Abstract
Objective(s):
Mesenchyme is a type of undifferentiated loose connective tissue that is derived mostly from mesoderm. Recently, mesenchymal stem cells (MSCs), as adult stem cells (ASCs) able to divide into a variety of different cells, are of utmost importance for stem cell research. In this research, ability of the liver extract to induce differentiation of rat derived omentum tissue mesenchymal stem cells (rOT-MSCs) into hepatocyte cells (HCs) was investigated.
Materials and Methods:
After isolation and confirmation of rOT-MSCs they were co-cultured with liver extract and hepatogenic differentiation was monitored. Expressions of mesenchymal stem cell markers were also analyzed via flow cytometry. Moreover, expressions of octamer-binding transcription factor-4 (Oct-4), Wilm’s tumor suppressor gene-1 (WT-1), albumin (ALB), alpha fetoprotein (AFP), cytokeratin-18 (CK-18), and mRNAs were analyzed using RT-PCR on days 16, 18 and 21. ALB production was analyzed by immunocytochemistry and western blot. Furthermore, glycogen and urea production were determined via periodic acid-Schiff (PAS) staining and colorimetric assays respectively.
Results:
The phenotypic characterization revealed the positive expressions of CD90, CD44 and negative expression of CD45 in rOT-MSCs. These cells also expressed mRNA of Oct-4 and WT-1 as markers of omentum tissue. Differentiated rOT-MSCs in presence of 6 µg/ml liver extract expressed ALB, AFP, CK-18, glycogen and urea as specific markers of HCs.
Conclusion:
These observations suggest that liver extract is potentially able to induce differentiation of MSCs into hepatocyte lineage and can be considered an available source for imposing tissue healing on the damaged liver.
Keywords: Hepatocyte, Liver extract, Mesenchymal stem cells, Omentum tissue
Introduction
Liver diseases affect approximately 17.5% of the population. According to World Health Organization statistics, more than a hundred million people worldwide are suffering from liver disease (1). Transplantation medicine is one of the most challenging and complex areas of modern medicine (2). Some of the key areas for medical management are transplant rejection and the need to immediately remove the organ from the recipient (3). Unfortunately, the only known treatment for liver failure is liver transplantation and serious shortages of liver donors, high cost, and risk of organ rejection are the major obstacles (4, 5). Many types of stem cells from different sources have been investigated for their ability to differentiate into hepatocytes (6). Recently, mesenchymal stem cells (MSCs) have been found a potential source for hepatic differentiation (7, 8). In particular, in vitro models using culture medium supplemented with a cocktail of growth factors achieved the trans-differentiation of MSCs into hepatic cells with functional properties such as albumin and urea production and glycogen storage (9). The healing potential of the omentum has been utilized clinically by transposing the omental pedicle or flap to injured organs (omental transposition) for many decades. Omentum is considered as a source of ASCs and the non-fat stromal cells in the expanded omental tissue, express marker of stem cell activity, such as stromal cell derived factor 1 (SDF1-α) chemokine receptor 4 (CXCR4), and Wilms’ tumor antigen 1 (WT1) (10).
Therefore, cultured omental cells could represent a readily available source of MSCs that could be used to repair and regenerate damaged tissue. Omental stromal cells (OSCs), however, are more easily obtainable in large quantities, can be harvested from the patient’s own omentum, are able to passage in culture and differentiate into target tissues (11, 12). Oct-4 is critically involved in the self-renewal of undifferentiated embryonic stem cells. As such, it is frequently used as a marker for undifferentiated cells (13). Based on the well-known healing property of omentum, cultured OSCs could qualify as potential stem cells from the adult (14). If so, omentum would be a convenient source of ASCs that could be used to repair and possibly regenerate damaged tissues (15). Recent study demonstrated that when omentum-induced regenerating liver stem cells (RLSCs) were grown on Matrigel in the presence of hepatocyte growth factor, these cells differentiated into hepatocyte-like cells in culture. These differentiated cells expressed α-fetoprotein (AFP), Cytokeratin-18 (CK-18) and secreted high levels of albumin (ALB) and urea (16). Cell treatment with tissue extract is a new strategy for differentiation; also tissue extraction involves the lowest cost (17, 18). Some researchers have emphasized that local environment and resident cellular populations are the major factors determining the fate of engrafted cells (19). Also cell extracts may prove useful for investigating the molecular mechanisms of stem cell differentiation. The cultured MSCs also express on surface CD73, CD90, CD44 and CD105, while lacking the expression of CD11b, CD14, CD19, CD34, CD45 and CD79a surface markers (20, 21). Liver extract is rich in different growth factors and molecules that are effective in their proliferation and differentiation. In this study, we decided to monitor differentiation of isolated rOT-MSCs to hepatocytes in the absence of specific extracellular matrices and growth factors.
Materials and Methods
Chemicals and cells
Five neonatal Wistar rats (Tehran University, Iran), phosphate-buffered saline (PBS), 0.25% trypsin, Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum (FBS, Gibco Bio Cult, UK), penicillin/streptomycin (Pen/Str, Sigma Aldrich Co, USA), rabbit anti-CD90, rabbit anti-CD45, rabbit anti-CD44 antibodies and rabbit polyclonal isotype control (dilution 1:200; ABCAM, UK), goat anti-rabbit IgG-FITC antibody (dilution 1:4; Razi Teb, Iran), sheep anti-rat albumin (dilution 1:200; Bethyl Laboratories, Montgomery, TX), goat anti–rat albumin FITC-conjugated IgG (dilution 1:200; Sigma Aldrich, USA), HRP-Labelled goat anti–Rat albumin IgG (dilution 1:2000; Santa cruz Biotechnology, CA, USA), bovine serum albumin (BSA) (Sigma, USA), Tripure isolating reagent (Ambion, USA), RNase-free DNase I (Thermo Fisher Scientific, Fermentas, USA), PCR master mix: Ampliqon Taq DNA polymerase 2× Master Mix Red (Pishgam Biotec, Iran), BioRT cDNA First Strand Synthesis Kit (Hangzhou Bioer technology, China), biosafe (NANOLYTIC bio), dimethyl sulfoxide (DMSO)(Sigma, USA), 4% paraformaldehyde (Dingguo, Beijing, China), normal donkey serum (Zsbio, Beijing, China), Triton X-100 (Sigma-Aldrich, USA), 4,6-diamidino-2-phenylindole (DAPI) (dilution 1: 1000; Roche, Germany), western blocker solution (Sigma-Aldrich, USA), periodic acid, Schiff’s reagent (MERCK, Germany), 25 cm2 plastic cell culture flask (Jet/Biofil, Italy), centrifuge (Hettich, Germany), phase contrast microscope (Olympus IX71, Japan), Flow cytometer (Beckton Dickinson, USA), SuperCycler Trinity (Kyratec, Africa), electrophoresis (UVIdocLCD gel documentation system), UV transilluminator (UVIdoc, UK), DNA ladder (NANOLYTIC bio), homogenizer (Jencons, USA), centrifuge (M-24OR, Germany), fluorescence microscope (Nikon TE-2000, Japan), chemiluminescence system (Pharmacia, Piscataway, NJ).
Isolation, culture, expansion and storage of rOT-MSCs
Cell isolation was performed according to Chen et al, with some modifications as described below (22). Omentum (1 mm2 segments) was isolated from neonatal Wistar rats (age 12 days/weight 15–16 g) through abdominal surgery, washed with PBS to remove any contamination and incubated with a solution containing 2 ml trypsin (0.25%) for 15 min at 37°C. After incubation, the cell suspensions were centrifuged at 1500 g for 5 min at 4°C and the remaining tissue was transferred to a new flask for the next round of digestion (the procedure was repeated for 15, 20 and 40 min). After centrifugation, the cell pellet was washed with DMEM containing 10% FBS and 1% Pen/Str, re-suspended in the same medium and plated in a 25 cm2 cell culture flask. Non-adherent cells were removed 3 days later by two brief washes with medium. The dishes were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C, and the medium was changed every three days. After the cultures reached the optimum confluence, cells were lifted by incubation with PBS containing trypsin (0.25%) and 0.02% EDTA at 37°C. Detached cells were suspended, washed in DMEM, centrifuged at 1500 g for 5 min, and seeded on three new 25 cm2 cell culture flasks (ratio 1:3). An equal volume of stromal medium was added to inactivate the trypsin and the suspension was transferred to a conical centrifuge tube. After centrifuging at 1500 g for 5 min, the cell pellet was re-suspended in 1 to 2 ml of cryopreservation medium (the same formulation as that used to propagate the cells with 20% FBS, 10% DMSO) and transferred to a liquid nitrogen container for long-term storage (23).
Liver extract preparation and treatment
For preparation of liver extract, livers from 12 day rats were isolated, weighted and 6 g of liver tissue washed with sterile PBS several times to remove any contamination. Liver tissues were divided into smaller segments in the 10 ml sterile DMEM, then samples were placed in a glass homogenizing tube at low speed for 20 to 30 sec on wet ice. The homogenate was transferred to centrifuge tubes, the tubes were balanced and the samples were centrifuged at 15000 rpm for 20 min at 4°C. The supernatant was removed with a sterile syringe (pore diameter 0.45) and stored in aliquots at -20°C until use (24). rOT-MSCs were plated at a number of 5×104 cells/cm2 in 25 cm2 plastic culture flasks without specific extracellular matrix coating and cultured in expansion medium at 37°C, 5% CO2. Undifferentiated cells were harvested after 3 days of culture at 70% confluence. For differentiation assay, when OSCs reached 70% confluence, they were treated with different concentrations (6 µg/ml, 18 µg/ml, 30 µg/ml and 60 µg/ml) of liver extract with 2%, 5%, 10% and 15% of FBS for 21 days respectively. For effective differentiation, the level of FBS was gradually increased at concentrations of 30 and 60 µg/ml. The same medium without liver extraction and 10% FBS was used as control during the differentiation. Medium changes were carried out every 4 days until day 21.
Flow cytometry
Cells were plated at a density of 200 cells/cm2 on fibronectin-coated culture dishes and harvested at about 50% confluence. 105–106 cells were incubated with FITC- or PE-conjugated anti-CD90, anti-CD45 and anti-CD44 for 30 min at 4°C in PBS. The primary and secondary antibody concentrations used were as follows: rabbit anti-CD90, rabbit anti-CD45, rabbit anti-CD44 dilution 1:200 and goat anti-rabbit IgG-FITC dilution 1:4. Isotype-matched irrelevant polyclonal antibodies were used as negative controls. For cell-surface staining, cells were incubated in darkness for 30 min at 4°C in PBS supplemented with BSA. After washing, the cells were re-suspended in PBS and measured by using a flow cytometer and the results were analyzed with the Win MDI 2.8 software (Scripps Institute, La Jolla, CA, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR analysis was performed for specific genes at days 16, 18 and 21 according to the standard protocol with some modifications (25). Total RNA was isolated from cell lysates using Tripure isolating reagent as described by the manufacturer (Roche Applied Science). Extracted RNA was solved in diethylpyrocarbonate (DEPC), treated with water and digested with RNase-free DNase I to remove contaminating genomic DNA. DNase I was dissolved in 10X reaction buffer with MgCl2 and 1 µg of DNase I was added per 1 µl of RNA and incubated for 30 min at 37°C. DNase I activity was arrested following addition of 1 µl of 25 mM EDTA and incubated at 65°C for 10 min. The RT-PCR procedure was carried out in one step using 1 µg of total tissue RNA and random hexamers as primers using the RT-PCR system. The system uses AMV Reverse Transcriptase (5 U/µl) for first strand synthesis and Taq DNA polymerase for second strand cDNA synthesis and amplification. cDNA was synthesized using BioRT cDNA First Strand Synthesis Kit. Semi-quantitative RT-PCR was carried out with 2 µl cDNA, 250 nM each of forward and reverse primers (Table 1) and 1U Taq polymerase in a final volume of 20 µl. Each cycle consisted of 2 min at 95°C, 15 sec at 58°C, 30 sec at 72°C (35 cycles) and finally 5 min at 72°C (SuperCycler Trinity, Kyratec, Africa). Amplified DNA fragments were electrophoresed on 1.7% agarose gel, stained with Biosafe and photographed on a UV transilluminator (UVIdoc, UK).
Table 1.
Primers used for reverse transcription polymerase chain reaction of specific gene expression
| Accession no | Name | Sequence | Product size (bp) |
|---|---|---|---|
| NM_134326.2 | Albumin | F: 5’-CTATCTGTCTGCCATCCTGAACC -3’ R: 5’-TGTCCACGAATTGTGCGAAGT-3’ |
317 |
| NM_012493.2 | α-fetoprotein | F: 5’-AATTTGCCACGAGACGGAACT-3’ R: 5’-TGCTGGAACTGCCTTGTCATAC -3’ |
283 |
| NM_053976.1 | Ck-18 | F: 5’-GAGGGCTCAGATCTTTGCGAAT-3’ R: 5’-TTGGGAGCATCCACTTCCAC-3’ |
315 |
| NM_031534 | WT-1 | F: 5’-TGAGAAACCATACCAGTGTGAC-3’ R: 5’-GTAGGTGAGAGGGAGGAATTTC-3’ |
396 |
| NM_001009178 | Oct-4 | F: 5’-GGAGATATGCAAATCGGAGACC-3’ R: 5’-CGAGTAGAGTGTGGTGAAATGG-3’ |
352 |
| NM_031144 | β-actin | F: 5’-TCATGAAGTGTGACGTTGACATCCGT-3’ R: 5’-CCTAGAAGCATTTGGGGTGCACGATG-3’ |
285 |
Fluorescent immune-cytochemistry assay
Hepatocyte markers in cells were monitored by immunocytochemical staining at days 16 and 18. After 16 and 18 days of cell culture under hepatocyte-conditioned medium (liver extract), cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. Subsequently, cells were washed with PBS and then permeabilized with blocking buffer containing 10% normal donkey serum and 0.3% (v/v) Triton X-100 for 45 min at room temperature to block nonspecific antibody binding. Corresponding primary antibodies including the sheep anti-rat albumin was then added to the cells and incubated overnight at 4°C. The cells were subsequently washed three times with PBS and incubated with a second fluorescence-labeled antibody containing goat anti–rat albumin FITC-conjugated IgG at room temperature for 1 hr in darkness. After washing with PBS the cells were incubated with 1 µg/ml 4, 6-diamidino-2-phenylindole (DAPI) for 2 to 5 min at room temperature with the purpose of nuclear staining. The cells were then visualized using a fluorescence microscope. To evaluate the number of immunostained cells, photographs of 10 random fields (×100) per slide were analyzed by NIH Image software (National Institutes of Health; Bethesda, MD). Negative control was performed avoiding primary antibody.
Western blotting analysis
Induction of hepatocyte phenotypes in cells was monitored by western blotting at days 16 and 18. Western blot analysis was performed as described previously (26). Cell lysates of proteins from different culture stages were subjected to 0.1% SDS-7.5% PAGE, and the separated proteins were transferred to a nitrocellulose membrane (Amersham) and membranes were blocked for 1.5 hr using a western blocker solution before being incubated with an antibody against ALB. The proteins were detected on immunoblots using primary antibody (sheep anti-rat Albumin Antibody) diluted with 0.05% Triton X-100 in Tris-buffered saline containing 1% gelatin, followed by HRP-conjugated secondary antibody (HRP-Labelled goat anti–Rat albumin IgG). Immunoreactive bands were developed by ECL (Amersham) and blots were exposed to medical X-ray film (Kodak, Rochester, NY, USA). The specific bands were detected by using the enhanced chemiluminescence system (Pharmacia, Piscataway, NJ).
Periodic acid-schiff staining (PAS)
Induction of hepatocyte phenotypes in cells was monitored by Periodic Acid Schiff (PAS) stain for glycogen synthesis at days 16 and 21. In the hepatocyte differentiation group (6 µl liver extract with 2% FBS), the medium was removed from the flasks, cells rinsed with PBS three times and fixed with 4% paraformaldehyde for 20 min at room temperature, washed 3 times with PBS for 2 to 3 min, oxidized for 15 min with 1% periodic acid, and washed 3× with deionized water. Cells were then stained with Schiff’s reagent for 30 min, washed 3 times with PBS for 2 to 3 min prior to microscopic examination and imaging.
Urea assay
Urea concentrations were determined by colorimetric assay. The control group was used as a negative control, which was cultured in DMEM supplemented with 10% FBS, and 1% Pen/Str. The method could detect at least 10 mmol/l urea.
Viability assay
Cell viability was determined by trypan blue exclusion assay. The percentage of cells excluding trypan blue was taken as a measure of cell viability in different concentrations (6 µg/ml, 18 µg/ml, 30 µg/ml and 60 µg/ml) of liver extracts.
Statistical analysis
One-way ANOVA was used for statistical analysis of more than one group of samples. For all other data, unpaired t-test was used. P-value of 0.05 was considered statistically significant.
Results
rOT-MSCs culture and characterization
The initial adherent spindle-shaped rOT-MSCs appeared as separate colonies at the bottom of culture flasks within 19 hr (Figure 1A). Cells in 2 to 5 day culture became more confluent (Figure 1B and C), and reached 75 to 80% of confluence within one week (Figure 1D). Morphologically verified cells were lifted and cultured in another 25 cm2 flask (1:3) until confluence was achieved. Cell population appeared to have more homogeneous spindle-shaped cells (Figure 1E) and following freeze-thaw stage these cells reattached to the culture flasks with a slow growth rate (Figure 1F). Molecular analysis (RT-PCR) confirmed that the cultured cells express omental (Oct-4 and WT-1) markers (Figure 2). Expression of cell surface mesenchymal (CD90 and DC44) markers was confirmed via Flow cytometry assay as well (Figure 3). Flow cytometry analysis revealed that the rOT-MSCs were positive for CD90 (84.05%), CD44 (88.22%) and relatively negative for CD45 (1.02%) (Figure 3).
Figure 1.
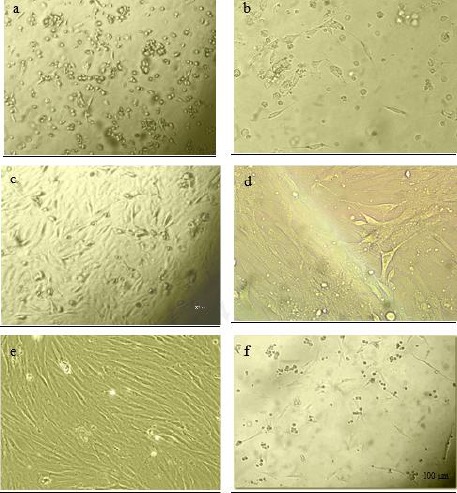
Cell morphology of cultured rat derived omentum tissue mesenchymal stem cells were observed with an Olympus phase contrast microscope. The initial adherent cells appeared as separate colonies after 19 hr (a), and 2 days (b). More confluent omental stromal cells after 5 days (c) and monolayer cultured mesenchymal stem cells after one week (d). Spindle-shaped morphology of cells after new consecutive passages (e), and re-attachment of cells to the culture flasks following thawing after freezing stage (f)
Figure 2.
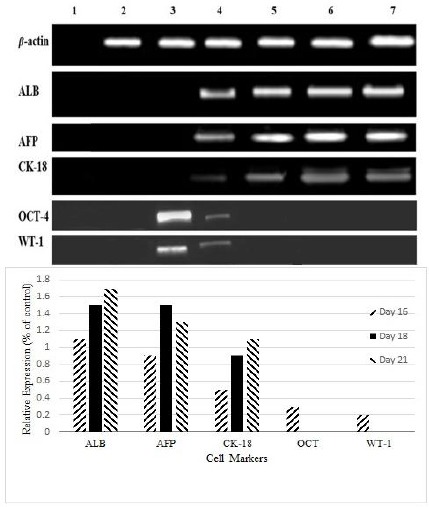
Reverse transcription polymerase chain reaction analysis of specific embryonic (octamer-binding transcription factor-4) and adult stem cell markers (Wilms’ tumor antigen 1) of rat derived omentum tissue mesenchymal stem cells and liver cell marker gene expression (albumin, alpha fetoprotein, and Cytokeratin-18) in differentiated mesenchymal stem cells. H2O negative control for polymerase chain reaction (Lane 1), breast cancer cell line SKbr3 as negative control for albumin (Lane 2), untreated rat derived omentum tissue mesenchymal stem cells (Lane 3), 16 day differentiated cells (Lane 4), 18 day differentiated cells (Lane 5), 21 day differentiated cells (Lane 6), cell line HepG2 as positive control (Lane7)
Figure 3.
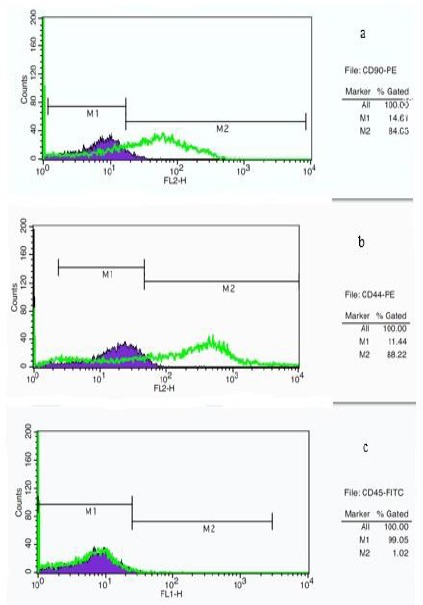
Flow cytometry analysis of cell surface markers on rat derived omentum tissue mesenchymal stem cells after one week culture. Cells were positive for CD90-PE (a), CD44-PE (b), and negative for CD45-FITC (c). M1: isotype control, M2: CD90, CD44 and CD45 antibody
Characterization of hepatocyte- like cells
A large number of cells died perhaps due to high concentrations of blocking enzymes present in the liver extract at concentrations of 60 µg/ml and 30 µg/ml (Figure 4a). Living cells were between the phase of hepatocytes and fusiform morphology of rOT-MSCs. The structure of hepatocyte-like cells vanished gradually and dose dependently, finally the cells trend to aging and apoptosis (Figure 4a). Observations showed that the effective concentration for hepatic differentiation was 6 µg/ml (Figure 4a). Under 6 µg/ml fresh liver extract treatment, rOT-MSCs gradually progressed toward the polygonal morphology of hepatocytes in a time-dependent manner. The morphology of the cells was observed under light microscopy and compared with rOT-MSCs as control (Figure 4b). The morphology of rOT-MSCs was changed and the cells developed a broadened, flattened morphology after 16 days of induction (Figure 4b). The multiplicity of induced mesenchymal cells was reduced gradually at day 16. The cuboidal morphology of hepatocyte-like cells and bi-nucleated cells was observed as early as 21 days after culturing under liver extract conditions and further matured by day 21 in the presence of 2% FBS (Figure 4b). In addition, morphological analysis of differentiated rOT-MSCs, induced by liver extract, showed that the nucleus to cytoplasm volume ratio increased (Figure 4b). Therefore the contraction of the cytoplasm progressed further during maturation, and most of the treated cells became quite dense with clear or double nuclei in the late stages of differentiation (Figure 4b). The control cells were still fibroblast-like and had fusiform shapes, no polygon-shaped cells were found in the control group (Figure 4b). Differentiation of cells to the hepatic phenotype was confirmed with expression of ALB, CK18 and AFP (Figure 2). Control cells were negative for AFP and CK18 (Figure 2). Undifferentiated cells, although also positive for albumin, did not secrete albumin as the differentiated cells did, suggesting that the differentiated state was characterized by high albumin secretion. However, AFP, an endodermal and early hepatic marker, was up-regulated on day 21, suggesting that some of the cells were possibly still in early stages of hepatic lineage. In addition, the high level of AFP expression on day 21 might be due to the proliferation of hepatic progenitors.
Figure 4.
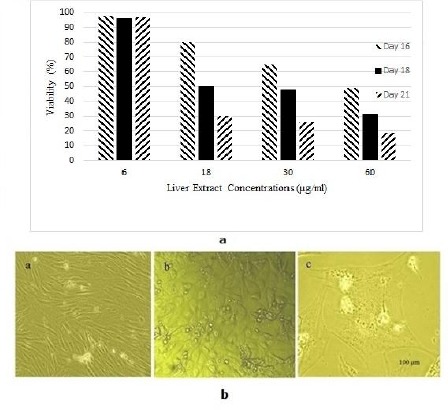
a) Viability assay of rat derived omentum tissue mesenchymal stem cells after 16, 18 and 21 day treatment with different concentrations of liver extract. b) Cell morphology of differentiated rat derived omentum tissue mesenchymal stem cells after 6 µg/ml liver extract treatment was observed with an Olympus phase contrast microscope. Rat derived omentum tissue mesenchymal stem cells as control cells with spindle-shaped morphology (a), cells after 16 day (b), and 21 day treatment (c)
Immunocytochemical analysis of ALB
The presence of albumin is a prominent feature of mature hepatocytes, as liver is the predominant site for the synthesis of albumin protein. The immunocytochemistry results showed that the cells expressed ALB on days 16 and 18. The percentage of albumin positive cells was 35 and 100% in the differentiated cells on days 16 and 18, respectively (Figure 5). Hence, immunocytochemical staining showed significantly greater levels of albumin after the 18 day differentiation as compared with the control (P<0.05).
Figure 5.
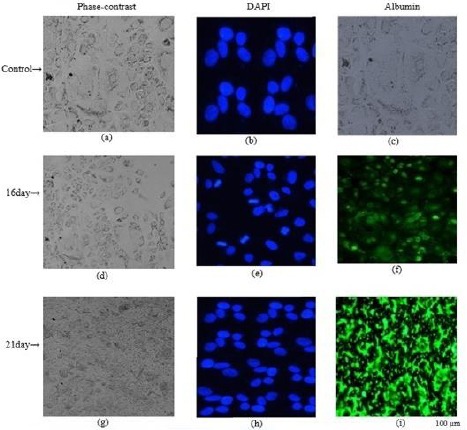
Immunocytochemical staining of DAPI and albumin in differentiated cells from rat derived omentum tissue mesenchymal stem cells after 6 µg/ml liver extract treatment. Untreated cells (a-c); 16 day differentiated cells (d-f), 21 day differentiated cells (g-i)
Western blotting analysis of ALB
To further confirm efficient hepatic induction, we checked the protein expression of ALB by western blot analysis. SDS-PAGE showed the presence of a prominent band of albumin in the media of differentiated cells at days 16 and 18, whereas there was little albumin in the media of undifferentiated (control) cells (Figure 6a). The rate of albumin secretion by the differentiated cells was 80 to 100 times higher than undifferentiated or control cells (Figure 6a).
Figure 6.
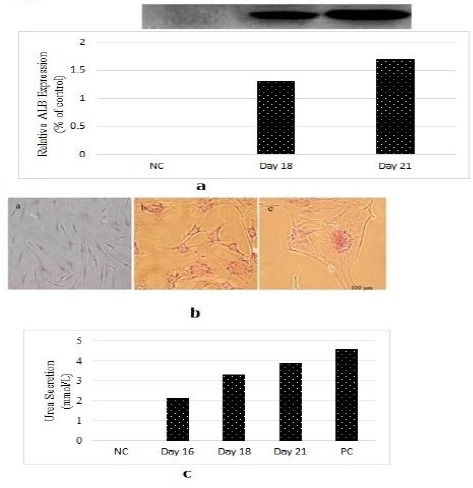
a) Protein expression of albumin was confirmed by western blot analysis after treatment of rat derived omentum tissue mesenchymal stem cells with 6 µg/ml liver extract. Undifferentiated rat derived omentum tissue mesenchymal stem cells as negative control (NC), 16 day differentiated cells and 21 day differentiated cells. b) Periodic acid-Schiff staining for glycogen production in the differentiation process of rat derived omentum tissue mesenchymal stem cells in presence of 6 µg/ml liver extract. Unstaining differentiated cells after 16 days (a), differentiated cells after 16 days (b), and 21 days (c). c) Urea secretion by rat derived omentum tissue mesenchymal stem cells was detected at various times throughout differentiation. Undifferentiated rat derived omentum tissue mesenchymal stem cells as negative control (NC), 16, 18 and 21 day differentiated cells, cell line HepG2 as positive control (PC)
Periodic acid-schiff (PAS) staining for glycogen
Glycogen production was used for examining whether differentiated hepatocytes from omentum MSCs were functionally competent or not. After 16 and 21 day differentiation cultures, induced omentum MSCs were analyzed for their glycogen-storage ability by PAS staining. As shown in Figure 6b the majority of rOT-MSC-derived hepatocytes were strongly positive for PAS staining at day 19. The positive rate was 50% to 60%, while undifferentiated MSCs were negative (Figure 6b).
Urea assay
Urea production and secretion by hepatocyte-like cells was detected at various time points throughout differentiation. Following treatment with 6 µg/ml fresh liver extract, urea produced by differentiated rOT-MSC to hepatocyte-like cells was detected with a concentration of 2.1±0.6 mmol/l on day 16, and 3.9±0.9 mmol/l on day 21 (Figure 6c).
Discussion
In this study rat omental stromal cells are shown for the first time to be able to directly differentiate into hepatic cells using liver extract. rOT-MSC-derived hepatocyte like cells were characterized in vitro at four levels (Morphology, RNA, Protein, and Activity). Some genes, like alpha-fetoprotein (AFP), are expressed very early in embryonic development and during the fetal stages, thus represent a reliable marker to discriminate between distinct developmental stages (27). Alternatively, most but not all, metabolic and detoxifying enzymes become functional during the terminal step of liver organogenesis (28). Therefore, to state the differentiation stage of the resultant hepatocyte-like cells, functional assays need to be carried out. At present, functional analysis is particularly focused on glycogen uptake, urea metabolism, and albumin (ALB) secretion. This study indicates that the extract of neonatal rat livers could effectively differentiate omental stromal cells into hepatocyte-like cells. The omentum has long been known to have the power to heal injured organs once it has adhered to the damaged site, either naturally or deliberately by surgery (29). It has previously been shown that the omentum, especially after its activation by injury, becomes a reservoir of stromal cells that express stem cell markers and growth factors (30, 31). At present, proposed therapies involving the use of embryonic cells pose ethical questions and carry the risk of uncontrollable growth and tumor induction (32). By contrast, adult cells with stem cell properties are safer and have greater practical use, having been employed for over 50 years to replace bone marrow in leukemia and non-hematological diseases without causing malignant transformation (33). Adult cells with stem cell properties have previously been identified in the bone marrow mesenchyme, skin, hair, dental pulp, kidney, and even peripheral blood (34). Omental stromal cells, however, are more easily obtainable in large quantities and can be harvested from the patient’s own omentum, thus obviating the need for immunosuppressive therapy. As these cells can be passaged in culture without loss of pluripotent markers (Oct–4, Nanog), they can be frozen in large numbers for long-term storage and later use (35). Based on the well-known healing property of the omentum, stem cell marker characterization, secretion of high amounts of VEGF, and the ability to recognize injured sites, cultured omental stromal cells could qualify as potential stem cells from adults. If so, the omentum would be a convenient source of adult stem cells and could be used to repair and possibly regenerate damaged tissues. In this study omental stromal cells adhered to plastic, were mesenchymal (CD90+, CD45-, and CD44+) and not hematopoietic in morphology and phenotype. They also expressed markers of adult (WT-1) as well as embryonic stem cells (Oct-4). Adhesion of cells to dish pre-coated with extracellular matrix compounds is crucial for cell differentiation and maintenance of mesothelium cells in culture (36). Our results showed that omental stromal cells could successfully grow in dish without extracellular matrix coating in presence of serum and 10% FBS. The most commonly used growth factors and cytokines in hepatic differentiation are HGF, FGF-4, and OSM (37). Also some research has reported that fibroblast growth factor-4 (FGF-4) and hepatocyte growth factor (HGF) could induce MSCs differentiation into functional hepatocyte-like cells (38, 39). Bone marrow-derived MSCs were placed on Matrigel, fibronectin or collagen matrix and differentiated into hepatocyte-like cells (40, 41); but in the present study, the omental stromal cells were cultured in plastic culture flasks without treatment with Matrigel or fibronectin. It seemed that the extracellular matrix can potentially modulate the local concentration of cytokines available with the stem cell microenvironment and cytokines can regulate stem cell proliferation and differentiation fate, but the differentiated hepatocyte-like cells maintained on plastic dishes often rapidly de-differentiate in culture. These yield-differentiated cells could provide a potential source of hepatocytes for drug screening in vitro and a valuable reference for cell therapy for liver patients. Adult stem cells comprise the first line repair mechanism, called into action by normal wear and tear of the body as well as by any serious damage or attack caused by disease or infection (42). Secondly, we found that there are controversies among various citations over MSC specific markers such as CD 90, CD 54, CD 44, CD 29, CD 105, CD 166, and CD 13 (43). Thus, it is unambiguous that prevalence of marker specificity for MSC with respect to all sources is quite vague. Hence, in order to avoid this uncertainty and to get a coherent clue of the presence of construed MSCs in these sources, we have categorized only CD 90 and CD 44 as positive markers, as reported and confirmed by previous research (44, 45). Nevertheless, our work contributes to the knowledge needed for the development of clinical stem cell treatment for liver failure. Our results also shed light on the underlying mechanism of liver regeneration.
Conclusion
MSCs were originally identified from bone marrow (BM) but recent studies have shown that cells with similar characteristics could be isolated from other mesodermal tissues such as omentum tissue. Results indicate that omentum tissue contains cells with stem cell functions among the CD90 and CD44 subset. Our data suggest that functional MSCs present in omentum can differentiate into hepatocyte lineages in presence of liver extract (6 µg/ml). These differentiated cells are an available source for imposing tissue healing on damaged tissues such as liver.
Acknowledgment
The results reported in this paper were part of a student thesis. This research was financially supported by the Cells and Molecular Research Center (Iran University of Medical Sciences) and the Iranian Research and Technology Support Center. We also thank Dr M Soleimani for lab assistantship.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: Lessons from the Dionysos study. J Hepatol. 2001;35:531–537. doi: 10.1016/s0168-8278(01)00151-9. [DOI] [PubMed] [Google Scholar]
- 2.Alpdogan O. Advances in immune regulation in transplantation. Discov Med. 2013;15:150–159. [PubMed] [Google Scholar]
- 3.Ekser B, Ezzelarab M, Hara H, Van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;18:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 4.Lacaille F. Liver transplantation and liver cell transplantation. Clin Res Hepatol Gastroenterol. 2012;36:304–307. doi: 10.1016/j.clinre.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Yang XY, Wang W, Li X. In vitro hepatic differentiation of human endometrial stromal stem cells. In vitro Cell Dev Biol-Anim. 2013;50:162–170. doi: 10.1007/s11626-013-9688-z. [DOI] [PubMed] [Google Scholar]
- 6.Jameson E. Cellular transplantation for liver diseases. Gastroen Res. 2008;1:8–13. doi: 10.4021/gr2008.11.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puglisi MA, Saulnier N, Piscaglia AC, Tondi P, Agnes S, Gasbarrini A. Adipose tissue-derived mesenchymal stem cells and hepatic differentiation: old concepts and future perspectives. Eur Rev Med Pharm Sci. 2011;15:355–364. [PubMed] [Google Scholar]
- 8.Khoshdel A, Lotfi AS, Soleimani M, Daliri M, Mota A. Evaluation of biochemical markers in hepatocyte like cells differentiated from adipose derivation stem cells. World Appl Sci J. 2012;16:693–698. [Google Scholar]
- 9.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Lowery E, Braun RK, Martin A, Huang N, Medina M, et al. Cellular basis of tissue regeneration by omentum. PLoS One. 2012;7:e38368. doi: 10.1371/journal.pone.0038368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhanasekaran M, Indumathi S, Harikrishnan R, Mishra R, Lissa RP, Rajkumar JS, et al. Human omentum fat-derived mesenchymal stem cells trans-differentiates into pancreatic islet-like cluster. Cell Biochem Funct. 2013;31:612–619. doi: 10.1002/cbf.2948. [DOI] [PubMed] [Google Scholar]
- 12.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 14.Ochiya T. Advances of stem cell-based liver regeneration: science and the future. Nihon Rinsho. 2011;69:2156–2160. [PubMed] [Google Scholar]
- 15.Herrick SE, Mutsaers SE. The potential of mesothelial cells in tissue engineering and regenerative medicine applications. Int J Artif Organs. 2007;30:27–40. doi: 10.1177/039139880703000611. [DOI] [PubMed] [Google Scholar]
- 16.Pancholi N, Patel J, Gudehithlu K, Kraus M, Dunea G, Arruda J, et al. Culture of omentum-induced regenerating liver yielded hepatocyte-committed stem cells. Transl Res. 2010;156:358–368. doi: 10.1016/j.trsl.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Liu YX, Yue W, Ji L, Nan X, Pei XT. Production of erythroid cells from human embryonic stem cells by fetal liver cell extract treatment. BMC Dev Biol. 2010;10:85. doi: 10.1186/1471-213X-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margl W, Prehn C, Bogumil R, Rohring C, Suhre K, Adamski J. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics. 2012;8:133–142. [Google Scholar]
- 19.Liu XN, Yin QI, Zhang HAO, Zhang H, Zhu SJ, Wei YJ, et al. Tissue extracts from infarcted myocardium of rats in promoting the differentiation of bone marrow stromal cells into cardiomyocyte-like cells. Biomed Environ Sci. 2008;21:110–117. doi: 10.1016/S0895-3988(08)60015-X. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Horwitz Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Kolf CM, Cho E, Tuan RS. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen KS, Chen WS, Chen HY, Lee CC, Kao HS, Chen YC, et al. Experience in primary culture of human peritoneal mesothelial cell. Chin J Physiol. 2012;55:274–283. doi: 10.4077/cjp.2012.baa040. [DOI] [PubMed] [Google Scholar]
- 23.Bruce A, Flaat M, Gagliardi C, Patel B. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miryounesi M, Nayernia K, Dianatpour M, Mansouri F, Modarressi MH. Co culture of mouse embryonic stem cells with sertoli cells promote in vitro generation of germ cells. Iran J Basic Med Sci. 2013;16:779–783. [PMC free article] [PubMed] [Google Scholar]
- 25.Ramroodi N, Niazi AA, Sanadgol N, Ganjali Z, Sarabandi V. Evaluation of reactive epstein–barr virus (EBV) in Iranian patient with different subtypes of multiple sclerosis (MS) Brazil J Infect Dis. 2013;7:156–163. doi: 10.1016/j.bjid.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanadgol N, Mostafaie A, Bahrami GH, Mansouri K, Ghanbari F, Bidmeshkipour A. Elaidic acid sustains LPS and TNF-αinduced ICAM-1 and VCAM-I expression onhuman bone marrow endothelial cells. Clin Biochem. 2010;43:968–972. doi: 10.1016/j.clinbiochem.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 27.Ogasawara K, Terada T, Asaka J, Katsura T, Inui K. Hepatocyte nuclear factor-4a regulates the human organic anion transporter 1 gene in the kidney. Am J Physiol Renal Physiol. 2007;292:1819–1826. doi: 10.1152/ajprenal.00017.2007. [DOI] [PubMed] [Google Scholar]
- 28.Gu N, Adachi T, Matsunaga T, Tsujimoto G, Ishihara A, Yasuda K, et al. HNF-1a participates in glucose regulation of sucrase-isomaltase gene expression in epithelial intestinal cells. Biochem Biophys Res Commun. 2007;353:617–622. doi: 10.1016/j.bbrc.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 29.Kania G, Blyszczuk P, Jochbeim A, Ott M, Wobus AM. Generation of glycogen and albumin producing hepatocyte-like cells from embryonic stem cells. Biol Chen. 2004;385:943–953. doi: 10.1515/BC.2004.123. [DOI] [PubMed] [Google Scholar]
- 30.Kang XQ, Zang WJ, Song TS, Xu XL, Yu XJ, Li DL, et al. Rat bone marrow mesenchymal stem cells differentiate into hepatocytes in vitro. World J Gastroenterol. 2005;11:3479–3484. doi: 10.3748/wjg.v11.i22.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KD. Applications of mesenchymal stem cells: an updated review. Chang Gung Med J. 2008;31:228–236. [PubMed] [Google Scholar]
- 32.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Renal Physiol. 2005:28931–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 33.Stieger B, Peters R, Sidler MA, Meier PJ. Hepatocyte transplantation: potential of hepatocyte progenitor cells and bone marrow derived stem cells. Swiss Med Wkly. 2006;136:552–556. doi: 10.4414/smw.2006.11419. [DOI] [PubMed] [Google Scholar]
- 34.Karaoz E, Aksoy A, Ayhan S, Sarıboyaci AE, Kaymaz F, Kasap M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunopheno- typic markers. Histochem Cell Biol. 2013 doi: 10.1007/s00418-009-0629-6. DOI 10.1007/s00418-009-0629-6. [DOI] [PubMed] [Google Scholar]
- 35.Prasajak P, Leeanansaksiri W. Developing a new two-step protocol to generate functional hepatocytes from Wharton's Jelly-Derived mesenchymal stem cells under hypoxic condition. Stem Cells Int 2013. 2013 doi: 10.1155/2013/762196. 762196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen KS, Chen WS, Chen HY, Lee CC, Kao HS, Chen YC, et al. Experience in primary culture of hman peritoneal mesothelial cell. Chin J Physiol. 2012;55:274–283. doi: 10.4077/cjp.2012.baa040. [DOI] [PubMed] [Google Scholar]
- 37.Peng L, Zhu HP, Zheng YB, Xie C, Chong YT, Gao ZL. Mesenchymal stem cells differentiate into hepatocyte-like cells under different induction systems in hepatitis B patients with liver failure. Afr J Biotechnol. 2011;10:840–847. [Google Scholar]
- 38.Snykers S, Kock JD, Rogiers V, Vanhaecke T. In Vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mardani M, Kabiri A, Esfandiari E, Esmaeili A, Pourazar AA, Ansar M, et al. The effect of platelet rich plasma on chondrogenic differentiation of human adipose derived stem cells in transwell culture. Iran J Basic Med Sci. 2013;16:1163–1169. [PMC free article] [PubMed] [Google Scholar]
- 40.Cho CH, Parashurama N, Park EY, Suganuma K, Nahmias Y, Tilles J, et al. Homogeneous differentiation of hepatocyte-like cells from embryonic stem cells: applications for the treatment of liver failure. FASEB J. 2008;22:898–909. doi: 10.1096/fj.06-7764com. [DOI] [PubMed] [Google Scholar]
- 41.Nikoozad Z, Ghorbanian MT, Rezaei A. Comparison of the liver function and hepatic specific genes expression in cultured mesenchymal stem cells and hepatocytes. Iran J Basic Med Sci. 2014;17:27–33. [PMC free article] [PubMed] [Google Scholar]
- 42.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 43.Dhanasekaran M, Indumathi S, Kanmani A, Poojitha R, Revathy KM, Rajkumar JS, et al. Surface antigenic profiling of stem cells from human omentum fat in comparison with subcutaneous fat and bone marrow. Cytotechnology. 2012;64:497–509. doi: 10.1007/s10616-012-9427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominici M, Le Blanc K, Mueller I, Cortenbach IS, Marini FC, Krausc DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]


