Abstract
Previous studies have shown that the effect of ethanol on glycine receptors (GlyRs) containing the α1 subunit is affected by interaction with heterotrimeric G proteins (Gβγ). GlyRs containing the α3 subunit are involved in inflammatory pain sensitization and rhythmic breathing and have received much recent attention. For example, it is unknown whether ethanol affects the function of this important GlyR subtype. Electrophysiologic experiments showed that GlyR α3 subunits were not potentiated by pharmacologic concentrations of ethanol or by Gβγ. Thus, we studied GlyR α1–α3 chimeras and mutants to determine the molecular properties that confer ethanol insensitivity. Mutation of corresponding glycine 254 in transmembrane domain 2 (TM2) found in α1 in the α3A254G –α1 chimera induced a glycine-evoked current that displayed potentiation during application of ethanol (46 ± 5%, 100 mM) and Gβγ activation (80 ± 17%). Interestingly, insertion of the intracellular α3L splice cassette into GlyR α1 abolished the enhancement of the glycine-activated current by ethanol (5 ± 6%) and activation by Gβγ (−1 ± 7%). Incorporation of the GlyR α1 C terminus into the ethanol-resistant α3SA254G mutant produced a construct that displayed potentiation of the glycine-activated current with 100 mM ethanol (40 ± 6%) together with a current enhancement after G protein activation (68 ± 25%). Taken together, these data demonstrate that GlyR α3 subunits are not modulated by ethanol. Residue A254 in TM2, the α3L splice cassette, and the C-terminal domain of α3 GlyRs are determinants for low ethanol sensitivity and form the molecular basis of subtype-selective modulation of GlyRs by alcohol.
Introduction
Glycine receptor (GlyR)–mediated neurotransmission is involved in key physiologic functions, such as motor rhythm generation, coordination of reflex responses, and sensory processing (Legendre, 2001; Dresbach et al., 2008; Lynch, 2009; Zeilhofer et al., 2012). These receptors are pentameric protein complexes arranged symmetrically as a ring around a central ion-conducting pore. Unlike nicotinic acetylcholine receptors (nAChRs) or GABAA receptors, only five GlyR isoforms have been identified to date (α1–α4 and β). These subunits can form homomeric or heteromeric GlyRs (Betz et al., 1999; Legendre, 2001; Grudzinska et al., 2005). Each of the GlyR subunits comprises a large extracellular amino-terminal domain, four α-helical transmembrane domains (TM1–TM4), and a large intracellular domain (ICD) between TM3 and TM4 (Laube et al., 2002; Lynch, 2009).
The α-subunits of GlyRs share most of their structural, biochemical, and biophysical properties (Lynch, 2009) mainly due to extensive sequence identity. More interestingly, it has been reported that GlyR isoforms have different pharmacological properties (Yevenes and Zeilhofer, 2011). In this context, the alcohol pharmacology of GlyRs has been studied extensively, mostly focusing on the α1 subunit (Malosio et al., 1991; Mascia et al., 1996b; Aguayo et al., 2004; Yevenes et al., 2008).
Although it is well accepted that the glycine-activated current through homomeric GlyRs containing the α1 subunit is potentiated by ethanol (Aguayo and Pancetti, 1994; Mascia et al., 1996a; Mihic et al., 1997), the mechanisms underlying this phenomenon are still not completely understood. Indeed, several hypotheses have been proposed to explain the alcohol effects on GlyRs (Mihic et al., 1997; Perkins et al., 2008; Borghese et al., 2012). The existence of ethanol binding pockets and regulatory sites for ethanol actions within the TM of α1 subunit GlyRs has been postulated. For instance, combining mutated GlyRs, molecular modeling, and covalent binding of alcohol analogs has determined that TM2 and TM3 residues jointly shape a water-filled cavity serving as an ethanol binding pocket (Ye et al., 1998; Mascia et al., 2000; Harris et al., 2008). Additional studies showed that residues in extracellular loop 2 and TM1 might contribute to the alcohol binding pockets, or exert modulatory roles on ethanol potentiation (Crawford et al., 2008; Lobo et al., 2008). However, it is also well documented that determinants of the ethanol sensitivity of α1 subunit GlyRs can be located intracellularly, such as protein kinase C phosphorylation (Mascia et al., 1998; Jiang and Ye, 2003) or direct modulation of the ion channel by G protein βγ subunits (Yevenes et al., 2003, 2006, 2008). In addition, the relevance of Gβγ signaling has been recently shown using intracellular blocking peptides designed to alter the interaction between the TM3-TM4 intracellular domain of GlyR and Gβγ (Guzman et al., 2009; San Martin et al., 2012).
Studies in recombinant and native GlyRs have consistently shown that receptors containing α1 are more sensitive to ethanol than those containing α2 (Mascia et al., 1996b; Tapia and Aguayo, 1998; Eggers et al., 2000; Yevenes et al., 2010). The low ethanol sensitivity of homomeric α2 GlyRs has been associated with divergent alcohol binding sites (Ye et al., 1998; Mascia et al., 2000) and the absence of functional modulation by Gβγ (Yevenes et al., 2010). Furthermore, we showed that all GlyR mutants that were sensitive to modulation by Gβγ were also modulated by ethanol, establishing a high degree of positive correlation (Yevenes et al., 2010). On the other hand, little is known about the effects of ethanol on α3 GlyRs (Lobo et al., 2008; Jonsson et al., 2009). Furthermore, it is also unknown whether this GlyR subtype is modulated by Gβγ. This subunit has clinical significance because it is involved in the control of rhythmic breathing (Manzke et al., 2010), and ethanol modulation of this GlyR subtype could be responsible for the respiratory depression induced by excessive alcohol consumption. In addition, modulation of α3 GlyRs by Gβγ through the activation of G protein–coupled receptors could be relevant for the regulation of glycinergic inhibition in other physiologic processes, such as central pain sensitization (Harvey et al., 2004; Manzke et al., 2010).
Here, we examined the molecular mechanisms underlying the sensitivity of α3 GlyRs to ethanol. We found that these GlyRs displayed a low sensitivity to ethanol and were not functionally modulated by Gβγ. Therefore, we examined the molecular characteristics behind this insensitivity and found that they were quite different than those described for α2 GlyRs. Thus, this study defines new sites that control the ethanol effects on GlyRs.
Materials and Methods
Cell Culture and Transfection.
Human embryonic kidney (HEK) 293 cells were cultured using standard methodologies. Cells were plated onto 18-mm glass coverslips in 20-mm culture plate wells and transfected using Xfect transfection reagent (Clontech, Mountain View, CA) with 0.5 µg of DNA for each plasmid studied per well. Expression of enhanced green fluorescent protein was used as a marker of positively transfected cells, and recordings were made after 14–18 hours.
cDNA Constructs.
Mutations were inserted using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) in cDNA constructs encoding rat GlyRs in a pCI vector (Promega, Madison, WI). The GlyR α3-α1 chimera was constructed by the introduction of restriction sites into homologous regions of the cDNAs encoding the rat GlyRs α1 and α3 subunit cDNAs. XbaI sites were engineered into the nucleotide sequences of GlyR α1 and α3S or α3L, corresponding to the region closest to the end of TM3, and SalI sites were incorporated into the cDNA sequences encoding the end of the C terminus, allowing us to combine DNA regions by standard subcloning. The α1-α3 ICD chimera was constructed using a SalI site added previously and a new site for XbaI added for exchange of the C-terminal region of the loop between TM3 and TM4 in α1 GlyRs. The replacement of the intracellular loop of α3L and α3S was accomplished by incorporating directed polymerase chain reaction products with the enzymes SacI and XbaI. Finally, the GlyR α1-α3-α1 chimera was constructed by site-directed mutagenesis. All constructs were confirmed by Sanger DNA sequencing.
Electrophysiology.
Whole-cell recordings were performed as previously described (Yevenes et al., 2003, 2008). A holding potential of −60 mV was used. Patch electrodes were filled with 140 mM CsCl, 10 mM N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-(carboxymethyl)glycine] tetracesium salt, 10 mM HEPES (pH 7.4), 4 mM MgCl2, 2 mM ATP, and 0.5 mM GTP. The external solution contained 150 mM NaCl, 5.4 mM KCl, 2.0 mM CaCl2, 1.0 mM MgCl2, 10 mM HEPES (pH 7.4), and 10 mM glucose. For G protein activation experiments, guanosine 5′-O-(3-thiotriphosphate) (GTPγS) (0.5 mM; Sigma-Aldrich, St. Louis, MO) was added directly to the internal solution, replacing GTP. The amplitude of the glycine current was assayed using a brief (1–6 seconds) pulse of glycine every 120 seconds. The modulation of the glycine current by ethanol (Sigma-Aldrich) was assayed using a pulse of glycine (EC10) coapplied with ethanol to each receptor studied, without any preapplication. In all experiments, a brief pulse of 1 mM glycine was performed at the end of the recording period to test that the glycine concentration corresponded to the actual EC10 in each single experiment. Cells that displayed responses ≤EC5 or ≥EC15 were discarded.
Construction of Glutathione S-Transferase Fusion Proteins and Glutathione S-Transferase Pull-Down Assays.
DNA fragments encoding wild-type GlyR α1, α3S, and α3L intracellular loops were first subcloned in the glutathione-S-transferase (GST) fusion vector pGEX-5X3 (GE Healthcare, Piscataway, NJ). GST fusion proteins were generated in Escherichia coli BL21 using 10 mM isopropyl 1-thio-β-d-galactopyranoside. After 3 hours, the cells were collected and sonicated in lysis buffer (1× phosphate buffer, 1% Triton X-100, and protease inhibitor mixture set II; Calbiochem, San Diego, CA). Subsequently, proteins were purified using a glutathione resin (Novagen, Madison, WI), and normalized amounts of GST fusion proteins were incubated with purified bovine Gβγ protein (Calbiochem). Incubations were made in 800 μl of binding buffer [200 mM NaCl, 10 mM EDTA, 10 mM Tris (pH 7.4), 0.1% Triton X-100, and protease inhibitor mixture set II] at 4°C for 1 hour. The beads were then washed five times, and bound proteins were separated on 10% SDS-polyacrylamide gels. Bound Gβγ was detected using a Gβ antibody (Santa Cruz Biotechnology, Dallas, TX) and a chemiluminescence kit (PerkinElmer Life Sciences, Waltham, MA). Finally, the relative amounts of Gβγ were quantified by densitometry.
Immunofluorescence, Image Visualization, and Analysis.
HEK293 cells on 18-mm glass coverslips were first fixed with 4% paraformaldehyde (0.1 M phosphate buffer, pH 7.4) and were then permeabilized (0.1% Triton X-100) and blocked (10% normal horse serum). Subsequently, all-night incubation with a monoclonal anti-FLAG M2 (Stratagene) and polyclonal anti-GlyR α1 (Synaptic Systems, Goettingen, Germany) or anti-GlyR α3 antibodies (Millipore, Billerica, MA) was carried out. Epitope visualization was performed by incubating the sample with two secondary antibodies conjugated to fluorescein isothiocyanate and cyanine dye (Jackson ImmunoResearch Laboratories, West Grove, PA). Finally, the coverslips were then mounted onto microscope slides using Fluorescence Mounting Medium (Dako Cytomation, Carpinteria, CA). For quantitative analysis, cells were chosen randomly for imaging using a Nikon confocal microscope (TE2000; Nikon, Tokyo, Japan). Single stacks of optical sections in the z-axis were acquired, and dual-color immunofluorescent images were captured in simultaneous two-channel mode. Colocalization was studied by superimposing both color channels. The cross-correlation coefficient (r) between both fluorescence channels was measured using computer software ImageJ (National Institutes of Health, Bethesda, MD) starting from separate immunoreactivity for GlyR and Gβ1-FLAG in the same cell. The theoretical maximum was 1 for identical images. Subsequently, the obtained data were compiled, analyzed, and plotted.
Molecular Modeling.
The GlyR α1 and α3 subunits were constructed by homology modeling using Modeller 9v10 software (http://salilab.org/modeller/) and the Caenorhabditis elegans glutamate-gated chloride channel structure (PDB ID 3RIF) as template (Hibbs and Gouaux, 2011). Due to the lack of sequence identity of ICDs with a known structure protein, these regions were predicted by ab initio technique using the QUARK server (Ann Arbor, MI; Xu and Zhang, 2012). All models were relaxed by energy minimization using a conjugate-gradient protocol with Maestro v9.3 (Schrödinger, LLC, New York, NY). The energy calculation and structural validation were performed by Prosa (Wiederstein and Sippl, 2007) and Procheck (Laskowski et al., 1993), respectively. All figures presented were created by PyMOL (Schrödinger, LLC).
Data Analysis.
Statistical analyses were performed using analysis of variance with values of P < 0.05 considered statistically significant. The values are expressed as arithmetic mean ± S.E.M. For all statistical analyses and plots, Origin 9.0 software (MicroCal, Northampton, MA) was used. Normalized values were obtained by dividing the current amplitude obtained with time of GTPγS dialysis by the current at minute 1. Although the effects of ethanol start at 10 mM (Aguayo and Pancetti, 1994), we and other groups routinely used 100 mM to facilitate statistical comparisons.
Results
Ethanol Sensitivity of Homomeric α3S and α3L Glycine Receptors.
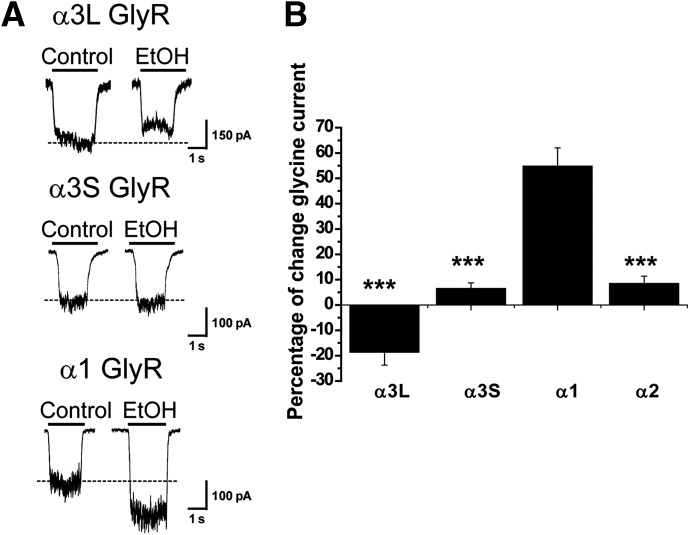
In the mammalian central nervous system, the GlyR α3 subunit is expressed in two splice variants denoted here as GlyR α3S and α3L (Nikolic et al., 1998). These receptors differ in the presence of a 15-amino-acid insertion within the TM3-TM4 intracellular domain of the α3L GlyR variant (KTEAFALEKFYRFSD, denoted as α3L INS). Using an EC10 glycine obtained from concentration-response curves (Table 1), we found that neither α3S nor α3L GlyRs were potentiated by 100 mM ethanol (Fig. 1) or by any ethanol concentration used (with incremental applications between 0.01 and 200 mM) (Supplemental Fig. 1). The glycine-evoked current through α3L GlyRs was slightly reduced by −19 ± 5%, whereas the current through α3S was not affected by a single application of 100 mM ethanol (7 ± 2%) (Fig. 1A). As described previously (Aguayo and Pancetti, 1994), α1 GlyRs were significantly potentiated by 100 mM ethanol (55 ± 7%). Intriguingly, the insensitivity of α3 subunits resembled the resistance of α2 GlyRs to ethanol, which has been previously reported by our group and others (Fig. 1B) (Mascia et al., 1996b; Perkins et al., 2008, 2012; Yevenes et al., 2010). These results suggest the existence of critical structural differences between α3 and α1 GlyRs associated with their differential alcohol sensitivity. In addition, due to the similar ethanol sensitivity and high sequence identity between α3 and α2 GlyRs (85 and 82% for α3S and α3L, respectively), it is likely that both GlyR isoforms share common molecular elements that explain their similar ethanol pharmacology.
TABLE 1.
Electrophysiologic properties of wild-type, mutated, and chimeric GlyRs
Values are given as the mean ± S.E.M. Values were obtained by fitting the data to the equation glycine = Imax(glycine)nH/(glycine)nH + (EC50)nH using computer software. Δ of current change (%) corresponds to change in the presence of 100 mM ethanol with respect to control. Δ of current change (%) at 15 minutes corresponds to change in the presence of intracellular GTPγS (500 μM) measured after 15 minutes of dialysis with respect to control current.
| GlyR | EC50 | Imax | nH | n | Δ of Current Change | Δ of Current Change at 15 Min |
|---|---|---|---|---|---|---|
| μM | pA | % | % | |||
| α1 WT | 45 ± 1 | 2771 ± 563 | 2.8 ± 0.1 | 5 | 55 ± 7 | 80 ± 13 |
| α3L WT | 109 ± 3** | 2041 ± 390 | 2.6 ± 0.2 | 4 | −19 ± 5** | −59 ± 6*** |
| α3S WT | 92 ± 3** | 3510 ± 420 | 2.2 ± 0.2 | 6 | 7 ± 2** | −46 ± 6*** |
| α3L A254G | 251 ± 9** | 2849 ± 692 | 2.9 ± 0.3 | 5 | 1 ± 3** | ND |
| α3S A254G | 92 ± 3** | 3422 ± 889 | 2.7 ± 0.2 | 6 | 11 ± 3** | ND |
| α3-α1 | 31 ± 1** | 3840 ± 691 | 2.3 ± 0.1 | 11 | 8 ± 2** | 10 ± 8*** |
| α3-α1 A254G | 50 ± 2 | 2831 ± 592 | 2.7 ± 0.2 | 8 | 46 ± 5 | 80 ± 5 |
| α1-α3L ICD | 61 ± 3** | 3545 ± 669 | 2.2 ± 0.1 | 5 | 10 ± 3** | 18 ± 10*** |
| α1-α3S ICD | 50 ± 1** | 3914 ± 760 | 2.5 ± 0.1 | 5 | 45 ± 5 | 72 ± 17 |
| α1 + α3L INS | 65 ± 2** | 3600 ± 650 | 2.0 ± 0.1 | 6 | 5 ± 6** | −1 ± 7*** |
| α1-α3S-α1CT | 51 ± 1** | 3278 ± 280 | 2.5 ± 0.1 | 7 | 49 ± 5 | 70 ± 16 |
| α3S A254G-α1CT | 49 ± 4 | 4232 ± 881 | 2.4 ± 0.4 | 5 | 40 ± 6 | 68 ± 15 |
| α1ΔCT | 116 ± 3** | 7650 ± 425 | 3.0 ± 0.2 | 7 | 51 ± 8 | ND |
INS, 15-amino-acid insert; ND, not determined; WT, wild type.
P < 0.01; ***P < 0.001 with respect to α1 WT.
Fig. 1.
Ethanol sensitivity of homomeric α3 GlyRs expressed in HEK293 cells. (A) Representative current traces evoked by glycine (EC10) in control and in the presence of 100 mM ethanol for GlyR α1 and α3 subunits. The segmented line shows the control current amplitude value. (B) Summary of the percentage potentiation obtained after application of a single concentration of 100 mM ethanol. Data with the α2 subunit were included for comparison. The results are the mean ± S.E.M., and all data were significant (***P < 0.001, analysis of variance with respect to GlyR α1). EtOH, ethanol.
G Protein Modulation of α3S and α3L GlyRs.
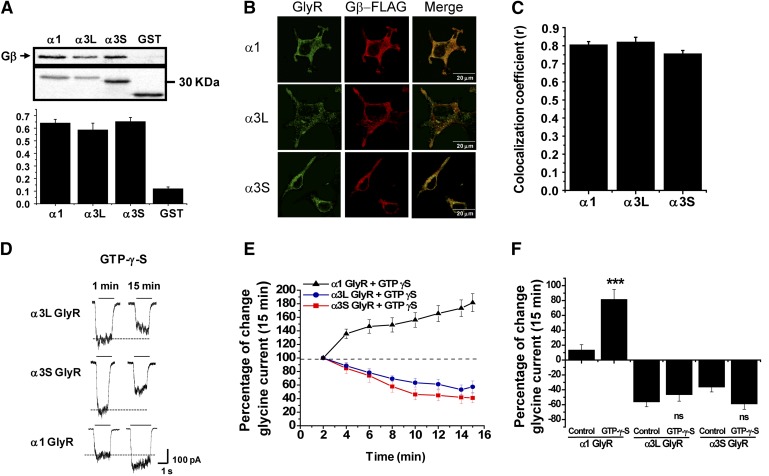
An important number of reports have shown that several intracellular signaling pathways can influence the ethanol sensitivity of several Cys-loop ion channels (Smart, 1997; Yevenes et al., 2003; Harvey et al., 2004; Fischer et al., 2005). For example, previous studies demonstrated that the ethanol potentiation of α1 GlyRs depends on the direct protein interaction between Gβγ with two basic motifs (316-320RFRRK and 385-386KK) of the TM3-TM4 ICD (Yevenes et al., 2003, 2006). Therefore, we first performed a sequence alignment between the large intracellular domains of α1, α3S, and α3L to examine these basic motifs. This sequence analysis revealed 100% identity of the relevant basic residues, suggesting that α3S and α3L TM3-TM4 intracellular domains are able to interact with Gβγ. To evaluate this potential ICD-Gβγ interaction, we designed and constructed GST fusion proteins with the ICD of α3S and α3L subunits. Once expressed and purified, these proteins were used for in vitro interaction assays with purified Gβγ protein. Similar to the results obtained with the α1 and α2 subunits (Yevenes et al., 2006, 2008), the intracellular loops of α3S and α3L were able to bind to Gβγ (Fig. 2A). To further evaluate these interactions in a more intact cellular context, immunocytochemical and confocal microscopy analyses were performed in HEK293 cells transfected with GlyRs and Gβγ. The cellular distribution of GlyRs and Gβγ showed a membrane pattern of colocalization in their expression (Fig. 2B). Correlation analysis yielded high coefficient values (Fig. 2C), giving support for a physical interaction between these GlyRs and Gβγ in a cellular context.
Fig. 2.
α3 GlyRs and G protein βγ interaction and functional modulation. (A) (Top) GST pull-down assays for GlyR α1 and α3 TM3-TM4 ICDs and Gβγ. (Bottom) Quantification of relative amount of bound Gβγ. (B) Transfected HEK293 cells stained with antibodies against GlyR α1 and α3 subunits and FLAG-M2 epitope (red) that recognizes tagged Gβ1. (C) The graph summarizes the correlation coefficients between GlyR subunits and Gβγ. (D) Representative glycine-evoked current traces at the initial time and after 15 minutes of intracellular dialysis of nonhydrolyzable analog GTPγS for GlyRs α1 and α3. The segmented line shows the control current amplitude value. (E) Time course of GTPγS dialysis on GlyR α1 and α3 subunits. (F) The graph summarizes the effects of normal internal solution and internal solution containing GTPγS after 15 minutes on the glycine-evoked current. For all panels, the results are the mean ± S.E.M. For (E), all points were significant after 4 minutes (***P < 0.001, analysis of variance with respect to α1). ***Represents statistical differences between normal internal solution (control) and GTPγS internal solution in GlyR α1. ns, lack of significance in control (GTP) and presence of GTPγS for α3S and α3L.
We next examined the effect of the nonhydrolyzable GTP analog GTPγS on glycine-evoked currents in α3 GlyRs to evaluate the presence of functional modulation by G proteins. In α1 GlyRs, intracellular dialysis of GTPγS significantly enhanced the glycine-evoked currents by 80 ± 13% after 15 minutes of dialysis (Fig. 2, D and E, solid triangles). This response has been shown to be linked to Gβγ modulation and independent of signaling pathways involving protein kinases (Yevenes et al., 2003, 2006). The experiments showed that α3S and α3L GlyRs were not potentiated by G protein activation (Fig. 2, E and F). After 15 minutes of intracellular GTPγS, the glycine-evoked currents of α3S and α3L GlyRs were diminished to −59 ± 6% and −46 ± 6%, respectively. These results could be suggesting a negative modulation of G protein activation on α3 GlyRs (Harvey et al., 2004). However, experiments using control internal solution (i.e., without GTPγS) showed similar percentages of current inhibition (i.e., current run-down) of α3 GlyRs after 15 minutes of whole-cell recording (−36 ± 6% for α3S; −56 ± 7% for α3L) (Fig. 2F), excluding inhibitory effects of the G protein activation on α3 GlyRs. These data demonstrate that α3 GlyRs can directly interact with Gβγ. Nevertheless, these interactions were not translated into a functional modulation of the ion channel, suggesting either the presence of molecular determinants that prevent Gβγ and ethanol modulation or the absence of key structural elements for alcohol binding and subsequent modulation.
Role of TM2-TM3 Domains in the Ethanol Sensitivity of α3 GlyRs.
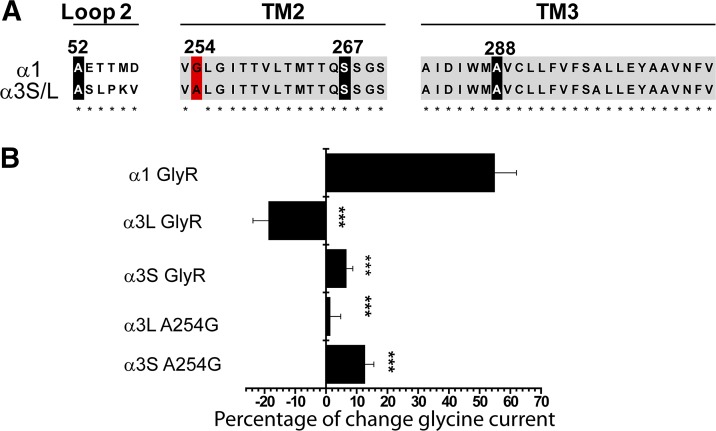
Compelling evidence has described the presence of critical residues for alcohol binding in the TM2 and TM3 regions of α1 GlyRs (Mihic et al., 1997; Harris et al., 2008). Residues in loop 2 have also been suggested in ethanol binding and modulation (Perkins et al., 2008, 2009, 2012). Thus, differences in the sequence within the extracellular loop 2 and the TM2-TM3 domains of α3 GlyRs could be a plausible explanation for their low ethanol sensitivity. However, we found only one nonconserved alanine residue at position A254 of the GlyR α3 (Fig. 3A). Interestingly, this amino acid in GlyR α1 and α2 has been found to be essential for ethanol potentiation and Gβγ modulation (Yevenes et al., 2010). On the other hand, several other key residues necessary for ethanol potentiation in α1 GlyRs described by our group and others (e.g., A52 in the extracellular loop 2, S267 in TM2, and A288/S296 in TM3) were 100% conserved (Mihic et al., 1997; Perkins et al., 2008; Yevenes et al., 2010) (Fig. 3A). Thus, it is possible that the single A→G substitution at position 254 in the TM2 region of α3 GlyRs may underlie alcohol insensitivity of this subtype. To examine this possibility, we constructed α3S and α3L GlyRs with the mutation A254G in the TM2 domain, finding that GlyR α3SA254G or α3LA254G mutants were not sensitive to ethanol (α3SA254G, 11 ± 3%; α3LA254G, 1 ± 3%) (Fig. 3B). Additionally, the mutations significantly changed the glycine sensitivity but not the maximal current amplitude (Table 1). These results indicate that the A254G substitution did not enhance the ethanol sensitivity of α3 GlyRs, thus the α1 phenotype was not rescued, and this is expressed as a statistically significant difference from α1 (Table 1, P < 0.001 with respect to α1 wild type). These data indicate that the mechanisms underlying the low ethanol sensitivity of α3 GlyRs are fundamentally different from those proposed for α2 GlyRs. Thus, we next aimed to identify the additional molecular elements in α3 GlyRs that determine their ethanol insensitivity.
Fig. 3.
Effects of the TM2 mutation A254G on the ethanol sensitivity of α3 GlyRs. (A) Multiple sequence alignment between GlyR α1 and α3 subunits, including important residues in loop 2 (A52), TM2 (G254 or A254, S267), and TM3 (A288) that are essential for potentiation of GlyR α1 by ethanol. (B) The graph summarizes the effects of 100 mM ethanol on GlyRs α1, α3, and the α3SA254G and α3LA254G mutants. The results are the mean ± S.E.M., and all data were significant (***P < 0.001, analysis of variance with respect to GlyR α1).
Molecular Elements in the TM3-TM4 Intracellular Domain Influence the Ethanol Sensitivity of α3 GlyRs.
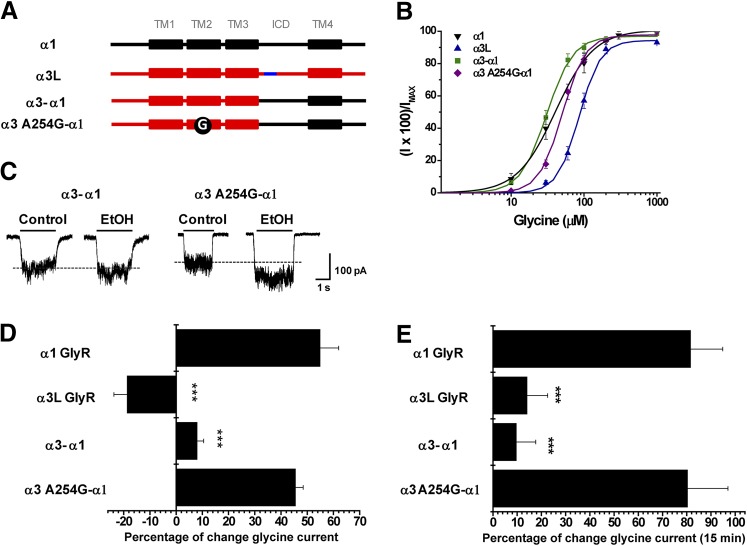
The previous results showed that modification in the TM2 domain of α3 GlyR was not sufficient to change the alcohol sensitivity. Therefore, we focused our attention on molecular elements downstream of the TM3 domain, since these showed the highest variability in different GlyR subtypes. To explore these elements, we first analyzed a chimeric GlyR which combines the coding region downstream of the TM3 domain of GlyR α1 with the region upstream of TM3 of GlyR α3 (GlyR α3-α1) (Fig. 4A). This chimeric GlyR showed an increased apparent affinity for glycine (Fig. 4B; Table 1) but did not display significant ethanol potentiation (8 ± 2%) or G protein modulation (10 ± 8%) (Fig. 4, C and D). This chimeric construct, however, still had residue A254, which is of known importance for the modulation of GlyR α1 by ethanol and was necessary to confer ethanol sensitivity to GlyR α2 (Yevenes et al., 2010). Thus, we also evaluated the ethanol potentiation and G protein modulation of an α3A254G -α1 GlyR mutant (Fig. 4, C and D). Interestingly, the glycine-evoked current of GlyR α3A254G -α1 displayed a robust potentiation, and α1-like phenotype, after G protein activation (80 ± 17%) (Fig. 4E) and significant ethanol potentiation (46 ± 5%, 100 mM) (Fig. 4D). In agreement with previous studies (Yevenes et al., 2008), these data support the existence of a high correlation between both modulations. These results indicate that the low ethanol sensitivity of GlyR α3 is determined by TM2 and other molecular elements downstream of the TM3 domain, which include the ICD, the TM4 domain, and the extracellular C terminus. Interestingly, because the low alcohol sensitivity of α2 GlyRs has been fully linked to extracellular loop 2 and TM2-TM3 domain residues (Yevenes et al., 2010), these so far unrecognized elements downstream from the TM3 domain of α3 GlyRs appear to be unique. Therefore, we next aimed to identify these elements using additional chimeric and mutant GlyRs.
Fig. 4.
Molecular elements downstream of the TM3-TM4 intracellular domain negatively regulate the ethanol sensitivity of α3 GlyRs. (A) Schematic representations of the chimeras GlyR α3-α1 and α3-α1A254G created from α1 (black) and α3 (red) subunits. The 15 additional amino acids present in α3L are displayed in blue, and the A254G mutation in TM2 is indicated by a black circle. (B) The graph shows the concentration-response relationship for wild-type and both chimeric GlyRs. (C) Representative current traces showing the effect of 100 mM ethanol on chimeras GlyR α3-α1 and α3-α1A254G. (D) The graph summarizes the effects of 100 mM ethanol on wild-type α1, α3, and chimeric GlyRs. (E) Modulation of chimeric GlyRs after 15 minutes of intracellular dialysis with GTPγS. For all panels, the results are the mean ± S.E.M. from normalized glycine-activated currents. Differences were significant for α1 (***P < 0.001, analysis of variance).
The Alternative Spliced Region of 15 Amino Acids within the TM3-TM4 Intracellular Domain of α3L GlyRs Negatively Influences Alcohol Effects and G Protein Modulation.
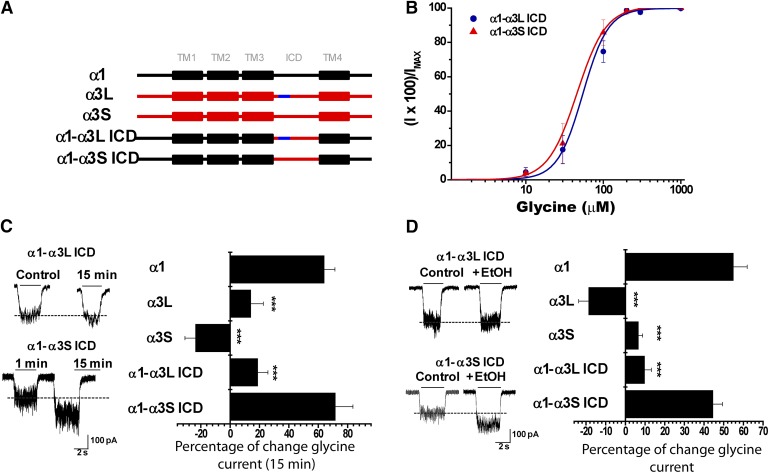
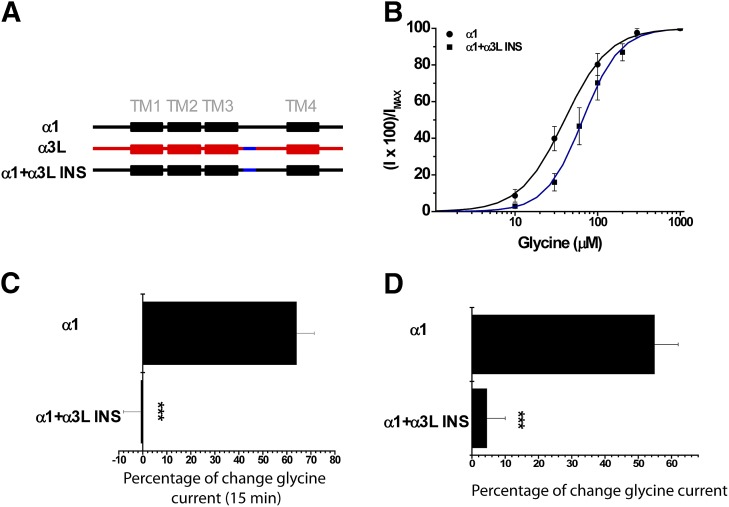
To identify novel residues downstream from the TM3 domain of α3 GlyRs that may exert negative effects on alcohol sensitivity, we studied two chimeric GlyRs in which only the ICDs of α3S and α3L isoforms were incorporated into the α1 GlyR background (α1-α3S ICD and α1-α3L ICD) (Fig. 5A). The glycine-activated current elicited by the α1-α3L ICD GlyR still displayed low sensitivity to ethanol (10 ± 3%) and to G protein activation (18 ± 3%), despite having glycine sensitivity closer to the values of wild-type α1 subunit GlyRs (Fig. 5, B–D; Table 1). Interestingly, the α1-α3S ICD GlyR construct showed a significant potentiation of the glycine-evoked currents after 15 minutes of dialysis with GTPγS (72 ± 17%) and following the application of ethanol (45 ± 5%) (Fig. 5, C and D). Since the only difference between these two constructs is the presence of the alternative spliced 15-amino-acid cassette within the TM3-TM4 domain in α3L GlyRs (Nikolic et al., 1998), these data suggest a negative regulatory role of the α3L cassette in ethanol sensitivity and Gβγ modulation. To further study the role of the α3L cassette on the GlyR pharmacology, we inserted it into a homologous position in the α1 GlyR ICD (α1+α3L INS) (Fig. 6A). Notably, the presence of the α3L cassette in α1 GlyRs significantly reduced the enhancement of the glycine-activated current by activation of G proteins (−1 ± 7%) and ethanol (5 ± 6%) (Fig. 6, C and D). Collectively, these results described an unrecognized intracellular element involved in the low ethanol sensitivity displayed by α3L GlyRs and demonstrate the critical influence of intracellular elements on alcohol pharmacology.
Fig. 5.
The alternatively spliced 15 amino acid cassette within the TM3-TM4 ICD of α3L GlyRs is a negative regulator of ethanol sensitivity and G protein modulation. (A) Schematic representations of chimeric receptors made replacing the TM3-TM4 ICD of GlyR α1 (black) by the corresponding region in GlyR α3 (red). The blue region represents the 15-amino-acid insert present in α3L. (B) Concentration-response curves for α1-α3 ICD using α3S and α3L isoforms. (C) Representative glycine-evoked current traces at the initial time and after 15 minutes of intracellular dialysis using GTPγS on chimeric GlyR α1-α3S or α1-α3L ICDs. The graph summarizes the effects of GTPγS on wild-type α1, α3 subunits and chimeric receptors GlyR α1-α3S or α1-α3L ICDs. (D) Representative current traces in the absence and presence of 100 mM ethanol. The graph summarizes the modulation of wild-type GlyR α1, α3 subunits and chimeric GlyR α1-α3S or α1-α3L ICDs by ethanol. For all panels, the results are the mean ± S.E.M. from normalized glycine-activated currents. Differences were significant for α1 (***P < 0.001, analysis of variance).
Fig. 6.
The presence of α3L INS sequence blunted the ethanol potentiation and G protein modulation of α1 GlyRs. (A) Schematic representation of chimera α1-α3L INS created by insertion of 15 amino acids (blue) of α3L (red) into the α1 GlyR subunit (black). (B) Concentration-response curves for wild-type GlyR α1 and α1 + α3L. (C) Modulation of chimera α1 + α3L by intracellular dialysis of GTPγS. (D) Effects of 100 mM ethanol on activity of chimera α1 + α3L. For all panels, the results are the mean ± S.E.M. from normalized glycine-activated currents. Differences were significant with respect to α1 (***P < 0.001, analysis of variance).
These results, however, still do not explain the low ethanol potentiation displayed by the α3S and the α3SA254G mutants (Fig. 3), which lack the α3L splice cassette. Therefore, additional molecular determinants within the TM4 or the C-terminal region could be negatively influencing the alcohol pharmacology of α3 subunit GlyRs.
Importance of the C-Terminal Region on the Ethanol Sensitivity and Gβγ Modulation of α3 GlyRs.
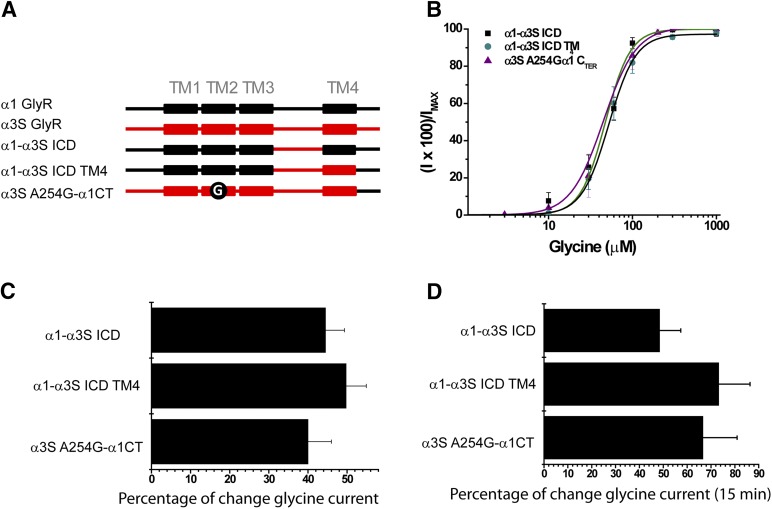
Several lines of evidence have demonstrated the importance of the TM4 domain in the Cys-loop family. Early studies on nAChRs suggested regulatory roles of the TM4 domain for their structure and function (Ortiz-Miranda et al., 1997). For example, mutations in the α- and β-subunit TM4 domains, which are postulated to be in close proximity to the lipid-protein interface, significantly altered ion channel gating (Ortiz-Miranda et al., 1997). More recent studies demonstrated additional critical roles of aromatic residues within the TM4 in the pentameric assembly and function of GlyRs (Haeger et al., 2010). Interestingly, other reports have detected residues in TM4 involved in the ethanol potentiation of α1 GlyRs (Lobo et al., 2006). Thus, it is plausible that the TM4 domain may play a role in the low alcohol sensitivity of α3 GlyRs. Evaluation of the primary sequences of α1 and α3 GlyRs revealed ∼70% amino acid identity, although most of the critical residues described for α1 GlyRs (Lobo et al., 2008) were fully conserved. To test for a possible role of TM4 in the low alcohol sensitivity of α3 GlyRs, we incorporated the TM4 domain into the construct α1-α3S ICD (α1-α3S ICD TM4) (Fig. 7A). The data show that incorporation of the TM4 domain of GlyR α3 did not affect the ethanol sensitivity (49 ± 5%) or G protein modulation (73 ± 10%) (Fig. 7, C and D), excluding a negative influence of α3 TM4 on the alcohol potentiation. We therefore focused our attention toward the putative extracellular C terminus.
Fig. 7.
The C terminus, but not the TM4 domain, influences the ethanol sensitivity and G protein modulation of GlyR α3. (A) Schematic representations of chimeric receptors created using α1 (black) and α3S (red) subunits, α1-α3S ICD generated by replacing only the ICD, α1-α3S ICD TM4 which includes the TM4 of α3S and the C-terminal domain of α1, and α3S-α1CT A254G. (B) Concentration-response curves for all chimeric GlyRs described previously. (C) The graph summarizes the potentiation of glycine-evoked currents by 100 mM ethanol on chimeric GlyRs. (D) Modulation of chimeric receptors by GTPγS after intracellular dialysis for 15 minutes. For all panels, the results are the mean ± S.E.M. from normalized glycine-activated currents. No differences were detected.
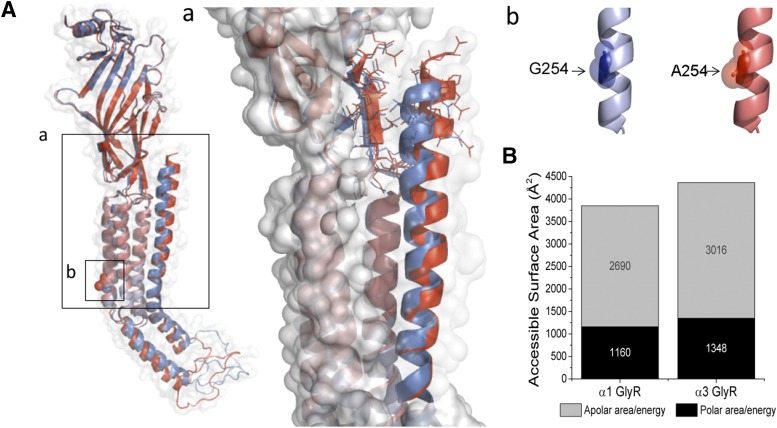
The exact role of the extracellular C terminus in the Cys-loop physiology and pharmacology has remained elusive. Nevertheless, studies on nAChRs have suggested that residues at the external end of TM4 may interact with the ligand binding domain via either an electrostatic interaction (Xiu et al., 2005) or protein-sugar interactions (Dellisanti et al., 2007), contributing to the gating of the ion channel. However, the influence of the C-terminal region on GlyR pharmacology is unknown. The amino acid sequences of the putative C terminus of GlyR α1 and α3 are not highly conserved (64% amino acid identity), raising the question of whether these differences may play a role in GlyR alcohol pharmacology. Therefore, we incorporated the C terminus (CT) of GlyR α1 into the template of the ethanol-resistant construct α3SA254G (α3SA254G-α1CT) (Fig. 7A). Notably, this GlyR mutant displayed a potentiation of the glycine-activated currents with 100 mM ethanol (40 ± 6%) together with a current enhancement after G protein activation (68 ± 25%) without changes in apparent affinity for glycine (Fig. 7, B–D). Finally, to analyze the structural relationship of the C terminus to ethanol sensitivity, in silico prediction showed differences in the C-terminal region for GlyR α1 and α3. The structural alignment, including TM4 to the C terminus, showed a deviation in the helical structure of α3 with respect to α1 (Fig. 8A), which combined with the greater length of the C-terminal segment generated a “less packed” structure for α3, associated with an increase in the accessible surface area compared with α1 (Fig. 8B). Additionally, in the model of α3, alanine 254 is found at a similar position to G254 on α1, and both are facing the inner side of TM2, explaining its critical role in the modulation of glycine receptors by ethanol and G protein (Fig. 8B). Finally, we tested the impact of the C terminus on the α1 subunit by truncating this region in α1ΔCT. Interestingly, the data showed that this receptor expressed a functional ion channel that was equally sensitive to ethanol as the α1 wild type (51 ± 8%) (Table 1). Taken together, our results demonstrate that the C terminus in GlyR α3, but not in α1, is another structural element that determines the low ethanol sensitivity of α3 glycine receptors, and suggest a pivotal role of C-terminal domains in alcohol pharmacology of some GlyRs.
Fig. 8.
Molecular modeling of GlyR α1 and α3. (A) Structural alignment between α1 (blue) and α3 (red) including the magnification of the segment TM4–C terminus and the complementary region of extracellular domain showing the residues involved in structural stabilization (a). The TM2 residues described in this study are also highlighted (b). (B) Calculation of accessible solvent surface for both GlyR subunits supporting the idea of a “less packed” conformation for GlyR α3.
Discussion
The wide range of ethanol effects on GlyRs have been explained by two hypotheses: 1) binding of alcohol within the ion channel (Mihic et al., 1997) or 2) indirect regulation of the channel caused by alcohol modulation of signal transduction pathways (Yevenes et al., 2006, 2008, 2010). A binding pocket formed by transmembrane residues I229 in TM1 (Lobo et al., 2008), S267 in TM2 (Mihic et al., 1997), A288 in TM3 (Mihic et al., 1997), and I409, Y410, and K411 in TM4 (Lobo et al., 2006) has been proposed to play a key role in alcohol binding to GlyRs. Residue A52 is another relevant amino acid, transferring binding energy into channel opening. Nevertheless, the lack of effect of ethanol on GlyR α3 cannot be explained by these elements, since all of these residues are conserved within the GlyR α3 subunit.
The second possibility postulates that ethanol modulates GlyR by an indirect mechanism through the activation or inhibition of intracellular signals. The most widely studied and recognized pathways involved in intracellular regulation of ligand-gated ion channel function involve phosphorylation through protein kinases. Indeed, the function of GlyR and other members of the ligand-gated ion channel superfamily is affected by activation of cAMP-dependent kinases and protein kinase C (Smart, 1997; Harvey et al., 2004). In this context, GlyR α3 is known to be negatively modulated by phosphorylation of S346 by protein kinase A (PKA) (Harvey et al., 2004). Interestingly, the GlyR α1 subunit does not have this PKA phosphorylation site, and the addition of the α3L cassette (S346 lies outside this region) prevented the functional modulation of GlyR α1 by alcohol. Furthermore, an equivalent PKA phosphorylation site is present in GlyR α2, where mutations upstream of TM3 confer ethanol and Gβγ modulation (Yevenes et al., 2010). In summary, phosphorylation by PKA does not seem to be important for ethanol action on GlyRs.
Besides the differences in protein sequence, the GlyR α3 subunit exhibits variants generated by alternative splicing that confer differential properties in the regulation by protein kinases (Lynch, 2004), extent and time course of desensitization (Breitinger et al., 2002), structural properties (Breitinger et al., 2009), ion channel gating (Breitinger et al., 2009), and receptor clustering and diffusion (Notelaers et al., 2012). In this framework, we analyzed the involvement of the intracellular domain and the GlyR α3L cassette in ethanol and Gβγ modulation. We found that the α3L cassette conferred loss of ethanol and Gβγ modulation on the GlyR α1 subunit (Fig. 6). These results highlight a new role for the α3L cassette as a negative regulatory domain for this allosteric modulator. This negative regulation can be explained by differences in intracellular domain structural flexibility, since the presence of the L-insert facilitates an α-helix structure to this region, not present in the α3S isoform (Breitinger et al., 2009). These results suggest that the lack of sensitivity of α3 is dependent on the presence of the α3L cassette and molecular requirements downstream of the ICD. We also found that both GlyR α1 and α3 bind Gβγ (Fig. 2), but only the GlyR α1 conformation allowed an effective conversion of Gβγ binding into functional allosteric modulation (Yevenes et al., 2006, 2008, 2010).
Three residues were described as necessary for Gβγ modulation and ethanol sensitivity in GlyR α1 and α2 subunits (Yevenes et al., 2010): residue A52 in loop 2 of the extracellular domain (Yevenes et al., 2010; Perkins et al., 2012), G254 in TM2 (Mihic et al., 1997; Yevenes et al., 2010), and S296 in TM3 (Yevenes et al., 2010) (using GlyR α1 numbering). We proposed that they allow a molecular transition between a resting closed state and a preopened closed state (denominated “flipped” state) of the glycine-bound GlyR (Plested et al., 2007) facilitating ion-channel opening after Gβγ binding (Yevenes et al., 2010). Here, we found that G254 in α3 was required, but not sufficient, for ethanol sensitivity and Gβγ modulation of homomeric α3 GlyRs (Figs. 3 and 4). Because G254 is important for gating properties, the presence of an alanine residue in α3 possibly interferes with the structural rearrangements associated with the binding of Gβγ, similar to that in GlyR α2 (Yevenes et al., 2010). The replacement of alanine by glycine in residue 254 contributes to a partial recovery of ethanol sensitivity (Fig. 3B), but only additional modifications allow complete recovery.
Participation of the TM4 and extracellular C terminus in GlyR physiology and pharmacology is largely unknown. It was recently proposed that TM4 and the C-terminal segment are involved in some physiologic and structural properties of GlyRs (Villmann et al., 2009). Furthermore, electrophysiological studies have shown that mutations in TM4 residues can influence the gating of nAChRs and α1 GABAA receptors (Jenkins et al., 2002), and even produce nonfunctional channels in AChR and GlyR (Haeger et al., 2010). Additionally, it was suggested that residues at the C terminus of TM4 in nAChR may interact with the ligand binding domain via either an electrostatic interaction (Xiu et al., 2005) or protein-sugar interactions (Dellisanti et al., 2007) contributing to channel gating (Xiu et al., 2005). Interestingly, the alignment of TM4 in GlyR α1 and α3 subunits showed nine nonconserved residues, in addition to the two residues present at the C terminus, which could be responsible for the insensitivity to modulation by Gβγ and ethanol. The present results discard TM4 as a negative influence on GlyR ethanol and Gβγ modulation (Fig. 7C). This result differs from other studies that have suggested that this region is important for closed and glycine-activated states (Han et al., 2013), indicating that these findings might be specific for GlyR α1 (Chen et al., 2009; Han et al., 2013). Furthermore, no significant differences in EC50 and Imax parameters were found in the chimeric receptors with changed TM4 or C-terminal domains (α1-α3S ICD TM4, α3S A254G-α1CT), and they were modulated by ethanol and Gβγ (Fig. 7). Thus, we propose that the difference in ethanol and Gβγ modulation is associated with a different degree of proximity or structural complementarity between TM4 and the C terminus with extracellular domains. Through predictive molecular modeling studies and comparative analysis between GlyR α1 and α3, we determined that α3 presents a shift in its helical structure (TM4 and C terminus) (Fig. 8A) in agreement with a previous study (Han et al., 2013). This structural difference in α3 would produce a conformation less favorable for Gβγ and ethanol modulation, and it is related to an increase in the volume of the α3 C-terminal region caused by the presence of larger side chains of the amino acids HQQD located at the sequence end of α3, accompanied by an increase in the accessible surface area of α3 compared with α1 (Fig. 8B). Along with this, replacement of the α3 C terminus by its corresponding segment of α1 in the chimeras α3-α1 A254G and α3S-α1CT A254G returns the complementarity between the C-terminal and extracellular domains being modulated by ethanol and G protein (Figs. 4 and 7). The situation is quite different for α1 since the elimination of its C terminal (α1ΔCT) did not affect its sensitivity to ethanol. This finding suggests that the C terminal of α1 affects the overall properties of α3, and that ethanol modulation depends on diverse regions along this protein.
In a physiologic context, GlyRs containing α3 participate in the spinal component of inflammatory hyperalgesia (Ahmadi et al., 2002; Harvey et al., 2004). They are also found in Botzinger complex, Pre–Botzinger complex, and spinal trigeminal nucleus in the brainstem where they appear to control rhythmic activity of respiratory networks (Manzke et al., 2010). Overall, our data support the conclusion that GlyRs containing α3 in these locations are relatively insensitive to ethanol and Gβγ modulation. We also conclude that residue G254, a key element for ethanol sensitivity and Gβγ modulation in GlyR α1 and α2 (Yevenes et al., 2010), is necessary but not sufficient for ethanol sensitivity and Gβγ modulation of α3 GlyRs. Rather, we found that the α3L cassette and the extracellular C terminus are novel determinants for ethanol sensitivity and Gβγ modulation for GlyRs containing α3.
Supplementary Material
Acknowledgments
The authors thank Lauren Aguayo for technical assistance during the study.
Abbreviations
- GlyR
glycine receptor
- GST
glutathione-S-transferase
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- HEK
human embryonic kidney
- ICD
large intracellular loop domain
- INS
insertion of 15 amino acids
- nAChR
nicotinic acetylcholine receptor
- PKA
protein kinase A
- TM
transmembrane domain
Authorship Contributions
Participated in research design: Sánchez, Burgos, Yévenes, Aguayo.
Conducted experiments: Sánchez, San Martin, Yévenes, Moraga-Cid.
Contributed new reagents or analytic tools: Harvey.
Performed data analysis: Sánchez, San Martin, Moraga-Cid, Burgos.
Wrote or contributed to the writing of the manuscript: Sánchez, Burgos, Yévenes, Harvey, Aguayo.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant R01-AA15150]. R.J.H. is supported by the Medical Research Council [G0500833, J004049].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aguayo LG, Pancetti FC. (1994) Ethanol modulation of the γ-aminobutyric acidA- and glycine-activated Cl− current in cultured mouse neurons. J Pharmacol Exp Ther 270:61–69. [PubMed] [Google Scholar]
- Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. (2004) Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev 47:33–45. [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. (2002) PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci 5:34–40. [DOI] [PubMed] [Google Scholar]
- Betz H, Kuhse J, Schmieden V, Laube B, Kirsch J, Harvey RJ. (1999) Structure and functions of inhibitory and excitatory glycine receptors. Ann N Y Acad Sci 868:667–676. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Blednov YA, Quan Y, Iyer SV, Xiong W, Mihic SJ, Zhang L, Lovinger DM, Trudell JR, Homanics GE, et al. (2012) Characterization of two mutations, M287L and Q266I, in the α1 glycine receptor subunit that modify sensitivity to alcohols. J Pharmacol Exp Ther 340:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitinger HG, Villmann C, Melzer N, Rennert J, Breitinger U, Schwarzinger S, Becker CM. (2009) Novel regulatory site within the TM3-4 loop of human recombinant α3 glycine receptors determines channel gating and domain structure. J Biol Chem 284:28624–28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitinger HG, Villmann C, Rennert J, Ballhausen D, Becker CM. (2002) Hydroxylated residues influence desensitization behaviour of recombinant α3 glycine receptor channels. J Neurochem 83:30–36. [DOI] [PubMed] [Google Scholar]
- Chen X, Webb TI, Lynch JW. (2009) The M4 transmembrane segment contributes to agonist efficacy differences between α1 and α3 glycine receptors. Mol Membr Biol 26:321–332. [DOI] [PubMed] [Google Scholar]
- Crawford DK, Perkins DI, Trudell JR, Bertaccini EJ, Davies DL, Alkana RL. (2008) Roles for loop 2 residues of α1 glycine receptors in agonist activation. J Biol Chem 283:27698–27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. (2007) Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 A resolution. Nat Neurosci 10:953–962. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Nawrotzki R, Kremer T, Schumacher S, Quinones D, Kluska M, Kuhse J, Kirsch J. (2008) Molecular architecture of glycinergic synapses. Histochem Cell Biol 130:617–633. [DOI] [PubMed] [Google Scholar]
- Eggers ED, O’Brien JA, Berger AJ. (2000) Developmental changes in the modulation of synaptic glycine receptors by ethanol. J Neurophysiol 84:2409–2416. [DOI] [PubMed] [Google Scholar]
- Fischer H, Liu DM, Lee A, Harries JC, Adams DJ. (2005) Selective modulation of neuronal nicotinic acetylcholine receptor channel subunits by Go-protein subunits. J Neurosci 25:3571–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. (2005) The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45:727–739. [DOI] [PubMed] [Google Scholar]
- Guzman L, Moraga-Cid G, Avila A, Figueroa M, Yevenes GE, Fuentealba J, Aguayo LG. (2009) Blockade of ethanol-induced potentiation of glycine receptors by a peptide that interferes with Gβγ binding. J Pharmacol Exp Ther 331:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger S, Kuzmin D, Detro-Dassen S, Lang N, Kilb M, Tsetlin V, Betz H, Laube B, Schmalzing G. (2010) An intramembrane aromatic network determines pentameric assembly of Cys-loop receptors. Nat Struct Mol Biol 17:90–98. [DOI] [PubMed] [Google Scholar]
- Han L, Talwar S, Lynch JW. (2013) The relative orientation of the TM3 and TM4 domains varies between α1 and α3 glycine receptors. ACS Chem Neurosci 4:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. (2008) Ethanol’s molecular targets. Sci Signal 1:re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, et al. (2004) GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304:884–887. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Andreasen A, Trudell JR, Harrison NL. (2002) Tryptophan scanning mutagenesis in TM4 of the GABAA receptor α1 subunit: implications for modulation by inhaled anesthetics and ion channel structure. Neuropharmacology 43:669–678. [DOI] [PubMed] [Google Scholar]
- Jiang ZL, Ye JH. (2003) Protein kinase C ε is involved in ethanol potentiation of glycine-gated Cl− current in rat neurons of ventral tegmental area. Neuropharmacology 44:493–502. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytiä P, Ericson M, Söderpalm B. (2009) Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res 1305 (Suppl):S27–S36. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Macarthur MW, Moss DS, Thornton JM. (1993) Procheck: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291. [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H. (2002) Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci 23:519–527. [DOI] [PubMed] [Google Scholar]
- Legendre P. (2001) The glycinergic inhibitory synapse. Cell Mol Life Sci 58:760–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Harris RA, Trudell JR. (2008) Cross-linking of sites involved with alcohol action between transmembrane segments 1 and 3 of the glycine receptor following activation. J Neurochem 104:1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Trudell JR, Harris RA. (2006) Accessibility to residues in transmembrane segment four of the glycine receptor. Neuropharmacology 50:174–181. [DOI] [PubMed] [Google Scholar]
- Lynch JW. (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84:1051–1095. [DOI] [PubMed] [Google Scholar]
- Lynch JW. (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56:303–309. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marquèze-Pouey B, Kuhse J, Betz H. (1991) Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J 10:2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hülsmann S, Ponimaskin E, Müller U, Smart TG, Harvey RJ, et al. (2010) Serotonin receptor 1A-modulated phosphorylation of glycine receptor α3 controls breathing in mice. J Clin Invest 120:4118–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Machu TK, Harris RA. (1996a) Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol 119:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. (1996b) A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol 50:402–406. [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Wick MJ, Martinez LD, Harris RA. (1998) Enhancement of glycine receptor function by ethanol: role of phosphorylation. Br J Pharmacol 125:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389:385–389. [DOI] [PubMed] [Google Scholar]
- Nikolic Z, Laube B, Weber RG, Lichter P, Kioschis P, Poustka A, Mülhardt C, Becker CM. (1998) The human glycine receptor subunit α3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J Biol Chem 273:19708–19714. [DOI] [PubMed] [Google Scholar]
- Notelaers K, Smisdom N, Rocha S, Janssen D, Meier JC, Rigo JM, Hofkens J, Ameloot M. (2012) Ensemble and single particle fluorimetric techniques in concerted action to study the diffusion and aggregation of the glycine receptor α3 isoforms in the cell plasma membrane. Biochim Biophys Acta 1818:3131–3140. [DOI] [PubMed] [Google Scholar]
- Ortiz-Miranda SI, Lasalde JA, Pappone PA, McNamee MG. (1997) Mutations in the M4 domain of the Torpedo californica nicotinic acetylcholine receptor alter channel opening and closing. J Membr Biol 158:17–30. [DOI] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Asatryan L, Davies DL, Alkana RL. (2012) Charge and geometry of residues in the loop 2 β hairpin differentially affect agonist and ethanol sensitivity in glycine receptors. J Pharmacol Exp Ther 341:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. (2008) Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J Neurochem 106:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Asatryan L, Alkana RL, Davies DL. (2009) Loop 2 structure in glycine and GABAA receptors plays a key role in determining ethanol sensitivity. J Biol Chem 284:27304–27314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested AJ, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. (2007) Single-channel study of the spasmodic mutation α1A52S in recombinant rat glycine receptors. J Physiol 581:51–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin L, Cerda F, Jimenez V, Fuentealba J, Muñoz B, Aguayo LG, Guzman L. (2012) Inhibition of the ethanol-induced potentiation of α1 glycine receptor by a small peptide that interferes with Gβγ binding. J Biol Chem 287:40713–40721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG. (1997) Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr Opin Neurobiol 7:358–367. [DOI] [PubMed] [Google Scholar]
- Tapia JC, Aguayo LG. (1998) Changes in the properties of developing glycine receptors in cultured mouse spinal neurons. Synapse 28:185–194. [DOI] [PubMed] [Google Scholar]
- Villmann C, Oertel J, Melzer N, Becker CM. (2009) Recessive hyperekplexia mutations of the glycine receptor α1 subunit affect cell surface integration and stability. J Neurochem 111:837–847. [DOI] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:W407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Hanek AP, Wang J, Lester HA, Dougherty DA. (2005) A unified view of the role of electrostatic interactions in modulating the gating of Cys loop receptors. J Biol Chem 280:41655–41666. [DOI] [PubMed] [Google Scholar]
- Xu D, Zhang Y. (2012) Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins 80:1715–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. (1998) Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. J Biol Chem 273:3314–3319. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Avila A, Guzmán L, Figueroa M, Peoples RW, Aguayo LG. (2010) Molecular requirements for ethanol differential allosteric modulation of glycine receptors based on selective Gβγ modulation. J Biol Chem 285:30203–30213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Guzmán L, Haeger S, Oliveira L, Olate J, Schmalzing G, Aguayo LG. (2006) Molecular determinants for G protein βγ modulation of ionotropic glycine receptors. J Biol Chem 281:39300–39307. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. (2008) A selective Gβγ-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci USA 105:20523–20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. (2003) Modulation of glycine-activated ion channel function by G-protein βγ subunits. Nat Neurosci 6:819–824. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Zeilhofer HU. (2011) Allosteric modulation of glycine receptors. Br J Pharmacol 164:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, Yévenes GE. (2012) Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev 92:193–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.