Abstract
Atmospheric CO2 concentration is continuously increasing, and previous studies have shown that elevated CO2 (eCO2) significantly impacts C3 plants and their soil microbial communities. However, little is known about effects of eCO2 on the compositional and functional structure, and metabolic potential of soil microbial communities under C4 plants. Here we showed that a C4 maize agroecosystem exposed to eCO2 for eight years shifted the functional and phylogenetic structure of soil microbial communities at both soil depths (0–5 cm and 5–15 cm) using EcoPlate and functional gene array (GeoChip 3.0) analyses. The abundances of key genes involved in carbon (C), nitrogen (N) and phosphorus (P) cycling were significantly stimulated under eCO2 at both soil depths, although some differences in carbon utilization patterns were observed between the two soil depths. Consistently, CO2 was found to be the dominant factor explaining 11.9% of the structural variation of functional genes, while depth and the interaction of depth and CO2 explained 5.2% and 3.8%, respectively. This study implies that eCO2 has profound effects on the functional structure and metabolic potential/activity of soil microbial communities associated with C4 plants, possibly leading to changes in ecosystem functioning and feedbacks to global change in C4 agroecosystems.
Atmospheric carbon dioxide (CO2) has been increasing at an accelerated pace since the Industrial Revolution, and is nearly 40% higher than it has been at any other time in the last 20 million years1. Such increases in CO2 concentration can affect, generally indirectly, soil microbial communities and their functions2,3,4, and subsequently, their mediated carbon (C) and nutrient cycling5,6,7. As soil contains the largest terrestrial C pool, shifts in microbial functional potential/activity may have great consequences in C stabilization and storage in soil, leading to either C sequestration or loss2,8,9. Therefore, understanding soil microbial responses to eCO2 is important for better predicting the contribution of terrestrial ecosystems to future climate8.
Unlike C3 plants, elevated CO2 (eCO2) should not directly stimulate the net CO2 assimilation rate of C4 plants, as C4 photosynthetic pathway is already CO2-saturated under current CO2 conditions10,11,12. However, eCO2 may indirectly promote C4 plant growth by increasing soil moisture10. Compared to C3 plants, our understanding of CO2 effects on C4 plants and their associated soil microbial communities is very limited. Although C4 plants only contribute ~25–30% of the global terrestrial productivity, many of them are ecologically and economically important (e.g., maize for grain, sugarcane and switchgrass for biofuel), and their cultivation is expected to increase in the future13,14. Therefore, it is necessary to understand the response of soil microbial communities to eCO2 in C4 agroecosystems.
The impact of eCO2 on the belowground microbial community is expected to be largely indirect, mediated through changes in soil nutrients, e.g., C, nitrogen (N) and soil properties3,15. As soil physiochemical parameters (e.g., nutrient availability, temperature, soil moisture) vary along the soil depth16,17,18, microbial communities may respond to eCO2 differently at different depths. Indeed, previous studies in other ecosystems have shown that eCO2 produces different effects on the microbial functional genes between soil depths (0–5 cm and 5–15 cm). For example, eCO2 significantly stimulated the abundances of many genes involved in C degradation and N cycling in the soil depth of 0–5 cm, but a majority of these genes remained unchanged in the depth of 5–15 cm7, indicating microbial responses to eCO2 differ along soil depths. Another study reported that soil organic C and N significantly increased in the soil depth of 5–15 cm, but remained unchanged in the depth of 0–5 cm under eCO2 in comparison to ambient CO219. However, most studies have examined the impact of eCO2 on soil microbial communities only at one depth (e.g., 0–15 cm). To fully understand the impact of eCO2 on soil microbial communities and their ecosystem processes, it is necessary to examine the response of soil microbial communities on a finer scale (e.g., different depths).
Maize (Zea mays L.) is the third most important food crop globally20. To discover the effect of eCO2 on the agronomy and productivity of important crops in the Midwestern USA, a free air CO2 enrichment experimental site (SoyFACE) was established in 2001 in a corn-soy agroecosystem (http://www.igb.illinois.edu/soyface/). In this study, we examined the response of soil microbial communities to maize fumigated with eCO2 in this FACE experiment. We hypothesized that eCO2 would alter the functional composition, structure and metabolic potential of soil microbial communities associated with maize cultivation, and that various microbial functional groups (e.g., autotrophs, heterotrophs, diazotrophs, nitrifiers and denitrifiers) would respond to eCO2 differentially between soil depths (0–5 cm and 5–15 cm). Our results demonstrated that eCO2 had significant effects on the functional structure and metabolic potential of soil microbial communities with similar trends in both soil depths, and that many key functional genes involved in C, N, and P cycling were stimulated by eCO2. This study provides new insights into our understanding the response of soil microbial communities to eCO2 in this C4 agroecosystem.
Resutls
Effects of eCO2 on plant yield and soil parameters
The grain biomass increased 12.5% when grown at eCO2 compared to aCO2, although this difference was not statistically significant (P = 0.25). The effects of eCO2 on some soil properties were different between soil depths. For example, soil NO3− level was significantly (P < 0.01) decreased in the depth of 0–5 cm under eCO2 compared to aCO2 but was significantly (P < 0.05) increased in the depth of 5–15 cm under eCO2. Soil moisture was not significantly different between two CO2 treatments in the depth of 0–5 cm, but was significantly (P < 0.05) increased in the depth of 5–15 cm at eCO2. eCO2 did not show any significant impacts on total C, total N, C:N ratio, or NH4+ contents at either soil depth (Table 1).
Table 1. Effects of eCO2 on soil properties at both depths.
| Moisture | NO3−-N | NH4+-N | Total nitrogen | Total carbon | |||
|---|---|---|---|---|---|---|---|
| (%, w/w) | (mg/kg) | (mg/kg) | (w/w, %) | (%, w/w) | TC/TN ratio | ||
| 0–5 cm | aCO2 | 24.0 ± 2.5B | 1.28 ± 0.11A | 30.4 ± 2.82A | 0.164 ± 0.011A | 2.43 ± 0.192A | 15.23 ± 0.90A |
| eCO2 | 24.1 ± 2.7b | 0.89 ± 0.06b | 36.4 ± 4.07a | 0.165 ± 0.013a | 2.12 ± 0.148a | 13.85 ± 0.61a | |
| P | 0.712 | 0.030 | 0.418 | 0.328 | 0.042 | 0.813 | |
| 5–15 cm | aCO2 | 36.5 ± 2.7A | 1.04 ± 0.07B | 32.61 ± 1.90A | 0.155 ± 0.008A | 2.24 ± 0.258A | 13.37 ± 0.79A |
| eCO2 | 38.5 ± 2.4a | 2.52 ± 0.59a | 31.34 ± 2.50a | 0.148 ± 0.007a | 1.91 ± 0.101a | 13.04 ± 0.51a | |
| P | 0.037 | 0.023 | 0.267 | 0.879 | 0.823 | 0.615 |
Soil variables from each depth were analyzed separately and significances between treatments (aCO2 and eCO2) or two soil depths were tested by t-test at the P< 0.05 level. A and B indicate significant changes between depths for aCO2, and a and b for eCO2.
Microbial metabolic potential
The metabolic capacity of soil communities collected from eCO2 and aCO2 conditions were similar across the incubation period of 144 hr in the soil depth of 0–5 cm (Figure 1). However, a significant (P < 0.05) stimulation of microbial C utilization capacity by eCO2 was observed in the soil depth of 5–15 cm after 48 hr of incubation and such eCO2-stimulated effects became greater overtime and lasted until the end of incubation (Figure 1).
Figure 1. Average well color development (AWCD) of the elevated CO2 (eCO2) and ambient CO2 (aCO2) samples in the soil depths of 0–5 cm (A) and 5–15 cm (B) measured by EcoPlate system.
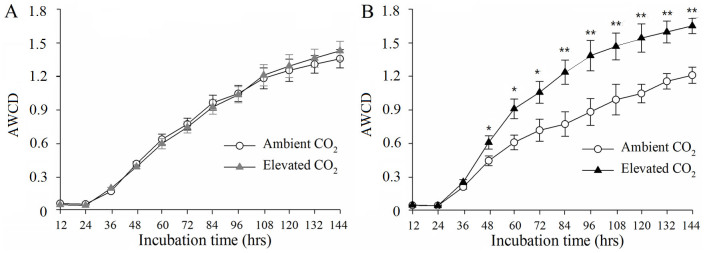
Error bars indicate ± SE (standard error) of the four blocks within each depth (n = 4). *: P< 0.05; **: P< 0.01 based on t-test between aCO2 and eCO2 at each time point.
Overview of functional and phylogenetic structure of soil microbial communities
A total of 6,491 genes were detected across 48 samples. The average number of detected genes (i.e., richness) was significantly (P = 0.044) greater (2,816 ± 200) under eCO2 than under aCO2 (2,202 ± 279) in the soil depth of 0–5 cm. This difference was even greater (P < 0.001) in the soil depth of 5–15 cm: 3,463 ± 189 genes detected under eCO2, 1,388 ± 137 genes detected under aCO2. Non-metric multidimensional scaling (NMDS) analysis based on the Bray-Curtis distance revealed that eCO2 dramatically altered the functional structure of microbial communities at both soil depths (Figure 2), and this was also the case for the phylogenetic structure based on the detected gyrB genes on GeoChip (Figure S1). Mantel tests indicated the phylogenetic structure was significantly correlated (r = 0.813, P < 0.001) with the functional structure. Those patterns were also confirmed by dissimilarity tests, showing significantly distinct functional structures between aCO2 and eCO2 at both depths, or between soil depths at both CO2 levels (Table 2). In addition, PERMANOVA revealed that eCO2 contributed 11.9% (P = 0.001) of the total variation of functional gene structure, while depth explained 5.2% of the variation (P = 0.014), and their interaction explained 3.8% (P = 0.034) (Table 3). Similarly, we observed significant differences in the phylogenetic structure due to eCO2 and depth (Table 3). Furthermore, such a pattern was observed at the functional gene category level, including C, N, P and CH4 cycling genes (Table S1).
Figure 2. Non-metric multidimensional scaling (NMDS) analysis of elevated CO2 (eCO2) and ambient (aCO2) samples in the soil depths of 0–5 cm (A) and 5–15 cm (B) based on Bray–Curtis values of detected functional genes (n = 12).
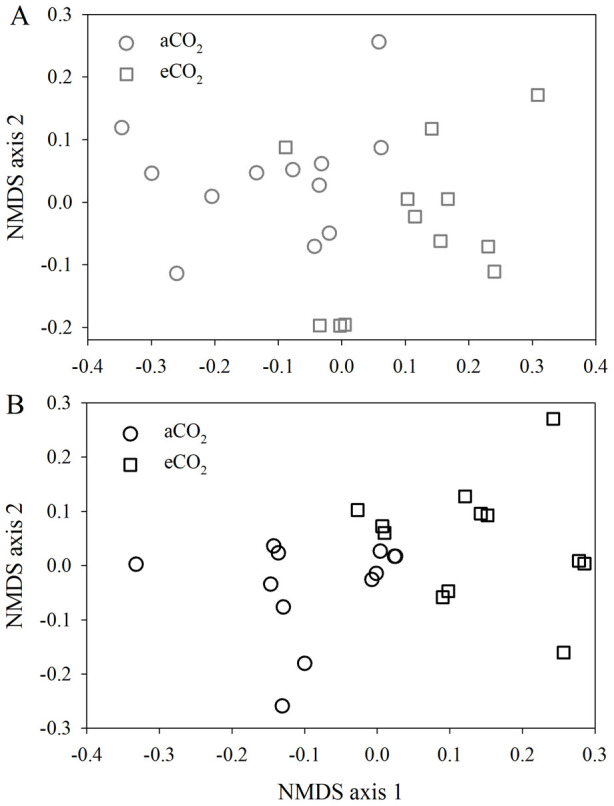
Table 2. Significance tests of the effects of CO2 and depths on the overall microbial community structure with three different statistical approaches.
| aCO2 vs. eCO2 | 0–5 cm vs. 5–15 cm | ||||
|---|---|---|---|---|---|
| 0–5 cm | 5–15 cm | aCO2 | eCO2 | ||
| Adonisa | F | 0.108 | 0.228 | 0.118 | 0.085 |
| P | 0.008 | 0.001 | 0.007 | 0.032 | |
| ANOSIMb | R | 0.210 | 0.424 | 0.115 | 0.055 |
| P | 0.004 | 0.001 | 0.014 | 0.134 | |
| MRPPc | δ | 0.514 | 0.453 | 0.483 | 0.484 |
| P | 0.005 | < 0.001 | 0.006 | 0.022 | |
aNon-parametric permutational multivariate analysis of variance (PERMANOVA) with the adonis function;
bAnalysis of similarities ANOSIM;
cNon-parametric procedure that does not depend on assumptions such as normally distributed data or homogeneous variances, but rather depends on the internal variability of the data.
Table 3. The effects of eCO2 and soil depth on the functional and phylogenetic structure of soil microbial community by non-parametric permutational multivariate analysis of variance (PERMANOVA) with the adonis function. The functional structure data were based on all detected genes by GeoChip while the phylogenetic structure data were based on gyrB only. R2 value is the constrained percentage of the parameter.
| CO2 | Depth | CO2:Depth | ||||
|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | |
| Functional structure | 0.119 | 0.001 | 0.052 | 0.014 | 0.038 | 0.034 |
| Phylogenetic structure | 0.103 | 0.001 | 0.049 | 0.016 | 0.027 | 0.155 |
Collectively, these results revealed that the diversity, composition, structure and functional potential of soil microbial communities were predominantly affected by eCO2 in this maize agroecosystem.
Genes involved in C cycling
A substantial number of Rubisco genes (74 from the soil depth of 0–5 cm and 58 from the depth of 5–15 cm) involved in C fixation were detected, and the abundance (signal intensity) of these genes was significantly (P < 0.05) higher under eCO2 than under aCO2 at both depths (Figure S2). Likewise, under eCO2, 12 unique rbcL genes were detected in the soil depth of 0–5 cm, while 27 unique genes were detected in the soil depth of 5–15 cm, compared with aCO2 at each depth (data not shown). Genes from the other two CO2 fixation pathways, CODH and Pcc/Acc, had significantly increased abundances under eCO2 in the soil depth of 5–15 cm, but their signal intensities did not differ significantly between two CO2 levels in the soil depth of 0–5 cm (Figure S2).
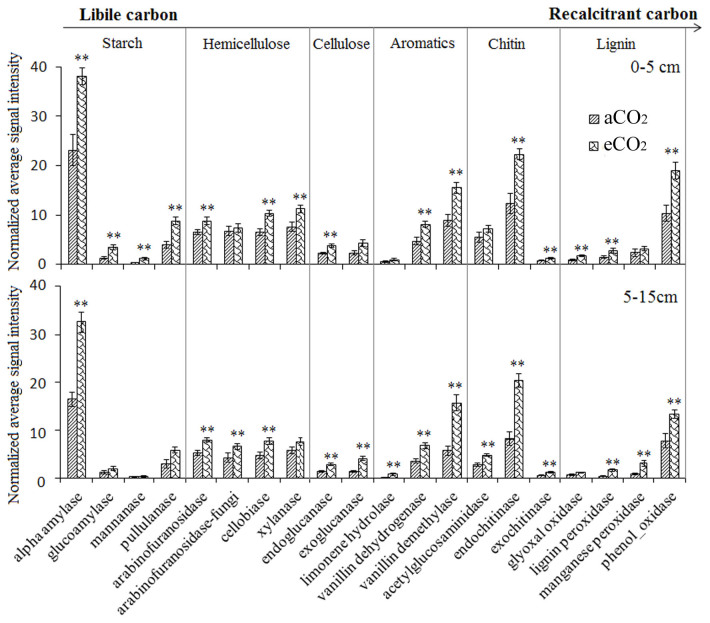
Cellulose, hemicellulose and lignin are the most abundant C sources derived from plant tissues in soil ecosystems. Here, most C degradation genes were significantly (P< 0.05) increased under eCO2 at both depths (Figure 3). For example, alpha-amylase, cellobiase, endoglucanase, vanillin dehydrogenase, endochitinase and phenoloxidase were all stimulated under eCO2. However, some genes responded differently to eCO2 along the soil depths (Figure 3). For example, the abundances of all four detected starch degradation genes were significantly (P < 0.05) increased under eCO2 in the soil depth of 0–5 cm, while only signal intensity of alpha-amylase was increased significantly under eCO2 in the soil depth of 5–15 cm. In addition, eCO2 increased the abundance of genes involved in CH4 cycling, including mcrA for methane production, and pmoA and mmoX genes for methane consumption (Figure S3). Apart from mmoX, where the abundance was significantly increased only in the soil depth of 5–15 cm, the significant increases of these genes were observed at both soil depths.
Figure 3. The abundance of detected key genes involved in C degradation.
All data are presented as the mean ± SE (standard error, n = 12). *: P < 0.05; **: P < 0.01based on t-test t between aCO2 and eCO2.
Genes involved in N cycling
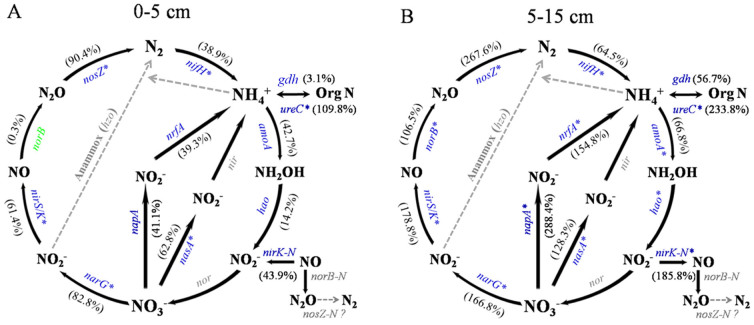
A total of 519 and 574 genes involved in N cycling were detected under aCO2 and eCO2, respectively, in the soil depth of 0–5 cm, and 287 and 570, respectively in the soil depth of 5–15 cm. eCO2 significantly (P < 0.05) increased the abundance of genes involved in N fixation (nifH), ammonification (ureC), denitrification (narG, nirS/K and nosZ) and assimilatory N reduction (nasA) at both depths (Figure 4A and 4B). Additionally, signal intensities of genes involved in nitrification (amoA and hao), and dissimilatory N reduction to ammonium (napA and nrfA) were only enhanced under eCO2 in the soil depth of 5–15 cm (Figure 4B).
Figure 4. The relative changes of the detected genes involved in N cycling at eCO2 compared to aCO2.
A: soil depth of 0–5 cm; B: soil depth of 5–15 cm soil. The signal intensity for each gene detected was normalized by all detected gene sequences using the mean. The percentage of a functional gene in a bracket was the sum of the signal intensity of all detected sequences of this gene divided by the grand sum of signal intensities of the detected N cycling genes, and weighted by the fold change of the signal intensity of this gene at eCO2 to that at aCO2. For each functional gene, colors mean that this gene had a higher (blue) or lower (green) signal intensity at eCO2 than at aCO2 with significance at P < 0.05 (*). Gray-colored genes were not targeted by this GeoChip, or not detected in those samples. It remains unknown if nosZ homologues exist in nitrifiers. Genes and their involved functional processes: N2 fixation by nifH encoding nitrogenase; Nitrification by amoA encoding ammonia monooxygenase; Denitrification by narG encoding nitrate reductase, nirS and nirK encoding nitrite reductase, norB encoding nitric oxide reductase, and nosZ encoding nitrous oxide reductase; Dissimilatory N reduction to ammonium by napA for nitrate reductase and nrfA for c-type cytochrome nitrite reductase; Ammonification by gdh encoding glutamate dehydrogenase and ure C encoding urease; Assimilatory N reduction, nasA encoding nitrate reductase.
Genes involved in P cycling
GeoChip 3.0 targets genes involved in exopolyphosphatase (Ppx) for inorganic polyphosphate degradation, polyphosphate kinase (Ppk) for polyphosphate biosynthesis in prokaryotes, and phytase for phytate degradation. Abundances of Ppk and Ppx genes were significantly increased (P < 0.05) under eCO2 compared to aCO2 at both depths, while phytase genes were significantly increased in the soil depth of 0–5 cm under eCO2 but remained unchanged in the soil depth of 5–15 cm (Figure S4).
Linking microbial functional structure to soil properties
Mantel tests were performed to examine the correlation between the microbial community structure and soil properties (TC, TN, NO3−, NH4+, and C:N ratio) and corn yield, no significant correlations were detected when all detected genes were considered. We then calculated the correlation between functional categories and soil variables. The results revealed that TN and TC were significantly (P < 0.05) correlated with the microbial community structure based on N fixation and nitrification genes, respectively (Table S2), whereas NO3−, NH4+and C/N ratio did not show significant correlations at the functional category level. We further examined the correlation of soil properties with individual functional gene families, and found that 30 functional gene families had significant (P < 0.05) correlations with soil properties, including those involved in C degradation, N cycling, CH4 consumption, bioremediation of aromatics, herbicides and pesticides (Table S3). For example, genes involved in C degradation (acetylglucosaminidase, pectinase, xylanase and amyAgenes), denitrification (norB), methane consumption (mmoX) and bioremediation/biodegradation of aromatics (pheA, oxdB, alkB and nagL), herbicides (pcpA and mhpC) and hydrocarbons (cpnA) were significantly (P < 0.05) correlated with soil properties, such as TC, TN and C:N ratio, and corn yield (Table S3).
Discussion
As soil microorganisms play important roles in mediating ecological processes (e.g., nutrient cycling, plant growth), understanding the response of soil microbial communities to eCO2 is critical to fully assess the impact of eCO2 on ecosystem functioning and stability and predict future climate change. In this study, we demonstrated that the functional structure and metabolic potential of microbial communities in a C4 maize agroecosystem were significantly altered under crops fumigated with eCO2 for eight years. The significant response to eCO2 was observed in samples of both soil depths, although some different microbial responsive patterns were observed between the two soil depths, and the abundance of many key functional genes was significantly increased and correlated with soil properties (e.g., nitrate, ammonia, soil C and N). CO2 was the dominant factor shaping the soil microbial functional structure, although depth and their interaction had significant contributions as well. Therefore, this study provides new insights into our understanding the response of soil microbial communities to eCO2 in C4 plant agroecosystems.
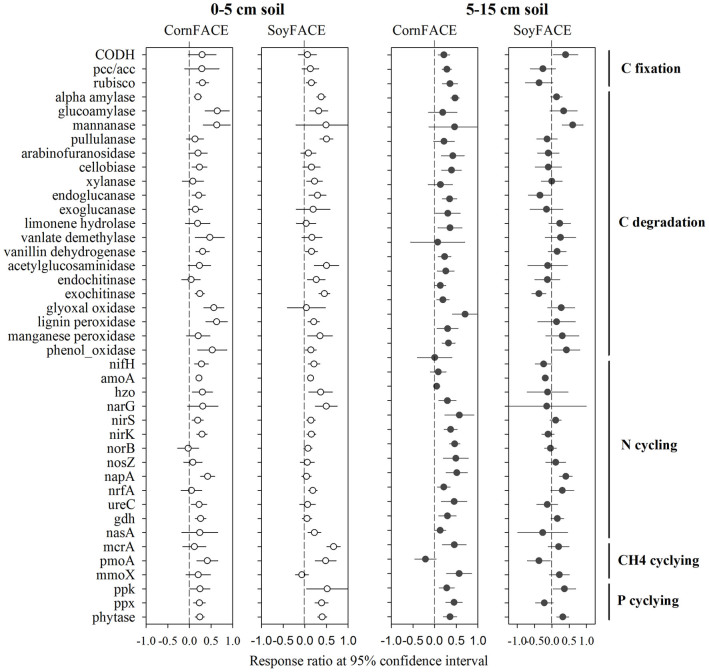
The C4 maize system examined in this study, and the C3 soybean plots that were the subject of a previous study7 were established in 2001 at the SoyFACE site (Champaign, IL, USA), and operated under typical Midwestern crop management practices21,38. While both studies revealed similar responses of soil microbial communities to eCO2 in the depth of 0–5 cm, a much greater stimulation of abundances of key functional genes involved in C and N cycling was observed in the depth of 5–15 cm in the maize field compared to the soybean field (Figure 5). This discrepancy may be due to the eCO2 enhanced water use efficiency for maize plants that would mitigate the drought stress on microbial activities21. Taken together, our data indicated that eCO2 may have comparable or even greater effects on microbial functional potential associated with C4 crops in comparison to C3 crops, which has not been recognized so far, which highlights the significance of this study.
Figure 5. Comparisons of effects of eCO2 on the abundance of functional genes with maize and soybean crops in the soil depths of 0–5 cm (open circle) and 5–15 cm (solid circle).
Significance was determined using the response ratio analysis5 at a 95% confidence interval (CI).
Elevated CO2 largely impacts soil microbial communities indirectly through increased plant C input, altered plant litter quality (e.g., C%, N%), and/or modified soil properties (e.g., soil pH and moisture)2,4,15,22. In the limited number of studies on C4 plants under eCO2, the stimulation of plant biomass and/or alteration of litter quality under eCO2 have not been previous observed21,23,24,25. Similarly, we did not detect a significant change in maize yield under eCO2 in this study. However, eCO2 could significantly improve, although the effect was small, C4 plant water use efficiency by reducing midday stomatal conductance or under drought26,27, thus indirectly stimulating the growth of C4 plants by delaying and ameliorating drought stress10. In 2008, the SoyFACE site experienced ‘atypical' precipitation year with wet early-season, a single extended drying event in the mid-season, and rewet late-season25. Soil samples used in this study were collected in the drought period in August 2008. The drought event could cause physiological stresses for maize plants, but growth under eCO2 might delay or relieve drought-induced reduction of net photosynthetic CO2 uptake, resulting in higher leaf-level photosynthetic C gain in the drought season25. The enhanced photosynthetic C gain, eventually increased plant C input may enhance C allocation into belowground in the form of root biomass and rhizodeposits under eCO2, which may result in the alteration of microbial functional structure and metabolic potential, and especially the stimulation of C degradation and N cycling genes.
Given the previously reported conservation of soil moisture by C4 crops under eCO221, we hypothesize that microbial activities were constrained by drought conditions under C3 plants, while the water-use efficiency of C4 plants mitigated the drought effects on microbial activities under the maize cropping system21,26. A previous study has demonstrated that the efflux of amino acids from maize roots was significantly enhanced under eCO2, although plant biomass remained unchanged28. Also, greater soil moisture under eCO2, due to the improved water use efficiency of C4 plants under elevated CO225, may alter the microbial community structure and function, particularly under drought condition29,30,31. For example, it has been shown that soil microbial activity was consistently enhanced in tallgrass prairie under eCO2 treatment due to improved soil water condition, which was closely correlated with soil water content in the depth of 0–5 cm19. Indeed, in this study, we found that soil moisture was significantly greater under eCO2 in the depth of 5–15 cm. Therefore, although it is beyond our research scope to identify mechanisms explaining how eCO2 shifts the microbial community structure and function, this study supports our hypothesis that eCO2 would alter the functional composition, structure, and metabolic potential of soil microbial communities associated with maize cultivation, possibly through increased soil moisture. Indeed, a recent study also showed that eCO2 increased soil moisture along with decreased maize evapotranspiration by 7–11%32. We expect this effect to be more evident under drought conditions21. Therefore, in this study, our results support the above hypothesis, evidenced by the comparison of the effects of eCO2 on the abundance of functional genes between maize and soybean crops. That is, eCO2 substantially stimulated the functional gene abundances at both depths in this maize FACE experiment (Figure 5), but only minor eCO2 effects were detected in the 5–15 cm soil planted with soybean at the SoyFACE site7.
It is also hypothesized that various microbial functional groups (e.g., C fixers, C degraders, diazotrophs and denitrifiers) would be stimulated differentially between two soil depths by eCO2. This study indicated that the abundance of key functional genes involved in C, N and P cycling was significantly stimulated under eCO2 at both soil depths. First, eCO2 increased the signal intensity of 75% (15 out of 20) of the detected functional genes involved in C degradation at both soil depths (0–5 cm and 5–15 cm), with 11 genes responding positively to eCO2 in two depths. These ‘common' eCO2-enriched genes in both soil depths, which are capable of decomposing a variety of C compounds present in plant materials and soil organic matter. Such responses of soil microbial communities to maize fumigated with eCO2 are generally consistent with previous studies2,6,7. The significantly enhanced C degradation genes under eCO2 may indicate the stimulation of microbially-mediated C decomposition in soil. However, this does not imply that soil C storage was reduced under eCO2, since soil C storage and stability are also largely affected by other factors, such as plant C input (e.g., quality and quantity), plant nutrient uptake (e.g., N, P), soil properties, and size and turnover of different C pools (e.g., native soil C pool, fresh plant litter pool, microbial C pool)2,5,23,33,34. Although some studies showed that eCO2 led to the loss of soil C8,9, other studies showed an increase in soil C35, or no significant effects on soil C content2,36, which is consistent with the current study. Especially in this SoyFACE, previous studies showed that management practices affected soil C and N stocks and dynamics more than eCO2-stimulated effects34,37. Such common responses at both soil depths are also reflected in N cycling genes. Although N fertilizer was yearly applied to the maize plots before planting38, key genes involved in N fixation (nifH) and ammonification (ureC) were significantly increased under eCO2 compared to aCO2. This finding agrees with other studies showing that microbial N fixation or the abundance of N fixation genes increased under eCO22,5,39. If such increased gene abundances are translated to increased N fixation and ammonification process rates, this may relieve progressive N limitation observed previously in other FACE sites40,41,42,43. Also, key genes involved in denitrification were generally stimulated under eCO2. For example, a previous study showed that the nirK abundance increased more than doubled while its diversity was significantly reduced in soil where trembling aspen was grown undereCO244. In contrast, the signal intensity of denitrification genes was not generally stimulated under eCO2 in a soybean agroecosystem (Figure 5)7, and a similar pattern was detected by qPCR analysis of amoA and nosZ gene abundances at this site in the same year, showing that eCO2 has limited effects on N transformations in soybean agroecosystem45. Based on the measurement of N2O fluxes, several studies have reported that denitrification is enhanced at eCO246,47, although exceptions were also reported48. Such a difference may be complicated by other factors, such as soil moisture, type and aggregate size. For example, the abundance of nosZ genes increased in the microaggregates under reduced precipitation but not by eCO2 or in the whole soil compared to ambient conditions34. If the increased denitrification genes indicate an enhanced denitrification processes under eCO2, this may result in increased N2O consumption and production, a possible positive feedback to global change.
However, some differential responses of soil microbial communities to eCO2 were also detected between the two soil depths. First, three key C fixation genes from three pathways, including Rubisco for the Calvin cycle, CODH for the reductive acetyl-CoA pathway, and PCC/ACC for the 3-hydroxypropinate/malyl-CoA cycle, increased significantly under eCO2 in the soil depth of 5–15 cm, while only Rubisco genes increased significantly in the depth of 0–5 cm. Second, the abundance of genes involved in nitrification (amoA and hao) and dissimilatory N reduction (napA and nrfA) was significantly enhanced in the soil depth of 5–15 cm, but unchanged in the depth of 0–5 cm. Third, the microbial metabolic potential as measured by EcoPlate increased significantly under eCO2 in the soil depth of 5–15 cm, but was similar in the depth of 0–5 cm. Also, the abundances of four starch degradation genes detected by GeoChip were all significantly increased under eCO2 in the soil depth of 0–5 cm, but only alpha-amylase gene was stimulated in the depth of 5–15 cm. Additionally, the response of P cycling genes to eCO2 appeared greater in the soil depth of 0–5 cm than that of 5–15 cm.
Several reasons may contribute to the subtle difference in microbial responses to eCO2. First, the composition and functional structure of microbial communities were significantly different between the two soil depths, thus resulting in differential functional potential/activity. Second, soil may contain more organic matter from plant residues in the depth of 0–5 cm than that of 5–15 cm, resulting in differences of nutrient availability for microbial growth and activities. Third, many soil physiochemical properties (e.g., O2 concentration, soil aggregate size, pH, moisture, temperature) may change with depths, and some of them (e.g., soil moisture, temperature) may experience wider fluctuations in the depth of 0–5 cm soil than that of 5–15 cm16,17,18, thus differentially affecting microbial responses to eCO219,49,50. For example, it has been shown that soil microbes responded differently to eCO2, warming, and their interactions49. Similarly, Castro and colleagues studied how microbes responded to multiple climate change factors (eCO2, warming and precipitation) and found complex responsive patterns with multiple factors50. Therefore, our results showed that microbial responses to eCO2 were consistent overall, and soil depth only had a minor effect, indicating eCO2 had a much greater impact on microbial structure and function than soil depth.
In summary, this study highlights the necessity and importance of examining the microbial response to eCO2 in C4 agroecosystem. The significant stimulation of a great number of key functional genes involved in C and N cycling at both soil depths may indicate the potential of altered C and N dynamics in soils planted with C4 crops. This study provides new insights into our understanding of soil microbial community responses to eCO2 in a C4 maize agroecosystem. However, further studies are needed to understand the mechanism by which microbial structure and function shift at the eCO2 environment and their feedbacks to ecosystem functioning, stability and services, especially with different C4 plant species.
Methods
Site description and sample collection
The SoyFACE experimental site at Champaign, IL, USA (40°03′N, 88°12′W, 228 m above sea level) was established in 2001 on tile-drained farmland that had been under cultivation for over 100 years. The crops are rotated between maize (Z. mays cv. 34B43, Pioneer Hi-Bred International) and soybean (Glycine max) on a yearly basis. The soil is Drummer–Flanagan series (fine-silty, mixed, mesic Typic Endoaquoll) and organic rich38. Fertilizer was applied to maize fields yearly at a rate of 202 kg N ha−1 (157 kg N ha−1 with 28% 1:1 urea: ammonium nitrate liquid before planting and 45 kg N ha−1 credit from previous soybean N2 fixation)38. Atmospheric CO2 of four replicate plots (each with a 20-m diameter) was maintained at the ambient CO2 (aCO2, 354 ppm) level, and four replicates are maintained at an elevated (~550 ppm) CO2 level in a randomized complete block design. To minimize the cross-contamination, aCO2 and eCO2 plots in each block were set with a 100-m interval. Three subsamples were collected in each plot at two soil depths (0–5 cm and 5–15 cm) from both CO2 treatments before harvest in August 2008, resulting in a total of 48 samples (4 plots x 3 subsamples x 2 CO2 treatments x 2 depths). Soil samples were sieved through a 2-mm sieve to remove visible plant materials. All soil samples were immediately stored at 4°C or −80°C until soil property analysis or DNA extraction.
Plant and soil analysis
Plant yields (grain biomass) were collected and analyzed at the end of the growing season. Soil total C and total N were measured by combustion (Muti N/C 3100, Jena, Germany). Soil NO3− and NH4+ were extracted with 20 ml of 2 M KCl and analyzed using a segmented flow analyzer (Skalar Sanplus, Breda, Netherlands).
Analysis of microbial metabolic potential
BioLog EcoPlateTM substrate utilization assays51 containing 31 sole carbon sources and control wells (without substrates) with three replicates in a 96-well plate were used to evaluate the metabolic potential of soil microbial communities. Three subsamples collected from the same plot were composited together, resulting in 4 biological replicates from aCO2 and eCO2 at both depths. The soil suspension was prepared by adding 5.0 g soil to 45 ml of double distilled H2O, followed by shaking for 45 min with 200 rpm at 4°C. Then samples were allowed to settle for 30 min before the supernatant was collected and serially diluted to 10−4 based on a pilot experiment. An aliquot of 100 µl of the diluted suspension from each soil sample was then inoculated into each EcoPlate well, and incubated at 25°C for 168 hours with in an OminLog System (BioLog Inc., Hayward, CA, USA). Well color development was automatically measured by OminLog System at 15 min intervals during the incubation. The average well color development (AWCD) presents the potential utilization of various carbon sources by a microbial community. AWCD was calculated by the differences between the OD590 of the wells containing individual carbon sources and the control wells according to AWCD = ∑(C-R)/31, where C is the OD590 value of each well, R is the OD590 value of the control well52.
DNA extraction
DNA was extracted from 5.0 g of soil samples using the method described previously53. DNA quality was assessed by the ratio of 260/280 nm and 260/230 nm using a ND-1000 spectrophotometer (NanoDrop Inc., Wilmington, NC) and DNA concentration was quantified with a Quant-ItTM PicoGreen (Invitrogen, Carlsbad, CA).
GeoChip analysis
Purified DNA was amplified using whole community genome amplification (WCGA) and labeled with fluorescent dyes as described previously54,55. The labeled DNA was then hybridized to GeoChip 3.0 at 42°C for 12 hrs56. After hybridization, the chips were scanned using a ScanArray 5000® Microarray Analysis System (PerkinElmer, Wellesley, MA) at 95% laser power and 75% PMT (photomultiplier tube gain), and the signal intensity of each spot was measured using ImaGene™ 6.1 Standard Edition (Biodiscovery Inc., El Segundo, CA). Spots with signal-to-noise ratio (SNR) < 2.0 were removed. Probe signal intensities were normalized by their own universal standards in the experiment. A probe was considered positive if it was detected in at least 3 out of 12 replicates. These positive spots were included for further analysis.
Statistical analysis
An unpaired t-test was conducted to test the significances of plant yield between aCO2 and eCO2, soil variables between aCO2 and eCO2 in each soil depth (0–5 cm or 5–15 cm), microbial metabolic potential between aCO2 and eCO2 at each time point in each soil depth, respectively57. The functional structure of microbial communities was ordinated using the NMDS based on the Bray-Curtis distance58. Non-parametric permutational multivariate analysis of variance (PERMANOVA), analysis of similarities (ANOSIM), and multi-response permutation procedure (MRPP) were used to evaluate the significance of the functional structure between aCO2 and eCO2 at each depth based on the null hypothesis58,59. The effect of eCO2 on the abundance of a given functional gene was analyzed by computing the response ratio5. Also, non-parametric permutational multivariate analysis of variance (PERMANOVA) was conducted to quantitatively evaluate the contribution of CO2 and depth to the microbial community structure using the ‘adonis' function58. Mantel tests were used to examine the correlation between the microbial community structure and environmental factors (soil properties and corn yield). All of the above analyses were performed with R v.2.8.1 project in the vegan package (v.1.15-1) (www.R-project.org).
Author Contributions
All authors contributed to the data set, discussed the results and commented on the manuscript. Z.H., A.K. and J.Z. designed this study. J.X. did the experiments, and J.X. and Y.D. did data analysis. J.X., Z.H. and S.S. wrote this paper with help from A.K. J.D.V., L.W. and J.Z.
Supplementary Material
Supplemental Information
Acknowledgments
Assistance with sample collection was provided by Ariane L. Peralta, Yu-rui Chang, Sara F. Paver, Diana N. Flanagan, and Anthony C. Yannarell. This work is supported by the US Department of Agriculture (Project 2007-35319-18305) through the NSF-USDA Microbial Observatories Program, and by Oklahoma Applied Research Support (OARS), Oklahoma Center for the Advancement of Science and Technology (OCAST) through AR062-034 and AR11-035, and the KC Wong Magna Fund of Ningbo University.
References
- Pearson P. N. & Palmer M. R. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406, 695–699 (2000). [DOI] [PubMed] [Google Scholar]
- He Z. et al. Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2. Ecol. Lett.13, 564–575 (2010). [DOI] [PubMed] [Google Scholar]
- Zak D. R., Pregitzer K. S., King J. S. & Holmes W. E. Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol. 147, 201–222 (2000). [Google Scholar]
- Drigo B. et al. Impacts of 3 years of elevated atmospheric CO2 on rhizosphere carbon flow and microbial community dynamics. Glob. Change Biol. 19, 621–636 (2013). [DOI] [PubMed] [Google Scholar]
- Luo Y., Hui D. & Zhang D. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87, 53–63 (2006). [DOI] [PubMed] [Google Scholar]
- Xu M. et al. Elevated CO2 influences microbial carbon and nitrogen cycling. BMC Microbiol. 13, 124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. et al. Distinct responses of soil microbial communities to elevated CO2 and O3 in a soybean agro-ecosystem. ISME J. 8, 714–726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney K. M., Hungate B. A., Drake B. G. & Megonigal J. P. Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc. Natl. Acad. Sci. U.S.A. 104, 4990–4995 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann M. & Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292 (2008). [DOI] [PubMed] [Google Scholar]
- Leakey A. D. et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876 (2009). [DOI] [PubMed] [Google Scholar]
- Wang D., Heckathorn S. A., Wang X. & Philpott S. M. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169, 1–13 (2012). [DOI] [PubMed] [Google Scholar]
- Twine T. E. et al. Impacts of elevated CO2 concentration on the productivity and surface energy budget of the soybean and maize agroecosystem in the Midwest USA. Glob. Change Biol. 19, 2838–2852 (2013). [DOI] [PubMed] [Google Scholar]
- Somerville C., Youngs H., Taylor C., Davis S. C. & Long S. P. Feedstocks for lignocellulosic biofuels. Science 329, 790–792 (2010). [DOI] [PubMed] [Google Scholar]
- Pingali P. L. Meeting world maize needs: technological opportunities and priorities for the public sector. (International Maize and Wheat Improvement Center, 2001). [Google Scholar]
- He Z. et al. The phylogenetic composition and structure of soil microbial communities shifts in response to elevated carbon dioxide. ISME J. 6, 259–272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N., Schimel J. P. & Holden P. A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 35, 167–176 (2003). [Google Scholar]
- Griffiths R. I., Whiteley A. S., O'Donnell A. G. & Bailey M. J. Influence of depth and sampling time on bacterial community structure in an upland grassland soil. FEMS Microbiol. Ecol. 43, 35–43 (2003). [DOI] [PubMed] [Google Scholar]
- Xiong J. et al. Assessing the microbial community and functional genes in a vertical soil profile with long-term arsenic contamination. PloS One 7, e50507 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. W., Garcia F. O., Hampton C. O. & Owensby C. E. Soil microbial response in tallgrass prairie to elevated CO2. Plant Soil 165, 67–74 (1994). [Google Scholar]
- Rosegrant M. W., Paisner M. S., Meijer S. & Witcover J. Global food projections to 2020: Emerging trends and alternative futures. International Food Policy Research Institute, Washington, DC, USA (2001). [Google Scholar]
- Leakey A. D. et al. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 140, 779–790 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair E. C., Reich P. B., Hobbie S. E. & Knops J. M. Interactive effects of time, CO2, N, and diversity on total belowground carbon allocation and ecosystem carbon storage in a grassland community. Ecosystems 12, 1037–1052 (2009). [Google Scholar]
- Ainsworth E. A. & Long S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372 (2005). [DOI] [PubMed] [Google Scholar]
- BALL A. Microbial decomposition at elevated CO2 levels: effect of litter quality. Glob. Change Biol. 3, 379–386 (1997). [Google Scholar]
- Markelz R. C., Strellner R. S. & Leakey A. D. Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated [CO2] in maize. J. Exp. Bot. 62, 3235–3246 (2011). [DOI] [PubMed] [Google Scholar]
- Conley M. M. et al. CO2 enrichment increases water-use efficiency in sorghum. New Phytol. 151, 407–412 (2001). [Google Scholar]
- Wall G. et al. Elevated atmospheric CO2 improved sorghum plant water status by ameliorating the adverse effects of drought. New Phytol. 152, 231–248 (2001). [Google Scholar]
- Phillips D. A., Fox T. C. & Six J. Root exudation (net efflux of amino acids) may increase rhizodeposition under elevated CO2. Glob. Change Biol. 12, 561–567 (2006). [Google Scholar]
- Hu S., Firestone M. K. & Chapin III F. S. Soil microbial feedbacks to atmospheric CO2enrichment. Trends Ecol. Evolu. 14, 433–437 (1999). [DOI] [PubMed] [Google Scholar]
- Manzoni S., Schimel J. P. & Porporato A. Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93, 930–938 (2012). [DOI] [PubMed] [Google Scholar]
- Schimel J. P., Gulledge J. M., Clein-Curley J. S., Lindstrom J. E. & Braddock J. F. Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biol. Biochem. 31, 831–838 (1999). [Google Scholar]
- Hussain M. Z. et al. Future carbon dioxide concentration decreases canopy evapotranspiration and soil water depletion by field-grown maize. Glob. Change Biol. 19, 1572–1584 (2013). [DOI] [PubMed] [Google Scholar]
- Feng X., Simpson A. J., Schlesinger W. H. & Simpson M. J. Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Glob. Change Biol. 16, 2104–2116 (2010). [Google Scholar]
- Pujol Pereira E. I., Chung H., Scow K. & Six J. Microbial communities and soil structure are affected by reduced precipitation, but not byelevated carbon dioxide. Soil Sci. Soc. Am. J. 77, 482–488 (2013). [Google Scholar]
- Jastrow J. D. et al. Elevated atmospheric carbon dioxide increases soil carbon. Glob. Change Biol. 11, 2057–2064 (2005). [DOI] [PubMed] [Google Scholar]
- Hungate B. A. et al. CO2 elicits long-term decline in nitrogen fixation. Science 304, 1291–1291 (2004). [DOI] [PubMed] [Google Scholar]
- Moran K. K. & Jastrow J. D. Elevated carbon dioxide does not offset loss of soil carbon from a corn–soybean agroecosystem. Environ. Pollu. 158, 1088–1094 (2010). [DOI] [PubMed] [Google Scholar]
- Leakey A., Bernacchi C., Dohleman F., Ort D. & Long S. Will photosynthesis of maize (zea mays) in the US corn belt increase in future [CO2] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE). Glob. Change Biol. 10, 951–962 (2004). [Google Scholar]
- Drake J. E. et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357 (2011). [DOI] [PubMed] [Google Scholar]
- Johnson D. W. Progressive N limitation in forests: review and implications for long-term responses to elevated CO2. Ecology 87, 64–75 (2006). [DOI] [PubMed] [Google Scholar]
- Finzi A. C. et al. Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87, 15–25 (2006). [DOI] [PubMed] [Google Scholar]
- Reich P. B. et al. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440, 922–925 (2006). [DOI] [PubMed] [Google Scholar]
- Norby R. J., Warren J. M., Iversen C. M., Medlyn B. E. & McMurtrie R. E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. U.S.A. 107, 19368–19373 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. J. et al. Elevated atmospheric CO2impacts abundance anddiversity of nitrogen cycling functional genes in soil. Microb. Ecol. 65, 394–404 (2013). [DOI] [PubMed] [Google Scholar]
- Pujol Pereira E. I. et al. Soil nitrogen transformations under elevated atmospheric CO2 and O3 during the soybean growing season. Environ. Pollu. 159, 401–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs E., Richter M., Hartwig U. & Cadisch G. Nitrous oxide emissions from grass swards during the eighth year of elevated atmospheric pCO2 (Swiss FACE). Glob.Change Biol. 9, 1214–1222 (2003). [Google Scholar]
- Robinson D. & Conroy J. P. A possible plant-mediated feedback between elevated CO2, denitrification and the enhanced greenhouse effect. Soil Biol. Biochem. 31, 43–53 (1998). [Google Scholar]
- Barnard R., Barthes L., Le Roux X. & Leadley P. W. Dynamics of nitrifying activities, denitrifying activities and nitrogen in grassland mesocosms as altered by elevated CO2. New phytol. 162, 365–376 (2004). [Google Scholar]
- Hayden H. L. et al. Changes in the microbial community structure of bacteria, archaea and fungi in response to elevated CO2 and warming in an Australian native grassland soil. Environ. Microbiol. 14, 3081–3096 (2012). [DOI] [PubMed] [Google Scholar]
- Castro H. F., Classen A. T., Austin E. E., Norby R. J. & Schadt C. W. Soil microbial community responses to multiple experimental climate change drivers. Appl. Environ. Microbiol. 76, 999–1007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland J. L. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 24, 289–300 (1997). [Google Scholar]
- Garland J. L., & Mills A. L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level, sole-carbon-source utilization. Appl Environ Microbiol 57, 2351–2359 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Bruns M. A. & Tiedje J. M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62, 316–322 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Liu X., Schadt C. W. & Zhou J. Microarray-based analysis of subnanogram quantities of microbial community DNAs by using whole-community genome amplification. Appl. Environ. Microbiol. 72, 4931–4941 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J. et al. Microbial communities and functional genes associated with soil arsenic contamination and the rhizosphere of the arsenic-hyperaccumulating plant Pteris vittata L. Appl. Environ. Microbiol. 76, 7277–7284 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. et al. GeoChip 3.0 as a high-throughput tool for analyzing microbial community composition, structure and functional activity. ISME J. 4, 1167–1179 (2010). [DOI] [PubMed] [Google Scholar]
- Ruxton G. D. The unequal variance t-test is an underused alternative to Student's t-test and the Mann–Whitney U test. Behav. Ecol. 17, 688–690 (2006). [Google Scholar]
- Anderson M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 (2001). [Google Scholar]
- Biondini M. E., Mielke P. W. Jr & Berry K. J. Data-dependent permutation techniques for the analysis of ecological data. Vegetatio 75, 161–168 (1988). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information