Abstract
Glycine receptors (GlyRs) are broadly expressed in the central nervous system. Ethanol enhances the function of brain GlyRs, and the GlyRα1 subunit is associated with some of the behavioral actions of ethanol, such as loss of righting reflex. The in vivo role of GlyRα2 and α3 subunits in alcohol responses has not been characterized despite high expression levels in the nucleus accumbens and amygdala, areas that are important for the rewarding properties of drugs of abuse. We used an extensive panel of behavioral tests to examine ethanol actions in mice lacking Glra2 (the gene encoding the glycine receptor alpha 2 subunit) or Glra3 (the gene encoding the glycine receptor alpha 3 subunit). Deletion of Glra2 or Glra3 alters specific ethanol-induced behaviors. Glra2 knockout mice demonstrate reduced ethanol intake and preference in the 24-hour two-bottle choice test and increased initial aversive responses to ethanol and lithium chloride. In contrast, Glra3 knockout mice show increased ethanol intake and preference in the 24-hour intermittent access test and increased development of conditioned taste aversion to ethanol. Mutants and wild-type mice consumed similar amounts of ethanol in the limited access drinking in the dark test. Other ethanol effects, such as anxiolysis, motor incoordination, loss of righting reflex, and acoustic startle response, were not altered in the mutants. The behavioral changes in mice lacking GlyRα2 or α3 subunits were distinct from effects previously observed in mice with knock-in mutations in the α1 subunit. We provide evidence that GlyRα2 and α3 subunits may regulate ethanol consumption and the aversive response to ethanol.
Introduction
Glycine receptors (GlyRs) constitute the major inhibitory neurotransmitter receptor system in the brainstem and spinal cord, and are also found in other parts of the central nervous system (Betz, 1991). For example, GlyRs are broadly expressed in brain (Lynch, 2009; Dutertre et al., 2012; Salling and Harrison, 2014) and are located extrasynaptically (Muller et al., 2008; Xu and Gong, 2010), presynaptically (Jeong et al., 2003; Xiong et al., 2014), and postsynaptically (Dumoulin et al., 2001), depending on the brain region and cell type studied. GlyRs from humans and rats contain three subtypes of α subunits (α1–3) and one β subunit (Grenningloh et al., 1990). In addition, the α4 subunit is found in mice (Matzenbach et al., 1994) and chicks (Harvey et al., 2000). GlyRs in adult animals consist of heteromeric αβ and homomeric α subunits (Lynch, 2009; Xiong et al., 2014).
GlyR activation enhances dopamine release in the striatum (Yadid et al., 1993), nucleus accumbens (NAc) (Ericson et al., 2006), and ventral tegmental area (Ye et al., 2004), thus potentially influencing the rewarding properties of drugs of abuse. Ethanol enhances the function of recombinant (Trudell et al., 2014) and native brain GlyRs (Badanich et al., 2013; Maguire et al., 2014). The work of Badanich et al. (2013) emphasized a selective effect of GlyRs in ethanol action in lateral orbitofrontal cortex neurons. Surprisingly, GABAA receptors made no contribution to the inhibitory effects of ethanol, and there was only a minor role for glutamate receptors; instead, most of the inhibition was due to enhancement of GlyR function. Some of the behavioral effects of ethanol are also likely due to enhancement of GlyR function because glycine microdialysis into the NAc increases extracellular dopamine and decreases ethanol consumption in a strychnine-sensitive manner (Molander et al., 2005). Microdialysis of strychnine also blocks tetrahydrocannabinol- and nicotine-induced dopamine increases in the NAc, indicating that accumbal GlyRs are involved in the actions of these drugs, but are not involved in cocaine or morphine actions (Jonsson et al., 2014). The alcohol use disorder therapeutic, acamprosate, was also shown to interact with GlyRs in the NAc to reduce dopamine release and ethanol consumption (Chau et al., 2010).
Other behavioral studies have further implicated GlyRs in alcohol actions. Ethanol-induced loss of righting reflex (LORR) in mice is augmented by the intracerebroventricular administration of glycine or its precursor serine (Williams et al., 1995), and these effects were blocked by strychnine (Ye et al., 2009). The hypnotic effects of ethanol were altered in spastic and spasmodic mice bearing dysfunctional GlyRα1 subunits (Quinlan et al., 2002). Studies using transgenic mice expressing a mutation (S267Q) in GlyRα1 subunits (Findlay et al., 2002), as well as heterozygous knock-in mice with mutations (Q266I, M287L, or D80A) in the α1 subunit, demonstrated changes in ethanol-induced incoordination and LORR (Blednov et al., 2012; McCracken et al., 2013a). Furthermore, knock-in mice with a mutation that reduces ethanol sensitivity of the GlyRα1 subunit show reduced duration of the LORR produced by ethanol (Aguayo et al., 2014).
Our understanding of alcohol action on GlyRs in vivo is based largely on studies of the α1 subunit, although α2 and α3 subunits are more abundantly expressed in some brain areas considered to be important for alcohol-related behaviors (Jonsson et al., 2009, 2012). For example, in the amygdala and NAc, GlyRα2 and α3 subunits show equal or greater expression compared with α1 (Jonsson et al., 2009, 2012; Delaney et al., 2010). GlyRs expressed in the spinal cord are predominantly α1β heteromers, whereas brain GlyRs, including those expressed in reward-related pathways, contain populations of α2 or α3 homomeric receptors (Muller et al., 2008; Eichler et al., 2009; Adermark et al., 2011; Chen et al., 2011; Weltzien et al., 2012). Furthermore, a human genetic linkage study in African Americans identified GLRA3 (which encodes the human glycine receptor alpha 3 subunit) as a candidate gene in alcohol dependence (Han et al., 2013). Based on this evidence, we examined the behavioral actions of ethanol in mutant mice lacking Glra2 (the gene encoding the glycine receptor alpha 2 subunit) or Glra3 (the gene encoding the glycine receptor alpha 3 subunit).
Materials and Methods
Animals.
Generation of Glra2 (B6;129S4-Glra2tm1Clc/J) knockout (KO) mice was described earlier (Young-Pearse et al., 2006). Breeding pairs were provided by M. McCall (University of Louisville, Louisville, KY). Because Glra2 is located on the X-chromosome, all behavioral analyses were performed on hemizygous (−/Y) and wild-type (WT) (+/+) male littermates generated from crosses between hemizygous and WT animals. Generation of Glra3 (B6;129OlaHsd-Glra3tm1.1Umu/J) KO mice was described earlier (Harvey et al., 2004). Breeding pairs were provided by H. Betz (Max Planck Institute for Brain Research, Frankfurt, Germany). Both colonies were backcrossed twice on a C57BL/6J genetic background. All behavioral analyses were performed on homozygous or hemizygous KO mice and WT littermates generated from crosses between heterozygous animals. Two different 129 substrains were used to generate the KO mice, which may account for the slightly different ethanol responses that we observed in WT mice from the two strains. Although we performed two backcrosses on a C57BL/6J background, the parental 129 strain genotype could still influence behaviors. However, all KO mice were analyzed with their WT counterparts, thus controlling for differences in genetic background between WT and corresponding KO mice. After weaning, mice were housed in the Animal Resources Center at University of Texas with ad libitum access to rodent chow and water with 12-hour light/dark cycles (lights on at 7:00 AM). Male mice between 8 and 12 weeks of age were used. Each mouse was used for one experiment, and all mice were ethanol-naive at the start of each study. All experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas (#AUP 2013-00061) and were conducted in accordance with National Institutes of Health guidelines regarding the care and use of animals in research.
Ethanol Preference Drinking, 24-Hour Access.
A two-bottle choice protocol was carried out as previously described (Blednov et al., 2003). Briefly, mice were allowed to acclimate for 1 week to individual housing. Two drinking bottles were continuously available to each mouse, and bottles were weighed daily. One bottle always contained water. Food was available ad libitum, and mice were weighed every 4 days. After 4 days of water consumption (both bottles), mice were offered 3% ethanol (v/v) versus water for 4 days. Bottle positions were changed daily to control for position preferences. The quantity of ethanol consumed (g/kg body weight per 24 hours) was calculated for each mouse, and these values were averaged for each concentration of ethanol. Immediately following 3% ethanol, a choice between 6% (v/v) ethanol and water was offered for 4 days, followed by 9, 12, and finally 15% (v/v) ethanol, with each concentration being offered for 4 days. Throughout the experiment, evaporation/spillage estimates were calculated daily from two bottles placed in an empty cage; one bottle contained water, and the other contained the appropriate ethanol solution.
Preference for Nonalcohol Tastants, 24-Hour Access.
WT or KO mice were also tested for saccharin and quinine consumption using a two-bottle choice protocol. One tube always contained water, and the other contained the tastant solution. Mice were offered saccharin (0.033 and 0.066%) and quinine hemisulfate (0.03 and 0.06 mM), and intakes were calculated. Each concentration was offered for 4 days, and bottle positions were changed daily. For each tastant, the low concentration was always presented first, followed by the higher concentration. Between tastant testing, mice had access to two bottles of water for 2 weeks.
Ethanol Drinking: 24-Hour Access Every Other Day (Intermittent Drinking).
Intermittent (every other day) access to ethanol increases voluntary ethanol consumption in rats (Wise, 1973; Simms et al., 2008) and mice (Melendez, 2011). We assessed ethanol consumption using intermittent access to 15% and 20% ethanol. Mice were given access to one bottle of ethanol and one bottle of water during 24-hour sessions every other day. The placement of the ethanol bottle was alternated with each drinking session to control for side preferences. The quantity of ethanol consumed was calculated as g/kg body weight per 24 hours.
Ethanol Drinking: Limited Access Drinking in the Dark Phase (One-Bottle Drinking in the Dark).
The limited access drinking test produces pharmacologically significant levels of ethanol in the blood (Rhodes et al., 2005). Beginning 3 hours after lights off, water bottles were replaced with bottles containing a 15% ethanol solution. The ethanol bottles remained in place for either 2 (first 3 days) or 4 hours (day 4) and then were replaced with water bottles. Other than these short periods of ethanol drinking, mice had unlimited access to water. The ethanol bottles were weighed before placement and after removal of the bottles from each experimental cage. The quantity of ethanol consumed was calculated as g/kg body weight per 2 or 4 hours.
Loss of Righting Reflex.
Sensitivity to the depressant effects of ethanol (3.6 g/kg) and other drugs (225 mg/kg flurazepam and 175 mg/kg ketamine) was determined using the standard duration of LORR (sleep time) test in mice. When mice became ataxic, they were placed in the supine position in V-shaped plastic troughs until they were able to right themselves three times within 30 seconds. Sleep time was defined as the time from being placed in the supine position until the righting reflex was regained.
Conditioned Taste Aversion.
Mice were adapted to a water-restriction schedule (2 hours of water per day) over a 7-day period. At 48-hour intervals over the next days (days 1, 3, 5, 7, 9, and 11), all mice received 1-hour access to a solution of saccharin (0.15% w/v sodium saccharin in tap water). Immediately after 1-hour access to saccharin, mice received repeated injections of saline or 2.5 g/kg ethanol (days 1, 3, 5, 7, 9, and 11). In separate experiments, 6 or 10 mEq/kg lithium chloride (LiCl) was used instead of ethanol. All mice received 30-minute access to tap water 5 hours after each saccharin-access period to prevent dehydration (days 1, 3, 5, 7, 9, and 11). On intervening days, mice had 2-hour continuous access to water at standard times in the morning (days 2, 4, 6, 8, and 10). Reduced consumption of the saccharin solution is used as a measure of conditioned taste aversion (CTA).
Conditioned Place Preference.
The conditioned place preference (CPP) protocol was carried out, as previously described (Blednov et al., 2003). Four identical acrylic boxes (30 × 15 × 15 cm) were separately enclosed in ventilated, light-, and sound-attenuating chambers (Med Associates, St. Albans, VT). Each box has two compartments separated by a wall with a door. The compartments each have a different type of floor (bars set in a grid or small round holes). Infrared light sources and photodetectors were mounted opposite each other at 2.5-cm intervals along the length of each box, 2.2 cm above the floor. Occlusion of the infrared light beams was used to measure general activity and location of the animal (left or right compartment) within the box. Total activity counts and location of the animal within the box were recorded by computer. The floors and the inside of the boxes were wiped with water, and the litter paper beneath the floors was changed between animals. The main principles of the CPP procedure were described previously (Cunningham et al., 1993). Briefly, the place-conditioning study involved one habituation session, eight conditioning sessions, and one test session. No pretest sessions were used. A 2-day weekend break occurred between the first four and last four conditioning sessions. For the habituation session, mice were injected with saline immediately before being placed in the conditioning box containing a smooth metal plate covering the floor for 30 minutes. During the habituation session, both compartments were available to the mice. The purpose of the habituation session was to reduce the stress associated with the novelty of the experimental procedure and exposure to the apparatus. Mice were not exposed to the distinctive floor textures to avoid latent inhibition. For conditioning, mice from the saline group were randomly assigned to one of two conditioning subgroups, floors with holes or bars, and were exposed to a Pavlovian differential conditioning procedure. On alternating days, one group received an injection of ethanol (2 g/kg i.p.) immediately before a 5-minute session on the bar floor (conditioned stimulus sessions). On intervening days, these mice received saline immediately before exposure to the floor with holes (unconditioned stimulus sessions). Conversely, the other group received ethanol paired with the floor with holes and saline paired with the bar floor. During conditioning trials, all mice had access to only one of the two compartments of the apparatus. The 2 g/kg dose of ethanol was chosen because it produces a strong preference for the paired tactile stimuli (Chester and Cunningham, 1998). The 5-minute session duration was used because it produces a stronger ethanol-induced CPP compared with longer sessions. For the 30-minute test session, all mice received saline. Both compartments of each box were available for exploration during the test session.
Ethanol-Induced Acute Withdrawal.
Mice were scored for handling-induced convulsion (HIC) severity 30 minutes before and immediately before ethanol administration, and the predrug baseline scores were averaged. Ethanol (4 g/kg) in saline was injected intraperitoneally, and the HIC score was tested every hour until the HIC level reached baseline. Acute withdrawal was quantified as the area under the curve but above the predrug baseline level (Crabbe et al., 1991). Briefly, each mouse is picked up gently by the tail and, if necessary, gently rotated 180°, and the HIC is scored as follows: 5, tonic-clonic convulsion when lifted; 4, tonic convulsion when lifted; 3, tonic-clonic convulsion after a gentle spin; 2, no convulsion when lifted, but tonic convulsion elicited by a gentle spin; 1, facial grimace only after a gentle spin; and 0, no convulsion when lifted and after a gentle spin.
Rotarod.
Mice were trained on a fixed speed Economex rotarod (Columbus Instruments, Columbus, OH; rod speed, 5 rpm), and training was considered complete when mice were able to remain on the rotarod for 60 seconds. Every 15 minutes after injection of ethanol (2 g/kg i.p.), the mouse was placed back on the rotarod and latency to fall was measured until the mouse was able to stay on the rotarod for 60 seconds.
Startle Reflex.
Acoustic startle responses were measured using SR-LAB test stations and software (San Diego Instruments, San Diego, CA). Startle responses were recorded, as described previously (Findlay et al., 2003). Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5-minute acclimation period. Over the next 8 minutes, mice were presented with each of seven trial types across five discrete blocks of trials for a total of 35 trials. The intertrial interval was 10–20 seconds. One trial measured the response to no stimulus (baseline movement). The other six trials measured the response to a startle stimulus alone, consisting of a 40-millisecond sound burst of 90, 95, 100, 105, 110, or 115 dB. Startle amplitude was measured every 1 millisecond over a 65-millisecond period beginning at the onset of the startle stimulus. The six trial types were presented in pseudorandom order such that each type was presented once within a block of six trials. The maximum startle amplitude (Vmax) over this sampling period was taken as the dependent variable. A background noise level of 70 dB was maintained over the duration of the test session.
Elevated Plus Maze.
Mice were evaluated for basal anxiety-related behaviors as well as ethanol-induced anxiolysis using the elevated plus maze, as described previously (Blednov et al., 2001). Mice were transported to the testing room 1 day before testing. Testing occurred between 10 and 12 AM under ambient room light. Mice were weighed and injected with ethanol (1.25 g/kg i.p.) or saline 10 minutes before testing. Each mouse was placed on the central platform of the maze facing an open arm. Mice were allowed to freely explore the maze for 5 minutes during which the following measurements were manually recorded: number of open arm entries, number of closed arm entries, total number of entries, time spent in open arms, and time spent in closed arms. A mouse was considered to be on the central platform or any arm when all four paws were within its perimeter.
Ethanol Clearance.
Animals were given a single dose of ethanol (4 g/kg i.p.), and blood samples were taken from the retro-orbital sinus 30, 60, 120, 180, and 240 minutes after injection. Blood ethanol concentration was determined spectrophotometrically using an enzyme assay (Lundquist, 1959).
Quantitative Reverse-Transcription Polymerase Chain Reaction Measurement of Glra1 and Glra3 mRNA.
Prefrontal cortex from WT and Glra2 KO mice (n = 17 for each genotype) and striatum from 20 WT and 18 Glra2 KO mice were dissected, flash-frozen in liquid N2, and stored at −80°C. Total RNA was isolated using the MagMax-96 for microarrays kit (Ambion, Austin, TX). RNA concentration and purity were determined by UV spectrometry (Nanodrop; Thermo Scientific, Wilmington, DE), and overall RNA integrity was assessed using a 2200 TapeStation (Agilent Technologies, Santa Clara, CA). Each RNA sample was reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), and control reactions without reverse transcriptase (RT) were included. Quantitative polymerase chain reaction was performed in triplicate for 60 ng of each cDNA using SsoAdvanced Universal Probes Supermix, according to manufacturer’s instructions (Bio-Rad, Hercules, CA). 6-Fluorescein amidite–labeled TaqMan Gene Expression Assays (Applied Biosystems) were used to amplify Glra1 (Mm00445061_m1), Glra3 (Mm00475507_m1), and Gusb (Mm01197698_m1 or Mm03003537_s1). RNA samples were evaluated for the presence of genomic DNA by comparing Gusb (s1 assay) Cq (quantitation cycle) values from RT+ and RT− reactions. Quantitative reverse-transcription polymerase chain reaction results were imported into qBase+ software, version 2.5 (Biogazelle, Gent, Belgium), in which the single-threshold Cq determination and ΔΔ cycle threshold methods were used (Hellemans et al., 2007). Data were normalized to the reference gene Gusb, which demonstrated minimal variation among mean sample Cq values (range of 0.62 for prefrontal cortex and 0.92 for striatum). WT and KO groups were compared using a two-sided corrected Mann–Whitney test.
Drug Injection.
All injectable ethanol (Aaper Alcohol and Chemical, Shelbyville, KY) solutions were prepared in 0.9% saline (20%, v/v) and injected intraperitoneally. Flurazepam (Sigma-Aldrich, St. Louis, MO; 225 mg/kg i.p.) and ketamine (Sigma-Aldrich; 150 mg/kg i.p.) solutions were dissolved in 0.9% saline and injected at 0.01 ml/g body weight. LiCl (Sigma-Aldrich) was freshly prepared in water and injected intraperitoneally at 6 or 10 mE/kg (0.01 ml/g body weight).
Statistical Analysis.
Values are reported as the mean ± S.E.M. The statistics software program GraphPad Prism (GraphPad Software, La Jolla, CA) was used. Analysis of variance (two-way analysis of variance followed by Bonferroni post hoc tests) and Student’s t tests were carried out to evaluate differences between groups.
Results
Ethanol Consumption.
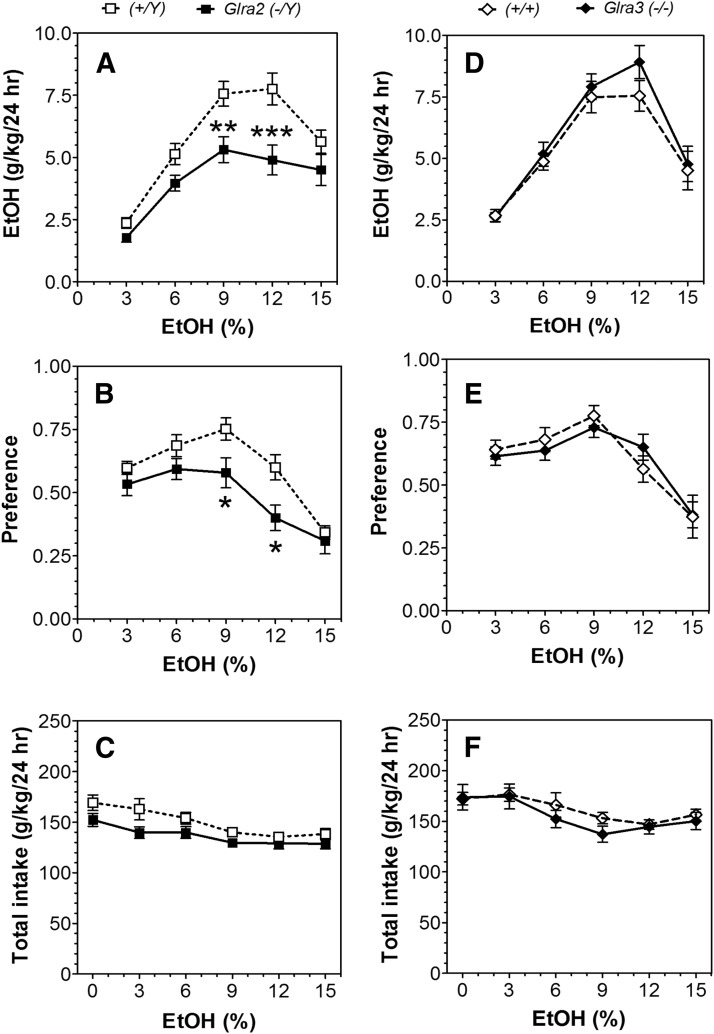
In a two-bottle free-choice paradigm in which mice could drink either water or a series of increasing ethanol concentrations, mice lacking Glra2 consumed less ethanol and showed lower preference compared with WT mice (Fig. 1, A and B; statistical analysis in Supplemental Table 1). Deletion of Glra3 did not alter ethanol consumption or preference in this test (Fig. 1, D and E). Neither mutation affected total fluid intake (Fig. 1, C and F). No differences between preference for sweet solutions of saccharin or avoidance of bitter solutions of quinine were found in the mutant mice (Supplemental Fig. 1; statistical analysis in Supplemental Table 2).
Fig. 1.
Decreased voluntary ethanol consumption in Glra2(−/Y) but not Glra3(−/−) mice (24-hour two-bottle choice). (A–C) Male Glra2(−/Y) and WT mice (n = 9–10 per genotype). (D–F) Male Glra3(−/−) and WT mice (n = 8–12 per genotype). (A and D) Ethanol consumption (g/kg per 24 hours). (B and E) Preference for ethanol. (C and F) Total fluid intake (g/kg per 24 hours). Values represent mean ± S.E.M. Data were analyzed by two-way repeated measures analysis of variance, followed by Bonferroni post hoc test (*P < 0.05, **P < 0.01, ***P < 0.001 versus WT). EtOH, ethanol.
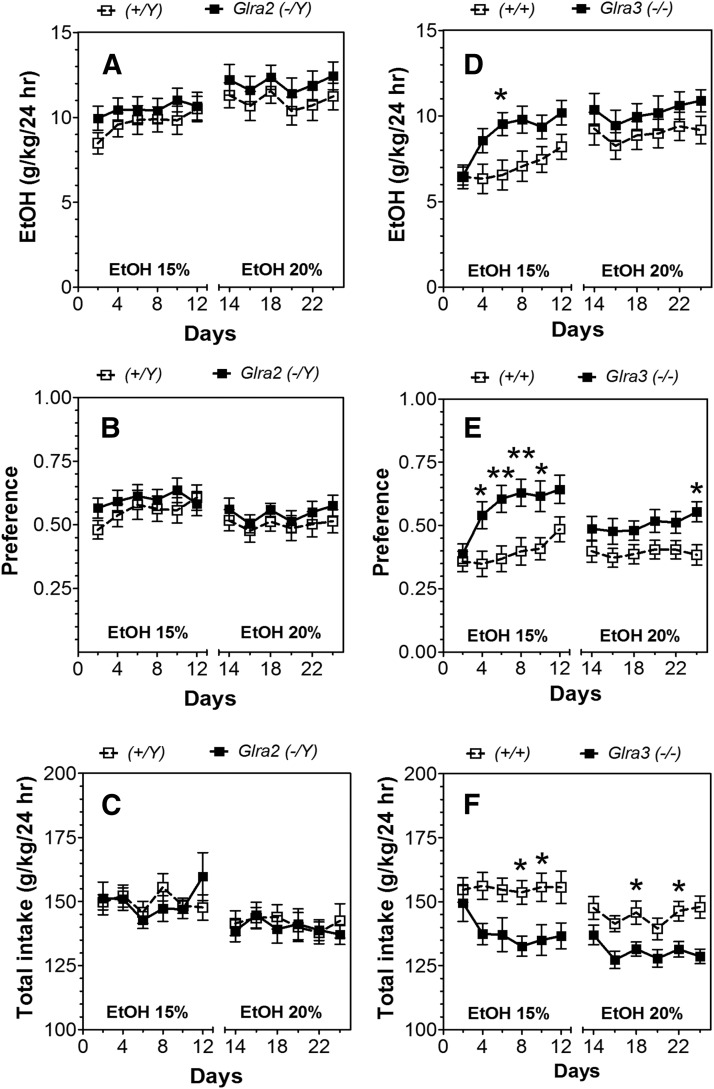
In the two-bottle choice test with intermittent access to ethanol in which mice could drink solutions containing high concentrations of ethanol, deletion of Glra2 did not alter ethanol consumption or preference (Fig. 2, A–C; statistical analysis in Supplemental Table 3). In contrast, mice lacking Glra3 consumed more ethanol (F1,27 = 4.3, P < 0.05) and showed greater preference (F1,27 = 7.7, P < 0.05) than WT for 15% ethanol (Fig. 2, D and E; statistical analysis in Supplemental Table 3). Although these mice did not consume significantly more of the 20% ethanol solution, they did show greater preference (F1,27 = 4.2, P < 0.05). Glra3 KO mice consumed significantly less fluid than WT (Fig. 2F). However, despite their differences, KO and WT mice from both colonies showed escalation of ethanol consumption with increased intake and preference over time (statistical analysis in Supplemental Table 3).
Fig. 2.
Increased voluntary ethanol consumption in Glra3(−/−), but not Glra2(−/Y) mice (24-hour two-bottle choice with intermittent access to ethanol). (A–C) Male Glra2(−/Y) and WT mice (n = 14–16 per genotype). (D–F) Male Glra3(−/−) and WT mice (n = 14–15 per genotype). (A and D) Ethanol consumption (g/kg per 24 hours). (B and E) Preference for ethanol. (C and F) Total fluid intake (g/kg per 24 hours). Values represent mean ± S.E.M. Data were analyzed by two-way repeated measures analysis of variance, followed by Bonferroni post hoc test (*P < 0.05, **P < 0.01 versus WT). EtOH, ethanol.
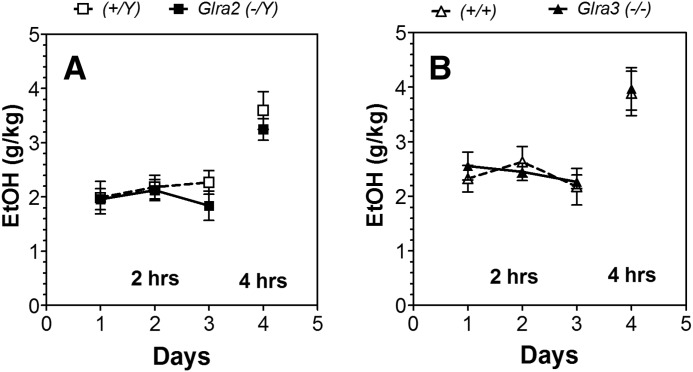
In the drinking in the dark (DID) limited access test, mutant mice did not differ from WT in consumption of 15% ethanol after 2 or 4 hours (Fig. 3).
Fig. 3.
Lack of Glra2 or Glra3 did not alter ethanol consumption in the one-bottle drinking in the dark test (limited access to ethanol). (A) Male Glra2(−/Y) and WT mice (n = 10 for each genotype). Ethanol consumption for days 1–3 (g/kg per 2 hours) and day 4 (g/kg per 4 hours). (B) Male Glra3(−/−) and WT mice (n = 8 for each genotype). Ethanol consumption for days 1–3 (g/kg per 2 hours) and day 4 (g/kg per 4 hours). Values represent mean ± S.E.M. Data were analyzed by two-way repeated measures analysis of variance (days 1–3) or Student’s t test (day 4). EtOH, 15% ethanol.
Conditioned Taste Aversion.
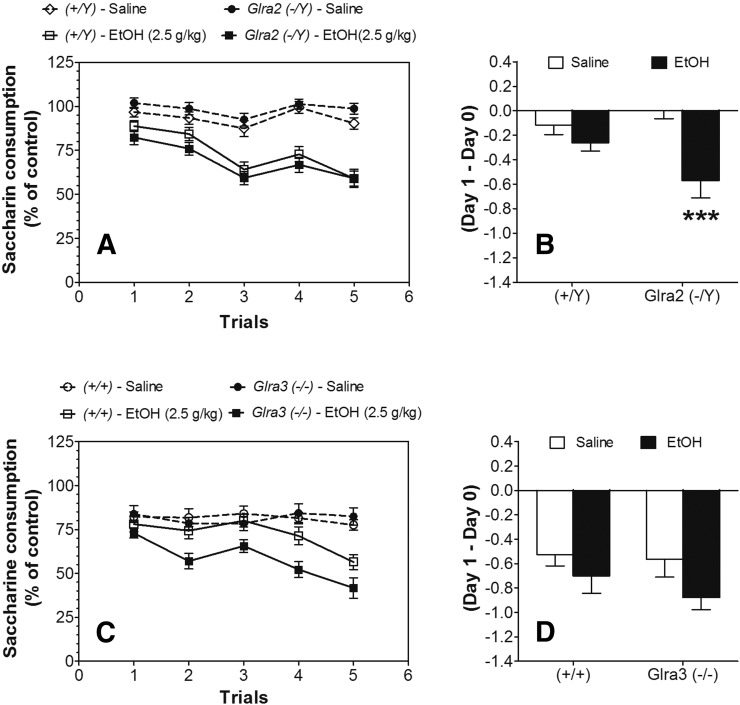
To normalize initial fluctuations in saccharin intake, intake was calculated as a percentage of the trial 0 consumption for each subject by dividing the amount of saccharin solution consumed on subsequent conditioning trials by the amount of saccharin solution consumed in trial 0 (before conditioning). Ethanol-saccharin pairings reduced saccharin intake across trials compared with saline-saccharin pairings, indicating the development of CTA in both Glra2(−/Y) and Glra3(−/−) mice and in WT (Fig. 4, A and C; statistical analysis in Supplemental Tables 4 and 5). No differences were found between ethanol-treated groups of WT or Glra2(−/Y) mice or between saline-treated groups of WT and mutant strains. However, Glra3(−/−) mice developed greater CTA following ethanol treatment compared with WT (Fig. 4C). Comparison of the initial response to ethanol (changes in saccharin intake after the first injection of ethanol) showed greater reduction of saccharin intake in Glra2(−/Y) compared with WT mice (F1,86 = 12.8, P < 0.001, effect of treatment and F1,86 = 4.6, P < 0.05, genotype × treatment interaction) (Fig. 4B). No differences in the initial response to ethanol were found between Glra3(−/−) and WT mice (Fig. 4D).
Fig. 4.
Increased initial aversive response to ethanol in Glra2(−/Y) and increased development of CTA in Glra3(−/−) mice. (A) Development of CTA in male Glra2(−/Y) versus WT mice (n = 20 for saline per genotype; n = 22–23 for ethanol per genotype). Changes in saccharin consumption produced by injection of saline or ethanol (2.5 g/kg) are expressed as a percentage of the control trial (Trial 0). (B) Initial aversive response to ethanol in male Glra2 (−/Y) versus WT mice. Values represent the difference between saccharin intake in the control trial (Trial 0) and intake after the first injection of ethanol (Trial 1). (C) Development of CTA in male Glra3(−/−) versus WT mice (n = 15 for saline per genotype; n = 14–15 for ethanol per genotype). Changes in saccharin consumption produced by injection of saline or ethanol (2.5 g/kg) are expressed as a percentage of the control trial (Trial 0). (D) Initial aversive response to ethanol in male Glra3(−/−) versus WT mice. Values represent the difference between saccharin intake in the control trial (Trial 0) and intake after the first injection of ethanol (Trial 1). Values represent mean ± S.E.M. Data were analyzed by two-way analysis of variance (B and D) or two-way repeated measures analysis of variance (A and C), followed by Bonferroni post hoc test (***P < 0.001 versus WT). EtOH, 2.5 g/kg ethanol.
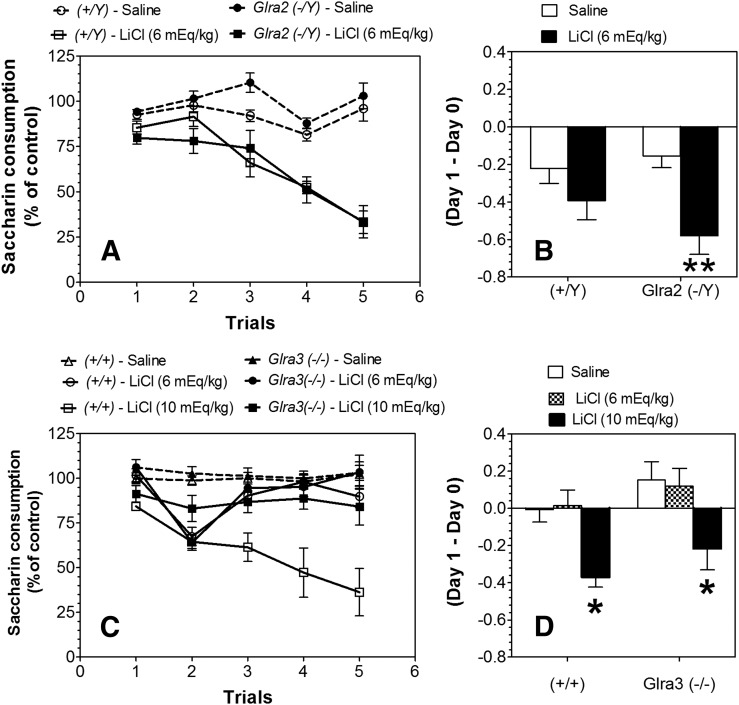
We then determined whether the greater CTA for ethanol would generalize to another aversive drug. LiCl-saccharin pairings reduced saccharin intake across trials compared with saline-saccharin pairings, indicating the development of CTA in both Glra2(−/Y) and Glra3(−/−) as well as in WT mice (Fig. 5, A and C; statistical analyses in Supplemental Tables 6–8). No differences were found between LiCl-treated (6 mEq/kg) groups of WT and mutant mice or between saline-treated groups of WT and mutants. However, after administration of LiCl at 10 mEq/kg, Glra3(−/−) mice showed weaker CTA compared with WT. Comparison of the initial response to LiCl (changes in saccharin intake after the first injection of LiCl) showed stronger reduction of saccharin intake in Glra2(−/Y) mice compared with WT (F1,32 = 11.7, P < 0.01, effect of treatment) (Fig. 5B). The initial response to LiCl in Glra3(−/−) was dose-dependent (F2,55 = 10.1, P < 0.001, effect of treatment), but no differences between KO and WT mice were observed (Fig. 5D).
Fig. 5.
Increased initial aversive response to LiCl in Glra2(−/Y) and decreased development of CTA in Glra3(−/−) mice. (A) Development of CTA in male Glra2(−/Y) versus WT mice (n = 9 for saline per genotype; n = 9 for LiCl per genotype). Changes in saccharin consumption produced by injection of saline or LiCl (6 mEq/kg) are expressed as a percentage of the control trial (Trial 0). (B) Initial aversive response to LiCl in male Glra2(−/Y) versus WT mice. Values represent the difference between saccharin intake in the control trial (Trial 0) and intake after the first injection of LiCl (Trial 1). (C) Development of CTA in male Glra3(−/−) versus WT mice (n = 11–17 for saline per genotype; n = 7–10 for LiCl per genotype). Changes in saccharin consumption produced by injection of saline or LiCl (6 or 10 mEq/kg) are expressed as a percentage of the control trial (Trial 0). (D) Initial aversive response to LiCl in male Glra3(−/−) versus WT mice. Values represent the difference between saccharin intake in the control trial (Trial 0) and intake after the first injection of LiCl (Trial 1). Values represent mean ± S.E.M. Data were analyzed by two-way analysis of variance (B and D) or two-way repeated measures analysis of variance (A and C), followed by Bonferroni post hoc test (*P < 0.05, **P < 0.01 versus WT).
Conditioned Place Preference.
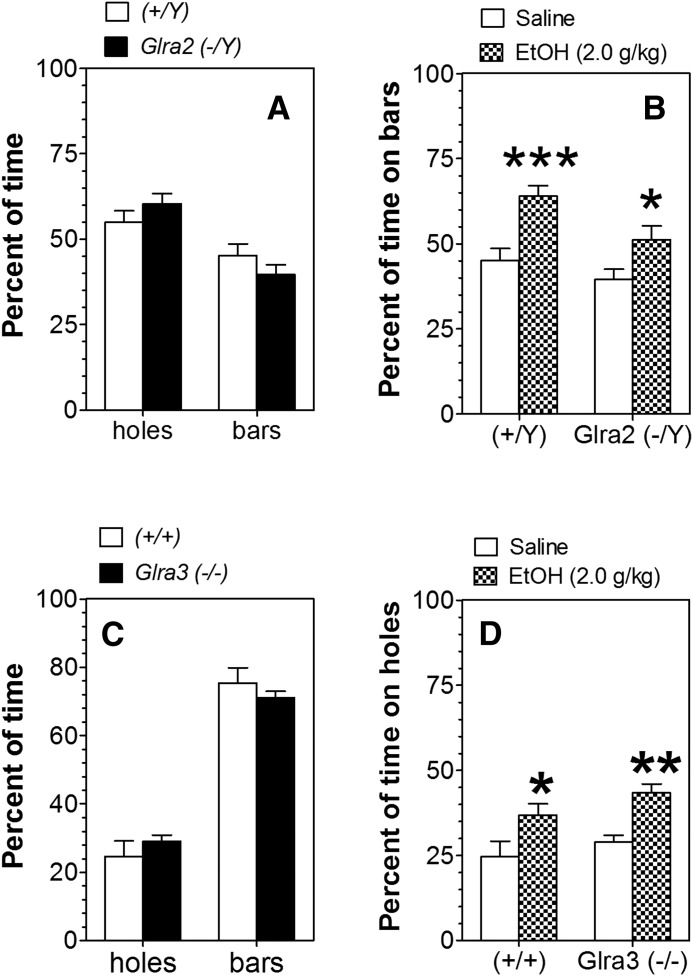
Following control saline injections, both Glra2(−/Y) and WT mice spent less time on the bar floor than on the floor with round holes (F1,66 = 22.9, P < 0.001, main effect of floor; Fig. 6A). However, both Glra3(−/−) and WT mice strongly preferred the bar floor compared with the floor with holes (F1,20 = 179, P < 0.001, main effect of floor; Fig. 6C). Because of this preference, ethanol was paired with the floor containing bars in Glra2(−/Y) and WT mice, whereas ethanol was paired with the floor containing holes in Glra3(−/−) and WT mice. For the Glra2(−/Y) colony, mice of both genotypes spent more time on the bar floor when it was paired with ethanol than when paired with saline, reflecting development of CPP (F1,74 = 19.1, P < 0.001, main effect of treatment). Post hoc analysis showed increased time spent on the bar floor for male mice of both genotypes [P < 0.001 for WT and P < 0.05 for Glra2(−/Y) mice]. This increase was also modestly dependent on genotype (F1,74 = 6.8, P < 0.05, main effect of genotype; Fig. 6B). For the Glra3(−/−) colony, mice of both genotypes spent more time on the floor with holes when it was paired with ethanol than when paired with saline, reflecting development of CPP (F1,23 = 17.1, P < 0.001, main effect of treatment). Post hoc analysis showed increased time spent on the bar floor for male mice of both genotypes [P < 0.05 for WT and P < 0.01 for Glra3(−/−) mice]. However, there was no difference in development of place conditioning between the genotypes (Fig. 6D).
Fig. 6.
Ethanol-induced conditioned place preference in Glra2(−/Y) and Glra3(−/−) mice. (A) The percentage of time spent on different floor types for male Glra2(−/Y) and WT mice (n = 16–19 per genotype). (B) Preference test in male Glra2(−/Y) and WT mice (n = 16–23 per group and genotype). Values represent the percentage of time spent on the less preferred bar floor after saline or ethanol injection. (C) The percentage of time spent on different floor types for male Glra3(−/−) and WT mice (n = 6 per genotype). (D) Preference test in male Glra3(−/−) and WT mice (n = 6–8 per group and genotype). Values represent the percentage of time spent on the less preferred floor with holes after saline or ethanol injection. Values represent mean ± S.E.M. Data were analyzed by two-way analysis of variance, followed by Bonferroni post hoc test (*P < 0.05, **P < 0.01, ***P < 0.001 versus saline group of corresponding genotype). EtOH, 2 g/kg ethanol.
Anxiety-Related Behavior.
In the plus-maze, locomotor activity was assessed by number of total entries and number of entries into the closed arms, whereas anxiety-related behavior was measured by time in the open arms and the percentage of time spent in open arm entries after injection of saline or ethanol. In both colonies, ethanol affected time in the open arms and the percentage of time spent in open arms (main effect of treatment: F1,16 = 22.5, P < 0.001 for Glra2 KO mice and F1,36 = 31.4, P < 0.001 for Glra3 KO mice) (Supplemental Figs. 2 and 3, A and B) as well as the number of entries into the open arms or percentage of open arm entries (main effect of treatment: F1,16 = 8.7, P < 0.01 for Glra2 KO and F1,36 = 23.7, P < 0.001 for Glra3 KO mice) (Supplemental Figs. 2 and 3, C and D). However, no genotype-dependent differences were found. The number of total entries (F1,16 = 11.8, P < 0.01, main effect of genotype; F1,16 = 25.3, P < 0.001, main effect of treatment) and number of closed arm entries (F1,16 = 9.6, P < 0.01, main effect of genotype) were significantly higher in Glra2(−/Y) compared with WT mice, and both parameters increased after injection ethanol (F1,16 = 56.4, P < 0.001, main effect of treatment; Supplemental Fig. 2, E and F). No differences in number of entries (total and closed arms) were found between Glra3(−/−) mice and WT (Supplemental Fig. 3, E and F). However, ethanol increased the number of total entries (F1,36 = 37.9, P < 0.001, main effect of treatment) but not the number of entries into the closed arms in both Glra3(−/−) and WT mice (Supplemental Fig. 3, E and F). Glra2(−/Y) spent more time in the center of the maze than WT mice (F1,16 = 8.6, P < 0.01, main effect of genotype; F1,16 = 17.1, P < 0.001, genotype × treatment interaction; Supplemental Fig. 2G), whereas no effect of genotype or ethanol treatment was found in Glra3(−/−) mice (Supplemental Fig. 3G).
Startle Reflex, Motor Incoordination, Acute Withdrawal, and LORR.
Changes in the function of GlyRs containing α1 subunits are often accompanied by changes in acoustic startle response, effects of ethanol and other sedative drugs on LORR, and effects of ethanol on motor incoordination and severity of ethanol-induced acute withdrawal (Quinlan et al., 2002; Findlay et al., 2003; Blednov et al., 2012; McCracken et al., 2013a). We next examined some of these behaviors in mice lacking Glra2 or Glra3.
Startle response was dependent on sound intensity but not genotype [F4,48 = 19.4, P < 0.001, effect of treatment of Glra2(−/Y) mice; F4,64= 97.9, P < 0.001, effect of treatment of Glra3(−/−) mice; Supplemental Fig. 4]. No differences in duration of LORR or latency to LORR between mutant and WT mice were found for ethanol, flurazepam, or ketamine (Table 1). Acute administration of ethanol (2 g/kg) produced motor incoordination in both genotypes, and KO mice showed a recovery similar to WT [F9,72 = 106, P < 0.001, effect of time for Glra2(−/Y) mice; F8,80 = 108, P < 0.001, effect of time for Glra3(−/−) mice; Supplemental Fig. 5].
TABLE 1.
Effects of GlyR deletion on the LORR produced by ethanol and other sedative drugs
Drugs were injected intraperitoneally. Values represent mean ± S.E.M.
| Drugs | LORR | Glra2 | Glra3 | ||
|---|---|---|---|---|---|
| (+/Y) | (−/Y) | (+/+) | (−/−) | ||
| dose | min | ||||
| Ethanol (3.6 g/kg) | Duration | 45.5 ± 2.64 | 43.4 ± 3.06 | 59.0 ± 0.78 | 59.9 ± 3.48 |
| n = 10 | n = 10 | n = 6 | n = 6 | ||
| Latency | 1.92 ± 0.08 | 2.11 ± 0.20 | 1.86 ± 0.13 | 2.20 ± 2.21 | |
| Flurazepam (225 mg/kg) | Duration | 99.1 ± 4.82 | 96.0 ± 1.48 | 96.1 ± 6.85 | 94.5 ± 7.24 |
| n = 4 | n = 5 | n = 4 | n = 5 | ||
| Latency | 4.82 ± 0.09 | 4.66 ± 0.17 | 9.35 ± 1.12 | 8.66 ± 1.25 | |
| Ketamine (175 mg/kg) | Duration | 39.1 ± 0.90 | 38.3 ± 0.53 | 40.9 ± 0.63 | 39.8 ± 0.64 |
| n = 5 | n = 5 | n = 4 | n = 4 | ||
| Latency | 1.34 ± 0.14 | 1.37 ± 0.11 | 0.87 ± 0.13 | 0.87 ± 0.21 | |
A single ethanol dose of 4 g/kg suppressed basal HIC in both KO and WT mice for about 5 hours, followed by increased HIC (Supplemental Fig. 6, A and C). KO and WT mice did not differ in basal levels of HIC. Animals of both genotypes demonstrated signs of withdrawal (HIC scores higher than the basal level). However, there were no differences in the area under the curve and above the basal level during withdrawal for Glra2(−/Y) (1.68 ± 0.36 and 1.47 ± 0.26 for WT and mutant mice, respectively) or Glra3(−/−) (2.05 ± 0.48 and 1.86 ± 0.36 for WT and mutant mice, respectively) (Supplemental Fig. 6, B and D).
Ethanol Clearance.
KO mice did not differ in blood ethanol clearance (4 g/kg) compared with their corresponding WT. The slopes for Glra2 KO and WT mice were −51.2 ± 4.3 and −46.5 ± 4.6, respectively; the slopes for Glra3 KO and WT were −77.9 ± 1.3 and −71.7 ± 3.4, respectively (Supplemental Fig. 7).
mRNA Levels in Brain Regions of Mice Lacking Glra2.
We compared the levels of Glra1 and Glra3 mRNAs in prefrontal cortex and striatum in WT and Glra2 KO mice. mRNA levels did not differ between WT and Glra2 KO mice in these regions. The ratios of prefrontal cortex mRNA levels (WT/KO) were 1.01 and 1.06 for Glra1 and Glra3, respectively. The ratios of striatum mRNA levels (WT/KO) were 1.00 and 1.02 for Glra1 and Glra3, respectively.
Discussion
Behavioral studies of ethanol and GlyRs have focused on knock-in mouse lines containing GlyRα1 mutations or on treatments that are not selective for GlyR subunits. The in vivo role of GlyRα2 and α3 subunits in alcohol responses has not been characterized despite their high levels of expression in reward-related pathways in the brain. We provide evidence that deletion of Glra2 or Glra3 alters specific ethanol-induced behaviors (see Table 2 for summary of phenotypes). The major changes produced by Glra2 deletion were reduced ethanol intake and preference in the 24-hour two-bottle choice test and increased initial aversive responses to ethanol and LiCl. The two-bottle choice test is considered the hallmark for voluntary drinking in animal models (Wahlsten et al., 2006) and is correlated with other measures of alcohol reinforcement (Green and Grahame, 2008). It is important to use an animal model in which there is voluntary access to alcohol, which induces specific changes in gene expression that cannot be mimicked by forced injection of the drug (Spanagel, 2003). Ethanol intake and preference in the two-bottle choice test were reduced in Glra2 KO mice only, indicating that activation of GlyRα2 subunits may help promote chronic ethanol drinking. There were no differences among the mutant and WT mice during the DID test, which is a model of binge-like drinking. However, mouse lines selected for high ethanol consumption during DID demonstrated altered gene coexpression networks (in the absence of ethanol exposure), and Glra2 was one of the genes found to have significant changes in connectivity (Iancu et al., 2013).
TABLE 2.
Summary of the behavioral effects of ethanol and other drugs in mutant mice
Results from Glra1 knock-in mice were taken from (Blednov et al., 2012).
| Test | Drug |
Glra2(−/Y) |
Glra3(−/−) |
Glra1 [Q266I] |
Glra1 [M287L] |
|---|---|---|---|---|---|
| Males | Males | Males | Males | ||
| Startle reflex | None | = | = | ↑↑ | ↑ |
| LORR | Ethanol | = | = | ↑ | ↓ |
| LORR | Ketamine | = | = | ↑ | ↑ |
| LORR | Flurazepam | = | = | ↑↑ | ↑ |
| Acute withdrawal | Ethanol | = | = | = | ↓ |
| Two-bottle choice (ethanol 3–15%) | Ethanol (g/kg per 24 hours) | ↓ | = | = | = |
| Preference | ↓ | = | ↓ | ↓ | |
| Fluid Intake (g/kg per 24 hours) | ↓ | = | ↑ | ↑ | |
| Two-bottle choice (saccharin) | Preference | = | = | ↓ | = |
| Fluid Intake (g/kg per 24 hours) | ↓ | ↑ | ↓ | = | |
| Two-bottle choice (quinine) | Preference | = | = | = | ↓ |
| Fluid Intake (g/kg per 24 hours) | ↓ | = | ↑ | = | |
| Two-bottle choice: Intermittent (15%) | Ethanol (g/kg per 24 hours) | = | ↑ | na | na |
| Preference | = | ↑ | na | na | |
| Fluid Intake (g/kg per 24 hours) | = | ↓ | na | na | |
| One-bottle DID (15%) | Ethanol (g/kg per 2 hours) | = | = | = | = |
| Ethanol (g/kg per 4 hours) | = | = | = | = | |
| Rotarod (recovery) | Ethanol | = | = | ← | = |
| CTA (ethanol 2.5 g/kg) | Initial | ↑ | = | = | = |
| Residual | = | ↑ | = | ↓ | |
| CTA (LiCl 6 mEq/kg) | Initial | ↑ | = | na | na |
| Residual | = | = | na | na | |
| CTA (LiCl 10 mEq/kg) | Initial | na | = | na | na |
| Residual | na | ↓ | na | na | |
| CPP | Ethanol | ↓ | = | na | na |
| Clearance | Ethanol | = | = | = | = |
| Anxiety (elevated plus-maze) | Ethanol | = | = | na | na |
=, no difference between mutant and WT mice; increased (↑, ↑↑) or decreased (↓) behavior in mutant compared with WT mice; na, not available.
Mice lacking Glra3 increased ethanol intake and preference in the 24-hour intermittent access test, but also showed stronger development of CTA to ethanol. Other ethanol effects, such as anxiolysis, motor incoordination, LORR, acoustic startle response, and ethanol clearance, were not altered in the mutants. LORR induced by flurazepam or ketamine was also not changed. There was a slightly lower level of ethanol-induced CPP in Glra2 KO mice. The ethanol sensitivity of homomeric GlyRα2 or α3 subunits is similar (McCracken et al., 2013b), suggesting that regional or cell-type differences in receptor expression may account for the different in vivo ethanol responses that we observed in the KO strains.
Overall, behavioral changes in mice lacking GlyRα2 or α3 subunits were distinct from the effects observed in mice with mutations in the α1 subunit. Mutations in GlyRα1 often result in a dramatic increase in the startle response (Harvey et al., 2008) and can affect other neurotransmitter systems such as GABAA, N-methyl-d-aspartate, or nicotinic acetylcholine (Quinlan et al., 2002; Blednov et al., 2012). Mutations in the GlyRα1 subunit that prevent G protein and ethanol modulation reduce the duration of LORR and increase the locomotor-stimulating actions without changing ethanol-induced ataxia (Aguayo et al., 2014). Thus, it appears that the GlyRα1 subunit regulates ethanol-induced motor activity and sedation. The current results suggest that receptors containing the GlyRα2 subunit may be important for promoting ethanol consumption (Table 2). It is interesting to note that an inhibitor of glycine transporter-1 was not effective in preventing alcohol relapse in humans (de Bejczy et al., 2014). Based on our study, a selective inhibitor of receptors containing GlyRα2 subunits might be more effective in reducing alcohol consumption.
KO of GlyRα2 subunits could be expected to increase levels of other GlyR subunits. Although levels of mRNAs coding for GlyRα1 and GlyRα3 were not altered, we only measured expression in prefrontal cortex and striatum, and cannot rule out the possibility that these (or other subunits) might be altered in select brain regions. Previous studies provide compelling evidence that GlyR-mediated activation in NAc, which expresses α1–3 subunits, reduces ethanol intake (Molander et al., 2005, 2007). The unexpected reduced ethanol consumption in Glra2 KO mice might thus be due to a role of α2 subunits in other brain regions that are normally important for promoting alcohol consumption.
As mentioned, altered gene coexpression networks were found in mouse lines bred for high levels of DID, and Glra2 was one of the genes showing significant changes in connectivity (Iancu et al., 2013). This study highlights the role of gene networks rather than differential expression of individual transcripts in the propensity for ethanol drinking and further supports the importance of the GlyRα2 subunit in ethanol drinking and preference. The lack of effect on DID in mice lacking GlyRα2 subunits may indicate the importance of associated gene networks rather than changes in individual mRNAs.
CTA is a valuable test for the formation of long-term nondeclarative memory (Rosenblum et al., 1997). CTA produced by drugs such as LiCl, as well as recreationally used drugs (Hunt and Amit, 1987), reflects negative hedonic effects (Garcia et al., 1974). There is a negative correlation between development of CTA (initial response after the first injection of ethanol) and ethanol intake and preference in the two-bottle choice test (Broadbent et al., 2002). In our study, CTA was altered in both mutants. The initial reduction of saccharin intake after injection of ethanol differed in mice lacking Glra2, but not Glra3. This is consistent with our finding that reduction of ethanol intake in the two-bottle choice test in mice lacking Glra2 was accompanied by an increased initial aversive response to ethanol in the CTA test. However, no correlation between ethanol intake and ethanol-induced CTA was found when residuals (averages from last trials) were used as an index of CTA (Risinger and Cunningham, 1998). This may explain why the absence of changes in ethanol consumption in the two-bottle choice continuous test in mice lacking Glra3 may be accompanied by development of greater CTA.
Glra3 KO mice demonstrate increased ethanol intake and preference, yet develop stronger CTA during intermittent access to ethanol. In this drinking test, mice demonstrate the ability to adapt (learn) to consume a high, initially aversive concentration of ethanol. Considering that CTA can be a test for long-term memory, the stronger ethanol aversion in these mice may reflect enhanced ability to learn the presentation of associated stimuli. CTA has also been associated with “reward comparison” (Grigson, 1997), which is related to anticipatory contrast, in which consumption of a preferred solution (e.g., saccharin) is reduced in situations when it predicts availability of a more preferred sucrose solution (Flaherty and Checke, 1982). Thus, reduced intake of saccharin could indicate greater preference for a more rewarding drug. This might explain the increased rewarding properties of ethanol and improved ability to overcome the initial taste aversion in the intermittent access test in mice lacking Glra3.
In summary, we provide the first evidence that the GlyRα2 and α3 subunits are important for selective in vivo effects of ethanol that are related to development of voluntary ethanol consumption and CTA. Moreover, our results show that changes in mice lacking GlyRα2 or α3 subunits are distinct from the effects previously observed in mice with mutations in the α1 subunit. The GlyRα2 subunit appears to be important for regulating alcohol consumption and preference based on the two-bottle voluntary drinking test and may be a relevant target for therapies designed to control alcohol intake.
Supplementary Material
Acknowledgments
The authors thank Heinrich Betz and Mary McCall for providing mice and Jody Mayfield for valuable comments and help writing and editing the manuscript.
Abbreviations
- CPP
conditioned place preference
- Cq
quantitation cycle
- CTA
conditioned taste aversion
- DID
drinking in the dark
- GlyR
glycine receptor
- HIC
handling-induced convulsion
- KO
knockout
- LORR
loss of righting reflex
- NAc
nucleus accumbens
- RT
reverse transcriptase
- WT
wild-type
Authorship Contributions
Participated in research design: Blednov, Harris.
Conducted experiments: Blednov, Benavidez, Black, Leiter, Osterndorff-Kahanek.
Performed data analysis: Blednov, Leiter, Osterndorff-Kahanek.
Wrote or contributed to the writing of the manuscript: Blednov, Harris.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant R01-AA013520, Integrative Neuroscience Initiative on Alcoholism, INIA-West (to Y.A.B. and R.A.H.) and Grant R01-AA006399 (to R.A.H.)].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Adermark L, Clarke RB, Ericson M, Söderpalm B. (2011) Subregion-specific modulation of excitatory input and dopaminergic output in the striatum by tonically activated glycine and GABA(A) receptors. Front Syst Neurosci 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG, Castro P, Mariqueo T, Muñoz B, Xiong W, Zhang L, Lovinger DM, Homanics GE. (2014) Altered sedative effects of ethanol in mice with α1 glycine receptor subunits that are insensitive to Gβγ modulation. Neuropsychopharmacology 39:2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ. (2013) Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 38:1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. (1991) Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci 14:458–461. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Homanics GE, Harris RA. (2012) Behavioral characterization of knockin mice with mutations M287L and Q266I in the glycine receptor α1 subunit. J Pharmacol Exp Ther 340:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. (2001) GIRK2 deficient mice: evidence for hyperactivity and reduced anxiety. Physiol Behav 74:109–117. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. (2003) GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther 305:854–863. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. (2002) Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci 116:138–148. [PubMed] [Google Scholar]
- Chau P, Höifödt-Lidö H, Löf E, Söderpalm B, Ericson M. (2010) Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol Clin Exp Res 34:39–45. [DOI] [PubMed] [Google Scholar]
- Chen RQ, Wang SH, Yao W, Wang JJ, Ji F, Yan JZ, Ren SQ, Chen Z, Liu SY, Lu W. (2011) Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology 36:1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. (1998) Modulation of corticosterone does not affect the acquisition or expression of ethanol-induced conditioned place preference in DBA/2J mice. Pharmacol Biochem Behav 59:67–75. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill C, Belknap JK. (1991) Acute dependence on depressant drugs is determined by common genes in mice. J Pharmacol Exp Ther 257:663–667. [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. (1993) Species difference in sensitivity to ethanol’s hedonic effects. Alcohol 10:97–102. [DOI] [PubMed] [Google Scholar]
- de Bejczy A, Nations KR, Szegedi A, Schoemaker J, Ruwe F, Söderpalm B. (2014) Efficacy and safety of the glycine transporter-1 inhibitor org 25935 for the prevention of relapse in alcohol-dependent patients: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 38:2427–2435. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Esmaeili A, Sedlak PL, Lynch JW, Sah P. (2010) Differential expression of glycine receptor subunits in the rat basolateral and central amygdala. Neurosci Lett 469:237–242. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonné S. (2001) IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci 21:6045–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Becker CM, Betz H. (2012) Inhibitory glycine receptors: an update. J Biol Chem 287:40216–40223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler SA, Förstera B, Smolinsky B, Jüttner R, Lehmann TN, Fähling M, Schwarz G, Legendre P, Meier JC. (2009) Splice-specific roles of glycine receptor alpha3 in the hippocampus. Eur J Neurosci 30:1077–1091. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Stomberg R, Söderpalm B. (2006) Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. Eur J Neurosci 23:3225–3229. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Phelan R, Roberts MT, Homanics GE, Bergeson SE, Lopreato GF, Mihic SJ, Blednov YA, Harris RA. (2003) Glycine receptor knock-in mice and hyperekplexia-like phenotypes: comparisons with the null mutant. J Neurosci 23:8051–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GS, Wick MJ, Mascia MP, Wallace D, Miller GW, Harris RA, Blednov YA. (2002) Transgenic expression of a mutant glycine receptor decreases alcohol sensitivity of mice. J Pharmacol Exp Ther 300:526–534. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Checke S. (1982) Anticipation of incentive gain. Anim Learn Behav 10:177–182. [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. (1974) Behavioral regulation of the milieu interne in man and rat. Science 185:824–831. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. (2008) Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol 42:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Pribilla I, Prior P, Multhaup G, Beyreuther K, Taleb O, Betz H. (1990) Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron 4:963–970. [DOI] [PubMed] [Google Scholar]
- Grigson PS. (1997) Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci 111:129–136. [PubMed] [Google Scholar]
- Han S, Gelernter J, Kranzler HR, Yang BZ. (2013) Ordered subset linkage analysis based on admixture proportion identifies new linkage evidence for alcohol dependence in African-Americans. Hum Genet 132:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, et al. (2004) GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304:884–887. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Schmieden V, Von Holst A, Laube B, Rohrer H, Betz H. (2000) Glycine receptors containing the alpha4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur J Neurosci 12:994–1001. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Topf M, Harvey K, Rees MI. (2008) The genetics of hyperekplexia: more than startle! Trends Genet 24:439–447. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T, Amit Z. (1987) Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev 11:107–130. [DOI] [PubMed] [Google Scholar]
- Iancu OD, Oberbeck D, Darakjian P, Metten P, McWeeney S, Crabbe JC, Hitzemann R. (2013) Selection for drinking in the dark alters brain gene coexpression networks. Alcohol Clin Exp Res 37:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Moorhouse AJ, Akaike N. (2003) Activation of presynaptic glycine receptors facilitates glycine release from presynaptic terminals synapsing onto rat spinal sacral dorsal commissural nucleus neurons. J Physiol 550:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Adermark L, Ericson M, Söderpalm B. (2014) The involvement of accumbal glycine receptors in the dopamine-elevating effects of addictive drugs. Neuropharmacology 82:69–75. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytiä P, Ericson M, Söderpalm B. (2009) Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res 1305:S27–S36. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Söderpalm B. (2012) Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res 1446:12–21. [DOI] [PubMed] [Google Scholar]
- Lundquist F. (1959) The determination of ethyl alcohol in blood and tissue. Methods Biochem Anal 7:217–251. [Google Scholar]
- Lynch JW. (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56:303–309. [DOI] [PubMed] [Google Scholar]
- Maguire EP, Mitchell EA, Greig SJ, Corteen N, Balfour DJ, Swinny JD, Lambert JJ, Belelli D. (2014) Extrasynaptic glycine receptors of rodent dorsal raphe serotonergic neurons: a sensitive target for ethanol. Neuropsychopharmacology 39:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzenbach B, Maulet Y, Sefton L, Courtier B, Avner P, Guénet JL, Betz H. (1994) Structural analysis of mouse glycine receptor alpha subunit genes: identification and chromosomal localization of a novel variant. J Biol Chem 269:2607–2612. [PubMed] [Google Scholar]
- McCracken LM, Blednov YA, Trudell JR, Benavidez JM, Betz H, Harris RA. (2013a) Mutation of a zinc-binding residue in the glycine receptor α1 subunit changes ethanol sensitivity in vitro and alcohol consumption in vivo. J Pharmacol Exp Ther 344:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, McCracken ML, Harris RA. (2013b) Zinc-dependent modulation of α2- and α3-glycine receptor subunits by ethanol. Alcohol Clin Exp Res 37:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI. (2011) Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Lidö HH, Löf E, Ericson M, Söderpalm B. (2007) The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male Wistar rats. Alcohol Alcohol 42:11–18. [DOI] [PubMed] [Google Scholar]
- Molander A, Löf E, Stomberg R, Ericson M, Söderpalm B. (2005) Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res 29:38–45. [DOI] [PubMed] [Google Scholar]
- Muller E, Le-Corronc H, Legendre P. (2008) Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Front Mol Neurosci 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JJ, Ferguson C, Jester K, Firestone LL, Homanics GE. (2002) Mice with glycine receptor subunit mutations are both sensitive and resistant to volatile anesthetics. Anesth Analg 95:578–582. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. (1998) Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res 22:1234–1244. [PubMed] [Google Scholar]
- Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. (1997) NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci 17:5129–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Harrison NL. (2014) Strychnine-sensitive glycine receptors on pyramidal neurons in layers II/III of the mouse prefrontal cortex are tonically activated. J Neurophysiol 112:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. (2003) Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol 17:507–518. [DOI] [PubMed] [Google Scholar]
- Trudell JR, Messing RO, Mayfield J, Harris RA. (2014) Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol Sci 35:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. (2006) Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA 103:16364–16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzien F, Puller C, O’Sullivan GA, Paarmann I, Betz H. (2012) Distribution of the glycine receptor β-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J Comp Neurol 520:3962–3981. [DOI] [PubMed] [Google Scholar]
- Williams KL, Ferko AP, Barbieri EJ, DiGregorio GJ. (1995) Glycine enhances the central depressant properties of ethanol in mice. Pharmacol Biochem Behav 50:199–205. [DOI] [PubMed] [Google Scholar]
- Wise RA. (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacology 29:203–210. [DOI] [PubMed] [Google Scholar]
- Xiong W, Chen SR, He L, Cheng K, Zhao YL, Chen H, Li DP, Homanics GE, Peever J, Rice KC, et al. (2014) Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nat Neurosci 17:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu TL, Gong N. (2010) Glycine and glycine receptor signaling in hippocampal neurons: diversity, function and regulation. Prog Neurobiol 91:349–361. [DOI] [PubMed] [Google Scholar]
- Yadid G, Pacak K, Golomb E, Harvey-White JD, Lieberman DM, Kopin IJ, Goldstein DS. (1993) Glycine stimulates striatal dopamine release in conscious rats. Br J Pharmacol 110:50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Sokol KA, Bhavsar U. (2009) Glycine receptors contribute to hypnosis induced by ethanol. Alcohol Clin Exp Res 33:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Wang F, Krnjevic K, Wang W, Xiong ZG, Zhang J. (2004) Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J Neurosci 24:8961–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Ivic L, Kriegstein AR, Cepko CL. (2006) Characterization of mice with targeted deletion of glycine receptor alpha 2. Mol Cell Biol 26:5728–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.