom WC, Abel S, Smuts CM, Marnewick J, Marasas WF, Lemmer ER, et al. Fumonisin-induced hepatocarcinogenesis: Mechanisms related to cancer initiation and promotion. Environ. Health Perspect. 2001;109:291–300. doi: 10.1289/ehp.01109s2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussein HS, Brasel JM. Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–134. doi: 10.1016/s0300-483x(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 6.Merrill AH, Jr, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, et al. Sphingolipids–the enigmatic lipid class: Biochemistry, physiology and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Wei H, Ma J, Luo X. The fumonisin B1 content in corn from North China, a high-risk area of esophageal cancer. J Environ Pathol Toxicol Oncol. 2000;19:139–141. [PubMed] [Google Scholar]
- 8.Goope NV, He Q, Sharma RP. Fumonisin B1-induced apoptosis is associated with delayed inhibition of protein kinase C, nuclear factor-kappaB and tumor necrosis factor alpha in LLC-PK1 cells. Chem Biol Interact. 2003;146:131–145. doi: 10.1016/s0009-2797(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 9.Schmelz EM, Dombrink–Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T, Merrill AH., Jr Induction of apoptosis by fumonisin B1 in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol Appl Pharmacol. 1998;148:252–260. doi: 10.1006/taap.1997.8356. [DOI] [PubMed] [Google Scholar]
- 10.Taranu I, Marin DE, Bouhet S, Pascale F, Bailly JD, Miller JD, et al. Mycotoxin fumonisin B1 alters the cytokine profile and decreases the vaccinal antibody titer in pigs. Toxicol Sci. 2005;84:301–307. doi: 10.1093/toxsci/kfi086. [DOI] [PubMed] [Google Scholar]
- 11.Taranu I, Pascale F, Lionide A, Burlacu R, Bailly JD, Oswald IP. Sex-related differences in the immune response of weanling piglets exposed to low doses of fumonisin extract. Br J Nut. 2006;95:1185–1192. doi: 10.1079/bjn20061773. [DOI] [PubMed] [Google Scholar]
- 12.Bracarense AP, Lucioli J, Grenier B, Drociunas Pacheco G, Moll WD, et al. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morpho-logical and immunological changes in the intestine of piglets. Br J Nutr. 2011;22:1–11. doi: 10.1017/S0007114511004946. [DOI] [PubMed] [Google Scholar]
- 13.Iijima K, Koike T, Abe Y, Inomata Y, Sekine H, Imatani A, et al. Extensive gastric atrophy: an increased risk factor for superficial oesophageal squamous cell carcinoma in Japan. Am J Gastroenterol. 2007;102:1603–1609. doi: 10.1111/j.1572-0241.2007.01257.x. [DOI] [PubMed] [Google Scholar]
- 14.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J Gastroenterol. 2007;17:21–27. doi: 10.1007/s00535-006-1924-9. [DOI] [PubMed] [Google Scholar]
- 15.Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298–305. doi: 10.1136/gut.2007.137364. [DOI] [PubMed] [Google Scholar]
- 16.Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma;a systematic review and meta-analysis. Ann Oncol. 2011;22:754–760. doi: 10.1093/annonc/mdq411. [DOI] [PubMed] [Google Scholar]
- 17.Sydenham EW, Theil PG, Marasas WFO, Shephard GS, Van Schalkwyk DJ, Koch KR. Natural occurrence of some Fusarium mycotoxins in corn from low and high esophageal cancer prevalence areas of the Transkei, Southern Africa. J Agric Food Chem. 1999;38:1900–1903. [Google Scholar]
- 18.Yoshizawa T, Yamashita A, Luo Y. Fumonisin occurrence in corn from high- and low risk areas for human esophageal cancer in China. Appl Environ Microbiol. 1994;60:1626–1629. doi: 10.1128/aem.60.5.1626-1629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghiasian SA, Rezayat SM, Kord-Bacheh P, Maghsood AH, Yazdanpanah H, Shephard GS, et al. Fumonisin production by Fusarium species isolated from freshly harvested corn in Iran. Mycopathologia. 2005;159:31–40. doi: 10.1007/s11046-004-3899-5. [DOI] [PubMed] [Google Scholar]
- 20.Alizadeh AM, Rohandel G, Roudbarmohammadi S, Roudbary M, Sohanaki H, et al. Fumonisin b1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac J Cancer Prev. 2012;13:2625–2628. doi: 10.7314/apjcp.2012.13.6.2625. [DOI] [PubMed] [Google Scholar]
- 21.Rheeder JP, Sydenham EW, Marasas WF, Thiel PG, Shephard GS, Schlechter M, et al. Ear-rot fungi and mycotoxins in South African corn of the 1989 crop exported to Taiwan. Mycopathologia. 1994;127:35–41. doi: 10.1007/BF01104009. [DOI] [PubMed] [Google Scholar]
- 22.Voss KA, Chamberlain WJ, Bacon CW, Riley RT, Norred WP. Subchronic toxicity of fumonisin B1 to male and female rats. Food Addit Contam. 1995;12:473–478. doi: 10.1080/02652039509374332. [DOI] [PubMed] [Google Scholar]
- 23.Sakagami T, Dixon M, O’Rourke J, Howlett R, Alderuccio F, Vella J, et al. Atrophic gastric changes in both Helicobacterfelis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249–1259. doi: 10.1046/j.1365-2036.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- 25.Dethloff LA, Robertson DG, Tierney BM, Breider MA, Bestervelt LL. Gastric gland degeneration induced in monkeys by the CCK-B/gastrin receptor antagonist CI-988. Toxicol Pathol. 1997;25:441. doi: 10.1177/019262339702500502. [DOI] [PubMed] [Google Scholar]
- 26.Brenner RM, Slayden OD, Rodgers WH, Critchley HO, Carroll R, Nie XJ, et al. Immuno-cytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometrium. Hum Reprod. 2003;18:1185–1193. doi: 10.1093/humrep/deg255. [DOI] [PubMed] [Google Scholar]
- 27.Alizadeh AM, Faghihi M, Khori V, Sohanaki H, Pourkhalili K, Mohammadghasemi F, et al. Oxytocin protects cardiomyocytes from apoptosis induced by ischemia-reperfusion in rat heart: Role of mitochondrial ATP-dependent potassium channel and permeability transition pore. Peptides. 2012;36:71–77. doi: 10.1016/j.peptides.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Alizadeh AM, Khaniki M, Azizian S, Mohagheghi MA, Sadeghizadeh M, Najafi F. Chemoprevention of azoxymethane-initiated colon cancer in rat by using a novel polymeric nanocarrier-curcumin. Eur J Pharmacol. 2012;689:226–232. doi: 10.1016/j.ejphar.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SM. Cell proliferation and carcinogenesis. Drug Metab Rev. 1998;30:339–357. doi: 10.3109/03602539808996317. [DOI] [PubMed] [Google Scholar]
- 30.Dragan YP, Bidlack WR, Cohen SM, Goldsworthy TL, Hard GC, Howard PC, et al. Implication of apoptosis for toxicity, carcinogenicity, and risk assessment: fumonisin B1 as an example. Toxicol Sci. 2001;61:6–17. doi: 10.1093/toxsci/61.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoodi M, Alizadeh AM, Sohanaki H, Rezaei N, Amini-Najafi F, Khosravi AR, et al. Impact of fumonisin B1 on the production of inflammatory cytokines by gastric and colon cell Lines. Iran J ` Allergy Asthma Immunol. 2012;11:165–173. [PubMed] [Google Scholar]
- 32.Mahmoodi M, Alizadeh AM, Amini-Najafi M, Khosravi AR, Hosseini SK, Safari Z, et al. Effects of fumonisin B1 on the stomach and colon cell lines in vitro. Tehran Univ Med J. 2012;69:744–753. [Google Scholar]
- 33.Chatzipantelis P, Konstantinou P, Voros D, Smyrniotis V, Kondi-Pafiti A. Apoptosis and gastric carcinogenesis: Immunohistochemical analysis of Bax and Bcl-2 proteins. Ann Gastroenterol. 2007;20:124–129. [Google Scholar]
- 34.Anagnostopoulos GK, Stefanou D, Arkoumani E, Sakorafas G, Pavlakis G, Arvanitidis D, et al. Bax and Bcl-2 protein expression in gastric precancerous lesions: immunohistochemical study. J Gastroenterol Hepatol. 2005;20:1674–1678. doi: 10.1111/j.1440-1746.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 35.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 36.Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 37.Craddock VM. Aetiology of oesophageal cancer: some operative factors. Eur J Cancer Prev. 1992;1:89–103. doi: 10.1097/00008469-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Houben GM, Stockbrugger RW. Bacteria in the aetio-pathogenesis of gastric cancer: a review. Scand J Gastroenterol Suppl. 1995;212:13–18. doi: 10.3109/00365529509090296. [DOI] [PubMed] [Google Scholar]
- 39.Lechevrel M, Casson AG, Wolf CR, Hardie LJ, Flinterman MB, Montesano R, et al. Characterization of cytochrome P450 expression in human oesophageal mucosa. Carcinogenesis. 1999;20:243–248. doi: 10.1093/carcin/20.2.243. [DOI] [PubMed] [Google Scholar]


