Abstract
As injectable therapies such as human insulin, insulin analogs, and glucagon-like peptide-1 receptor agonists are used to manage diabetes, correct injection technique is vital for the achievement of glycemic control. The forum for injection technique India acknowledged this need for the first time in India and worked to develop evidence-based recommendations on insulin injection technique, to assist healthcare practitioners in their clinical practice.
Keywords: Adherence, aspart, degludec, detemir, diabetes, education, glargine, glucagon-like peptide 1 receptor agonists, glulisine, injections, injection sites, injection technique, insulin, insulin analogs, lipohypertrophy, lispro, needle length, skin-fold, storage
INTRODUCTION
An alarmingly rising prevalence of diabetes has been reported in recent years.[1] According to current studies, 67 million Indians are affected with diabetes.[2] By 2030, the prevalence of diabetes among Indians is projected to reach 87 million.[3] Insulin remains the mainstay of treatment in diabetes[4] and about 3.2 million Indians depend on insulin injections for the management of diabetes.[5] Many people with type 2 diabetes and all with type 1 diabetes need insulin for survival, and intensive insulin therapy is recommended in them.[6] However, incorrect technique of injecting insulin may increase the risk of poor glycemic control, due to mismatch of peak insulin effect and maximal glucose load.[7,8] Shorter needles are beneficial over longer ones as they avoid the risk of intramuscular administration even if injected at 90° angle to the skin surface.[9] It has been demonstrated that the use of shorter needles provides equivalent glycemic control compared to longer needles, without increasing the incidence of leakage events. Shorter needles also help in combating the psychological fear of needles in patients and hence improve their acceptance of insulin injection therapy.[10]
Improper use or reuse of injection devices, like needles, may lead to undesirable consequences including pain with bleeding and bruising, breaking off and lodging under the skin, contamination, dosage inaccuracy, and lipohypertrophy.[11] According to a study, correct injection technique and use of shorter needles (4 mm) are associated with improved glucose control, greater satisfaction with therapy and lower consumption of insulin after only a 3-month period.[12] Appropriate injection technique, thus, is an indispensable part of diabetes management.
NEED FOR INDIAN RECOMMENDATIONS
Appropriate injection technique is crucial for the success of insulin therapy. Several issues such as lack of knowledge, time constraints of healthcare providers (HCPs), and scarcity of local recommendations may contribute to the lack of appropriate injection technique.[13] Physicians’ awareness and willingness to convey basic information such as the dosage, injection site selection, depth of injection, and the correct method of injection go a long way in preventing faulty injection technique.
However, there are some factors such as dexterity, visual and hearing impairment, and learning skills which at times cannot be modified. These factors can be addressed by choosing appropriate insulin devices, suited to the individual.
Recommendations or consensus statements on an insulin technique that suit the local needs of developed countries are available. Such documents have not addressed certain issues relevant to developing countries such as India. Hence, a need for Indian recommendations for best practice in insulin injection technique was felt.
MATERIALS AND METHODS
The first draft of the forum for injection technique (FIT) India recommendations, 2012, was prepared by a core writing group of three endocrinologists. A psychiatrist was co-opted into the core writing group to provide expert opinion on the psychological aspects of injectable therapy.
The methodology of literature search, medical writing strategy, and evidence grading was finalized after consensus approval and validation by a board of 13 HCPs from various medical disciplines, including endocrinologists, diabetologists, diabetes educators, psychiatrists, and psychologists. Simultaneously, the draft was also reviewed by 76 clinicians from India and six experts from South Asia (South Asian referral group). Another five eminent members were added to the FIT India advisory board in November, 2014.
These recommendations are based on the evidence collated from published literature, specific to the subject of injection technique. The grading method followed by Frid et al. (2010), which includes an activities-specific balance confidence scale for the strength of recommendation and 123 scale for scientific support, has been used to grade the evidence [Figure 1].[14] Certain recommendations, which are supported by manufacturer advice or drug authority guidance, have been ranked 1 in scientific support.
Figure 1.
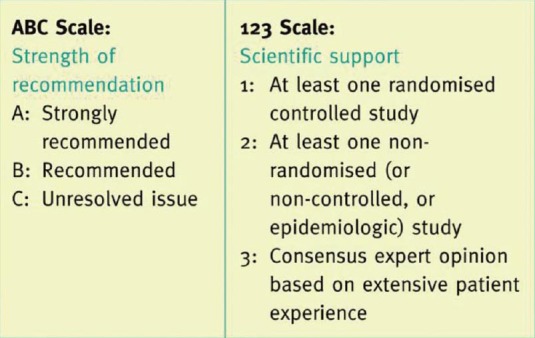
Grading criteria (Frid et al., 2010)
An abridged version of the recommendations was published in Indian Journal of Endocrinology and Metabolism. An addendum was published in 2013 to cover certain issues which had not been included in the original FIT India document. An expert on insulin pump therapy was co-opted while writing the addendum.
Another addendum has been published in 2014, with the aim of making FIT India more comprehensive.
The revised FIT India recommendations, 2015, have been prepared carefully to ensure that no relevant information is omitted, and no superfluous information added.
INJECTION TECHNIQUE RECOMMENDATIONS
Preinjection assessment
Clinical assessment
A thorough patient assessment should precede therapy initiation (B3). Optimization of injection technique with respect to the individual patient needs is critical for the success of injectable therapy.[11]
Environmental assessment
The use of correct type and device of insulin should be ensured. As the insulin is sensitive to extreme temperatures, it is essential to inquire about storage conditions of injection supplies (B2). Availability of stock for at least 1–3 months should be ensured so that no dose is missed. If injection cold storage facilities are not readily available, insulin pens can be used instead of vials (C1).
Sociocultural sensibilities
Sociocultural sensibilities of the community should be respected. It is advised to discuss the site of injection beforehand in Indian women so that their sensibilities are not offended.
Preinjection counselling
More than ¼ of patients may refuse insulin therapy after prescription.[15] This phenomenon is often termed as psychological insulin resistance (PIR). The most pronounced reasons associated with PIR are personal failure, anticipated pain, low self-efficacy, and lack of awareness about the benefits of insulin.
It is thus essential to encourage shared decision-making with active participation of the patient. Specification of pain-minimizing devices, like pens requiring low pressure for injection, in the doctor's prescription, could help in insulin therapy initiation.[14]
Children
In the early stages of diabetes, especially in childhood, the diagnosis of diabetes creates a sense of distress and anxiety in both parents and children. This hinders parents’ ability to administer insulin or encourage children to self-administer insulin (A1). Helping children to overcome their fear of needles is mandatory.
Initially, to overcome anxiety, it may help to allow parents and children to administer saline, insulin diluent or one unit of insulin themselves. Explanation of the role of insulin in diabetes management and the need for regular injections allays misconceptions and concerns. One may demonstrate injection technique on oneself, or on a toy doll. Play therapy is a useful method of explaining injection technique.
Adolescents
Adolescents may exhibit the sub-optimal compliance to insulin injection schedule due to several factors such as peer pressure, lack of seriousness, pain, and frustration (A2). Adolescents, especially girls, may skip insulin injections because of the fear of weight gain. It is important to help them overcome any possible misconceptions related to insulin by sharing information with them (B2). Re-emphasizing the benefits of insulin administration helps increase acceptance of insulin injection among adolescents (B1).
Adults
All newly diagnosed persons with diabetes should be educated about the course of diabetes and the need to start insulin therapy at some time in the future[14] (A3). It is important to explore and acknowledge concerns of the patients.
Elderly
Counseling geriatric patients for self-injection can be a challenging task. Limited dexterity, visual impairment, and hearing impairment are of utmost importance in this vulnerable group of patients.[16]
The importance of modern pen devices is reflected best in the field of geriatrics. Their discreetness, simplicity, and convenience of use, dosage accuracy, and being less painful to inject allows for widespread acceptability amongst the elderly.[17,18,19] Easy preselection of the prescribed insulin dose has also been well-documented.[20] Various recommendations for diabetes management in the elderly have discussed these points and suggested some practical remedial steps.[21]
Technical superiority of a product (analog vs. conventional) or an injection device (pen vs. syringes), should be given due consideration.
Injection storage
It is recommended that specific storage recommendations provided by the manufacturer be followed. Insulin should be stored in a cool and dark place. Insulin pens and vials, which are not being used, should be refrigerated, but not frozen (A1). If frozen, insulin should be discarded. Insulin being used can be kept at room temperature to limit local irritation at the injection site, which may occur when cold insulin is used. The insulin vial should be taken out and kept at room temperature for at least 30 min before use.[22] Pens should never be stored with needles on, because the higher risk of the air entering through the needles may clog them and hence affect dosage delivery.[11] In rural areas or in places where a refrigerator is not available, it is advisable to put the vial in a plastic bag, tie a rubber band, and keep it in a wide-mouthed bottle or earthen pitcher filled with water. Insulin should be kept out of reach of children.
Travel: Surface
While travelling, insulin should be stored in a flask with ice, or in a handbag, or in a proper container if the outside temperature is >30°C. Insulin should never be kept in the glove compartment of a car or left in a locked car with closed windows.
Travel: Air
While travelling by air, one should ensure that:
Physician should be consulted if travelling to a place with a time-zone difference of 2 or more h because it may require a change in insulin injection schedule[23]
Insulin should not be placed in the baggage hold of the plane due to the risk of exposure to extreme temperatures[23]
Extra insulin pen or vial should be carried so that insulin therapy is not interrupted in the event of device breakdown/malfunction
The shelf life of insulin should be adequate for the duration of the trip.
Device selection and use
Prior to injection, the patient must verify the expiry date and the dose, and whether injection with the correct type of insulin is being prepared. Depending on the type of insulin, significant variations in the expiry dates of insulin vials or pens after opening may be reported (A1). Resuspension of cloudy insulin is important to ensure proper absorption of injected insulin and to maintain appropriate concentrations of the remaining insulin in the vial or pen[18] (A2).
Syringe and vial compatibility
Dose
Both U-40 and U-100 insulin concentrations are available in India. While initiating insulin therapy, the patient should be informed that U-100 vials should be used with U-100 insulin syringes and U-40 vials with U-40 insulin syringes only. Insulin syringes of U-100 have an orange cover and black scale markings denoting two units each while U-40 syringes have a red cover and red scale marking denoting one unit each.[24,25]
Needle size
Insulin syringes with three different needle lengths 6, 8, and 12.7 mm are available. Furthermore, three gauge sizes, 31, 30, and 29, are available in insulin syringes. The higher the needle gauge, the thinner the needle. A 31-gauge syringe is available in both 6- and 8-mm needle lengths.
Pens and pen needles
Insulin pens carry insulin in a self-contained cartridge.[26] Different brands and models of insulin pens are available.[27] Insulin pens are of two types: Reusable insulin pens, where cartridge can be reloaded; and disposable insulin pens, which are disposed once emptied.
Different types and brands of insulin are available for the pen. One should consider the number of insulin units the pen can hold when full and the largest dose that can be injected with it. Certain features of the insulin pen such as adjustments of the dose by the pen based on the markings (two-, one- or half-unit increments) and indications on the pen to make sure whether or not there is enough insulin left in it for the entire dose, have additional benefits to the patient. The design and material of the pen, numbering on the pen dose dial and its magnification and the amount of strength and dexterity needed to operate the pen should be checked. Furthermore, one should check whether corrective measures are available if a wrong dose is inadvertently dialed into the pen.[27]
Pen needles
Pen needles are available in 4, 5, 6, and 8 mm sizes and are of 32, 31, and 30 gauge.[28] The shorter needles alleviate the risk of intramuscular injections while avoiding intradermal delivery as well. They are long enough to pass through the skin into the fat layer but are short enough not to reach the muscle tissue.[29]
Needle length
A subcutaneous injection aims to deliver medication directly into the subcutaneous tissue without any discomfort or leakage. Ultrasound measurements reveal a mean skin thickness of about 2.2 mm. Multivariate analyses (of age, body mass index, ethnicity and gender in adults with diabetes) demonstrate that variation in skin thickness is not clinically significant.[7] Hence, there is no medical reason to recommend needles longer than 4–6 mm to either children or adults. Extremely lean patients should be using a skin fold to inject even with a 4 mm and 5 mm needle.
Clinical studies have also reported equal efficacy and safety/tolerability of shorter needles (4 mm) in comparison to longer ones. A randomized trial compared the efficacy and tolerability of 4 mm and longer needles (5 mm and 8 mm) in adult diabetes patients. In addition to providing equivalent glycemic control and alleviating the risk of intramuscular injections, a 4-mm needle resulted in less painful injections and did not increase leakage events compared to longer needles. This study also reported that shorter needles were preferred by patients. Thus, shorter needles may obviate PIR and thereby help improve patient adherence to insulin injection therapy [Tables 1 and 2].[10]
Table 1.
Needle length: Recommendations

Table 2.
Estimated intramuscular injection risk by body site
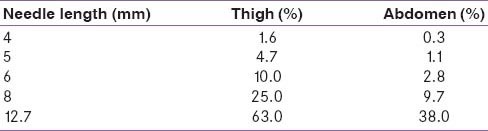
Needle length in children
Shorter needle length (4-mm) is considered safe and efficacious in children. Currently, a 4 mm pen needle is considered safest for all the children. However, when used in children aged 2–6 years, it should be used with a pinched skin-fold.[30]
Injection site
A subcutaneous injection is the most commonly employed route for insulin administration in ambulatory patients. Other routes such as intravenous (I.V.), infusion, or intramuscular routes are employed only during ketoacidosis or stressful conditions.[31]
Anterior abdomen
Abdomen is the most common site for insulin injections.[14] The space around a horizontal line drawn 2.5 cm above and below the umbilicus and lateral to vertical lines drawn 5 cm away from the umbilicus may be utilized for subcutaneous insulin injections.
Upper arm
Over the arm, the injection site includes the upper lateral mid-third of the arm between the shoulder and elbow joint.
Anterior thigh
Over the thigh, the preferred site is in the anterior and outer aspect of the mid-third of the thigh, between the anterior superior iliac spine and knee joint.
Buttock
The upper outer quadrant of the buttock should be used. The upper outer quadrant may be located by placing index finger on the iliac crest and making a right angle between the index finger and the thumb. This site is not used routinely in adults. It can be used in infants and toddlers.
The order of the rates of absorption at these sites is abdomen >arm >thigh >buttock.[32] The presence of a fat layer and only a few nerves in these regions makes injections convenient. Proper care of the injection site should be taken, as poor hygiene can lead to local infections.[32]
Seven-step injection site care process
Prior to injection, the site has to be inspected and palpated for lipohypertrophy and inspected for wounds, bruises or blisters (A3)
If the injection site shows any signs of lipohypertrophy, inflammation, edema or infection, a different site should be selected (A2)
Injection should be given at a clean site with clean hands (A2)
If the injection site is found unclean, it should be cleansed prior to injection (A3)
The injection site has to be inspected at every visit or at least every 6 months, or as part of the investigation into sub-optimal or erratic blood glucose control (A2)
Rotate injection sites systematically (A2)
Ideally do not reuse needles (A2)
Cleansing
Cleanliness of the injection site should be ensured before giving the injections. Cleansing is the single most important procedure for preventing healthcare-associated infections. The injection site may be thoroughly cleansed either with cotton balls dipped in water or with alcohol swabs (A2). Cleansing should be started in the middle and moving outwards in a circular motion, the whole area of the injection site should be properly cleansed. Alcohol on the skin should be completely dry before injection.[14]
Skin-folds
Injections into skin-folds are considered when the presumptive distance from the skin surface to muscle is less than the length of the needle (A3). Lifting a skin-fold at the abdomen and thigh is relatively easier than in the buttocks and is virtually impossible in the arm. Ideally, the thumb and index finger are used to lift a skin-fold properly (possibly with the addition of the middle finger).[14] Use of the whole hand while lifting the skin risks lifting muscle and can lead to intramuscular injections.[33] Use of skin-fold avoids soft-tissue compression and prevents the penetration of drug to a level deeper than intended.
Systematic rotation of injection sites
Systematic rotation of the injection sites is important as it:
Helps maintain healthy injection sites
Optimizes insulin absorption
Reduces the risk of lipohypertrophy.
Organizing a site rotation scheme
Definition
Correct site rotation is defined as administering insulin injections using a new injection site for each injection, in a systematic manner, to avoid repeated local tissue damage, while ensuring stable insulin absorption by:
Rotating between injection sites
Rotating within injection sites, at least 1 cm apart
Changing injection sites.
A common and effective scheme is to divide the injection site into quadrants (abdomen) or halves (thighs, buttocks and arms). One quadrant or half should be used for 1 week and then move either in a clockwise or an anticlockwise fashion to another quadrant or half, next week[34] (A3). Proper and consistent rotation of injection sites safeguards normal subcutaneous tissue.[14]
Injection technique
Syringe and vial
A syringe is the primary injecting device commonly used in India.[7] While injecting insulin, one should confirm that the insulin is at room temperature; it should be taken out of refrigerator 30 min prior to injection. Before use, insulin bottle should always be inspected for expiry date, possible damage to the bottle and possible spoilage of insulin.
For steps of injecting insulin with a syringe, please refer to:
Kalra S, Balhara YS, Baruah MP, Chadha M, Chandalia HB, Chowdhury S, et al. First injection technique recommendations for patients with diabetes, FITs India. Indian J Endocr Metab 2012;16:876-85.
Mixing insulins
For a mixed dose, care should be taken to follow the right sequence of mixing. Regular insulin should be filled first followed by neutral protamine hagedorn (NPH) insulin. Reversal of this order can contaminate the regular insulin vials:
Steps 1–12 would be similar for this too. However, care should be taken while mixing the insulins
One must ensure that the right number of insulin units has been drawn as the insulin cannot be pushed back into the vial.[35]
For steps of injecting insulin with a syringe, please refer to:
Kalra S, Balhara YS, Baruah MP, Chadha M, Chandalia HB, Chowdhury S, et al. First injection technique recommendations for patients with diabetes, FITs India. Indian J Endocr Metab 2012 Nov; 16:876-85.
Mixing insulins recommendations
Although a wide range of premixed insulin preparations is available, preparing split-mix insulin, by mixing rapid- or short- and intermediate- or long-acting insulin, may be necessary at times. Admixture of such combinations is needed in some patients to maintain euglycemia. The formulations and particle size distribution of insulin preparations vary, and on mixing, physicochemical changes may occur in the mixture either immediately or over time. This may result in variations in physiological responses to admixtures of insulin as compared to both component insulins being injected separately.
Human short-acting (regular) insulin can be mixed with intermediate-acting insulin (NPH) in the same syringe in any ratio as desired[36]
When rapid-acting (lispro, aspart, and glulisine) and NPH are mixed, a slight decrease is seen in the absorption rate, but not in the total bioavailability. However, in clinical trials, postprandial blood glucose response was similar with this combination[22]
It is advised not to mix glargine (which has an acidic pH) with any other insulin; the time/action profile of glargine may change and precipitation of the preparation may occur[37] (A2).
Pens
The method of device preparation is the same for both reusable and prefilled disposable pens. In the case premixed insulin is being used, one should ensure that the insulin has been re-suspended by rolling the pen. The edge of the pen should be cleaned with a swab, and a new pen needle screwed on. Insulin pen should be primed with two units of insulin as the first step. It should then be discarded, and the actual dose should be dialed in. The appropriate dose dialed can be seen on the device's display window and can be heard as audible clicks in many pen devices.[38]
The selected injection site should be cleaned with an alcohol swab by starting in the middle of the area and then moving in a circular motion outwards. Stinging may be reduced by ensuring that alcohol on the skin is completely dry before injection.[39] The needle should be inserted in the skin and injection button pushed to deliver the insulin dose. Waiting for a while, by counting till 10, before withdrawing the needle, is advised to deliver the full dose and prevent leakage of the medication (A1). A longer waiting period may be required for higher doses.
The pen needle should then be removed from the pen and disposed safely.[14] It is advised to release the pinch up only after the needle is taken out of the injection site. It is advised to remove and dispose the needle immediately after injection, instead of being left attached to the pen. This will prevent the entry of the air into the cartridge as well as leakage of the medication, which can affect subsequent dose accuracy (A2).
Injection–meal time gap
The efficacy of insulin may be affected by the time gap between injection and meal. Hence, the timing of injection with respect to meal is critical in controlling glycemic levels.[40] Patients should always follow physician's advice.
Short- and rapid-acting insulins
Ideally, human short-acting (regular) insulin should be administered 30 min before meal as it has a delayed onset;[40] whereas rapid-acting insulins (lispro, aspart, and glulisine) can be injected before or immediately after a meal.[41] The efficacy of insulin may be accentuated or reduced by altering the time gap between insulin injection and meal, hence modulating the glucose-lowering effect. Such a strategy should be practiced only under a physician's guidance.
Intermediate- and long-acting insulins
Intermediate-NPH and long-acting insulins (detemir and glargine) should be injected at the same time every day but need not be related to meal times. The ultra-long-acting insulin degludec can be injected at any time, without regard to timing of meals or timing of the previous day's injections.
Glucagon-like peptide-1 receptor agonists
Various glucagon-like peptide-1 receptor agonists are now available, which are administered by different injection techniques. Exenatide is administered twice-daily, 30 min before meals. Liraglutide is injected once daily, without regard to meal timings. Some physicians advise liraglutide at bedtime to reduce gastrointestinal discomfort. Long-acting release exenatide is injected with a 23 gauge needle, once a week, at any time of the day. Dulaglutide and albiglutide are also injected once a week with 29 gauge needle.
Troubleshooting
Pain
Patient adherence to insulin therapy is often affected by the pain following an injection. Pain due to insulin injection is infrequent, unless the needle irritates the nerve endings. Good injection practices can minimize or avoid injection-associated pain[Table 3].[14,42]
Table 3.
Minimizing pain associated with insulins

Local site reaction to subcutaneous insulin injection
Lipodystrophy
Lipohypertrophy: A thick soft to firm swelling with “rubbery” consistency
Lipoatrophy/Lipodystrophy: A scarring lesion with depression
Amyloidosis
Bruising and bleeding.
Lipohypertrophy
Lipohypertrophy, often caused by repeated reuse of needles, manifests as a localized lesion at the repeated injection site[Table 4].
Table 4.
Lipohypertrophy

Chronic reuse of needles and injections at the same site may result in localized lipohypertrophy, or degeneration and atrophy. Patients tend to inject frequently in the lipohypertrophic sites because of reduced pain sensation. Injecting into lipohypertrophic sites may result in significantly unpredictable and delayed absorption which can lead to hyperglycemia and/or hypoglycemia. Further, unnecessarily larger doses may be used in such cases.[14,43] Thus, in patients with uncontrolled diabetes, the sites of insulin injection should be inspected and palpated before making significant changes to the dose or type of insulin.
A study showed that combined intervention of injection technique and injection site assessment by trained HCP followed by general and individualized injection technique education to avoid lipohypertrophy and use correct rotation along with switch to 4 mm needles lead to:
Almost 0.6% (0.58%) mean fall in hemoglobin A1c (Hb1Ac) in 3 months
Mean fall in fasting plasma glucose of 14 mg/dL
Reduced insulin daily dose of 2 units.
These results are highly significant to patients and clinicians as the correct insulin technique can:
Reduce the risk of complications significantly
Support well-being and satisfaction of the patient
Support treat to target Hb1Ac while minimizing need for additional insulin.[12]
Bleeding and bruising
Occasional bleeding or bruising may occur due to reuse of needles. Clinical studies have reported that shorter needles are associated with less frequent bleeding and bruising incidents. Bleeding and bruising appear to have no adverse clinical consequences for the absorption or action of injectable therapies.[14]
Amyloidosis
Amyloidosis is a systemic or local disease in which amyloid substances are deposited extracellularly and impair tissue function.[44] It has been shown that local amyloid deposition very infrequently takes place at the site of repeated insulin injection in patients with insulin-requiring diabetes.[45,46,47]
The nature of amyloid in the insulin injection site is considered to be insulin itself or insulin-related substance and has been identified as amyloid insulin type (A Ins).[46,47,48,49,50]
Blood glucose control improves markedly shortly after resection of the tumor or after the change of the injection site. The presence of the amyloid mass itself, perhaps due to poor penetration of insulin, may contribute to insulin resistance.[51]
Trypanophobia (Belonephobia)
The fear of self-injecting insulin compromises glycemic control and emotional well-being. Similarly, the fear of pricking can be a source of distress and may seriously hamper self-management. Needle phobia may be associated with factors like a perceived loss of control over life, a lack of confidence that the demands of insulin therapy will be handled, a belief that insulin therapy equates to a personal failure and injection-related anxiety.[11,14]
Needle-stick injuries
Needle-stick injuries commonly occur while recapping needles. Safety needles effectively protect health professionals against contaminated needle-stick injuries. Education and training are needed to ensure that safety practices are followed.[14]
Injection through clothing
Injection through clothing is a common practice among patients, especially when in a hurry, or in a public place. This practice should, however, be firmly discouraged.
Needle/syringe hygiene
The United States Food and Drug Administration recommends injection needles for a single use only. Syringes should ideally be used only once. On the contrary, in India, patients often reuse syringes and needles for economic reasons. It is advised to counsel such patients about the potential hazards of reuse while explaining the technique of single-handed scoop recapping the needle cover aseptically after each use (although against National AIDS Control Organization [NACO] recommendations).[52] The importance of not sharing syringes between individuals should also be emphasized. HCPs should adopt a pragmatic approach on this issue.
Following aspects of reusing the needles should be discussed with patients:
When reusing, the thin tip of the needles gets damaged, needles bend, and the silicone lubricant coating of the needles is also lost. All these contribute to a more painful injection, with bleeding and bruising. Repeat usage can also result in breaking off and lodging of the needles under the skin. Furthermore, there is a higher chance for insulin to get deposited within the needle with reuse, making it harder to press on the plunger and deliver proper insulin doses
Reuse of needles increases the risk of contamination and infection. In an attempt to be hygienic, some patients clean the needle with alcohol prior to reusing it. This practice removes the silicone lubricant and results in a more painful injection. This practice should be discouraged
Repeated use of insulin needles can also result in damage to the tissues and an increased risk of lipohypertrophy[45]
Insulin pen delivery systems, when used properly, are extremely accurate. Improper use of pens with needles left attached after use bears more chance for the air to pass into the insulin chamber and an increased risk of contamination. Furthermore, there is a higher chance for dosage inaccuracy due to the air bubble formation. Hence, manufacturers recommend removing insulin pen needles immediately after use.
Periodic clinical audits
A periodic audit of injection practices in diabetes patients by their clinicians is highly recommended. Mutual audits can be performed in pairs by members of diabetes clubs or patient organizations.
Injection device disposal
According to the recommendations developed by NACO, it is recommended to collect the used needles or syringes in a puncture-proof box or safety box, labeled as “biohazard.” When filled, these boxes should be handed over to appropriate centers such as waste management agencies, medical colleges, or hospitals, wherein disinfection and disposal of sharps are carried out.[53] Needle clipping devices that remove insulin syringe needle and pen needles safely and easily, should be used.[54]
Many people in India do not have access to sharps disposal devices. An easy and practical method is to collect used sharps, including needles and lancets, in a strong cardboard/glass container, label and seal it, when full, and dispose it off at the nearest healthcare facility. Public facilities should be encouraged to provide sharps disposal devices in restrooms and retiring areas.
Missing injections
Insulin injections may be missed by patients either by design or because of unavoidable circumstances. All patients should be counseled about the negative effects of missing injections (A2). If a patient is admitted in the hospital and there is uncertainty about his current insulin regimen, human insulin should be administered until further information is available. This should always be done under medical supervision.[22]
Special populations
Pregnancy
Insulin is required in about 10–20% of all antenatal women with diabetes, which complicates about one-sixth of all pregnancies. Patients should be reassured that insulin is not only safe in pregnancy, but also contributes to maternal and fetal well-being. The abdomen is a safe site for insulin administration in pregnancy.
First trimester
Women should be reassured that no change in insulin site or technique is needed.
Second trimester
Lateral parts of the abdomen can be used to inject insulin, staying away from the skin overlying the fetus.
Third trimester
Insulin can be injected over the abdomen while ensuring the skin fold is properly raised. Apprehensive patients may use the thigh or upper arm to inject themselves.
Dermatological disease
Insulin injection should be avoided at sites of active or recently healed infection or inflammations, such as skin and soft-tissue infections and psoriasis. Injection should not be administered into keloids or scars. However, stable vitiligo is not a contraindication for insulin injection. Acanthosis nigricans is also not a contraindication.
Surgical diseases
In patients with recent surgical wounds or open fistulas/ileotommies/colostomies, a different quadrant of the abdomen, should be used for insulin injection. Adequate preinjection cleansing must be done. Apprehensive patients with recent abdominal surgery may use thigh or upper arm for injection.
Elderly
It is recommended that elderly patients should be assisted by a caregiver and the importance of injection therapy, as well as prevention and treatment of hypoglycemia should be emphasized.
Sensory motor impairment: Visual, tactile and lack of manual dexterity
In visually impaired patients, nonvisual insulin measurement devices, syringe magnifiers, needle gauges, and vial stabilizers help ensure accuracy and aid in insulin delivery. In patients with both visual and dexterity impairment, prefilled syringes may be helpful (A2). Pens, which require low pressure for plunging, should be preferred.
In patients with low hearing and those who use hearing aids, therapy-related discussions should be conducted in a noise-free environment (A2). The instructor should face the person with sufficient light falling on his/her face which facilitates lip-reading. In addition, speaking slowly and clearly with normal intonation will also be a benefit. In people with dexterity problems, use of injection devices with preset doses and easy featuring devices may be beneficial.
Immunocompromised individuals
Hypoglycemia is a major concern in some immunocompromised patients, including those with HIV and hepatitis.[55] Hence, early initiation of insulin therapy should be considered in immunocompromised patients as it improves therapeutic outcomes.[55] Personnel giving injections and those handling the sharp material are at high risk of exposure and transmission of blood-borne pathogens (HIV and hepatitis) through injections and finger sticks administered to affected patients.[56] Therefore, needles, syringes or lancets should never be reused (A2).
Insulin pump infusion
The insulin pump is a pager-sized device, which provides continuous subcutaneous delivery of rapid-acting insulin with the help of an infusion set. One end of the infusion set connects to the reservoir, which is filled with insulin and is kept inside the insulin pump, and the other end of the infusion set is connected to the needle placed subcutaneously.[57]
In India, unlike in the Western world, pumps are more commonly used in type 2 diabetes. As an alternative delivery system, insulin pumps are a replacement for syringes/pens, provided the subject fulfills the essential criteria for a pump candidature. Since there are no reimbursement policies for pump users in India, most of the subjects have innovated and improvised their own ways and means of reducing the cost by prolonging the use of infusion sets and reusing reservoirs.
Such peculiar circumstances have created an urgent need for formulating insulin pump infusion recommendations. These need to be customized for the Indian subcontinent, considering education, ethnicity, dressing styles, resources, and weather, while at the same time ensuring clinical efficacy and avoidance of infections.
Infusion site
The preferred infusion site for pumps is the abdomen, while the upper arm and thighs are alternate sites. Select a site in the abdomen 5 cm away from the umbilicus and every new site should be 2.5 cm away from the previous site. Following a systematic pattern will help ensure that the longest possible time will pass before using the same site again. In pregnancy, the outer thigh or hip may be used.
Cannula selection
Plastic cannulae are preferred by almost all doctors recommending an insulin pump. Steel needle infusion sets are recommended in pregnancy, for patients who have reactions to plastic cannulae and who have frequent kinks in plastic cannulae. Short length cannulae (6 mm for 90° sets, 13 mm for 30–45° angled infusion sets) are most preferred. Shorter tubing is recommended for most patients while longer tubing is preferred by patients who feel comfortable keeping the pump in the socks or underneath the pillow while sleeping.
Angle of insertion
Insertion angle 90° is widely used by pump users. 30–45° angles are preferred by dexterous lean or muscular patients and pregnant women.
Selection of infusion sets
The most popular infusion set is a 90°, soft cannula infusion set. Variable angle, soft cannula infusion sets are also available for patients who are lean or lead an active lifestyle. Steel needle infusion sets and 90°, soft cannula infusion sets that combine the infusion set and insertion device into one unit are also available.
Troubleshooting
Adhesive tape allergy
This is extremely rare in Indian scenario, and the use of oral antihistaminics for a few days usually suffices.
Infusion site infection prophylaxis
The infusion site must be clean and dry before insertion of the cannula. Washing the site with an anti-bacterial soap or solution is sufficient. Changing the infusion set once in every 2–3 days is recommended. In India, most of the pump users retain the same infusion set for 5–7 days due to the high cost of consumables.[58] With this practice, the potency of insulin will be lost; there are chances of the site getting infected as well. Customized advice and recommendations are to be made based on affordability, work pattern, and level of education.
Lipohypertrophy
Lipohypertrophy has been described in the earlier part of the recommendations.
Loss of insulin potency
If patients use the same reservoir and insulin for more than the recommended 3 days, the potency of insulin may be compromised. If patients fill the reservoir with fresh insulin without changing the infusion set, adequate instructions have to be given by the diabetes care team. For subjects living in extremely hot environments, pump may not be a suitable option.
Pump occlusion
The newer cannula rarely kink and block. If the occlusion persists, the cannula and infusion set have to be changed.[59,60]
Disaster management
Avoiding or missing insulin therapy in persons with type 1 diabetes can be life-threatening. Hence, disaster planning is essential and should include the precautionary steps to be taken if a disaster strikes. A portable, insulated, and waterproof diabetes disaster kit should be kept handy. The kit should have a supply of insulin syringes for at least 30 days and insulin vials or pens and needles along with cold packs. In addition, it should also contain blood-testing supplies including lancets, test strips, and a glucose meter (preferably two) with extra batteries. A separate sharps container for the disposal of lancets and needles should also be included.
At least a 3-day supply of nonperishable food and bottled water is also recommended.[11]
Barriers to insulin therapy
Patient-related barriers
Several myths, misunderstandings, and negative approaches act as barriers in the use of insulin among type 2 diabetes patients.[9]
Clinicians may help patients discuss their concerns by asking open-ended and nonjudgmental questions: Effective solutions can thus be found and implemented
Encouraging shared decision-making with active participation of the patient is important
Giving patients a sense of control over their treatment plan improves acceptance and enhances adherence.
Physician-related barriers
Physicians are often concerned about the patient-related barriers in early initiation of insulin therapy
Physician-related barriers include concerns about adherence and the patient's perceived adverse effects of hypoglycemia and weight gain, lack of supporting staff and counseling/motivational skills, and the desire to prolong noninsulin therapy
Physicians have a misconception that insulin therapy is expensive; however, insulin therapy actually reduces costs by decreasing complication rates and management burden.
Health care system-related barriers
Lack of resources also acts as an important barrier in insulin therapy. A financial barrier exists for patients who lack insurance
For HCPs, lack of trained diabetes educators is an important issue
-
This can be linked to the lack of resources, ability,
and/or facilities for training diabetes educators
Use of reusable insulin pens; providing training to HCPs; and hiring a diabetic educator and setting up an educational program may be helpful in increasing the availability and lowering the cost of insulin therapy.[54]
Improving adherence
Counseling forms an integral part of diabetes management. Counseling about drug-, patient-, and physician-related factors can improve adherence to injection therapy. These factors are listed in Box 6.21.
Encouraging patients to ask questions and clarify doubts is important. Arranging periodic refresher sessions with patients is helpful in addressing any new issues that arise during the course of therapy.[61] The message should be personalized, and information relevant to the patient's perspective should be provided.[62]
The WATER approach explained below has been suggested to fulfill the purpose[Table 4].
The patient must be Welcomed Warmly in the clinic, from the outpatient counter onwards
The clinicians should Ask and Assess carefully making use of various cues and sequencing the questions appropriately
They should tell truthfully making use of metaphors analogies, keeping in mind both verbal, as well as nonverbal cues from the patient
They should explain with empathy, making use of experience sharing, practical demonstration, and imparting coping skills training
Finally, the clinicians must reassure the patient and tell him/her to Return for any clarifications[63]
Optimization of insulin-related and device-related factors can help improve adherence to insulin therapy.
CONCLUSION
The FIT India recommendations 2015 have been designed to address the needs of people with diabetes and HCPs in India. Their relevance, however, is glocal that is, global as well as local. These recommendations provide a reader-friendly, yet comprehensive, source of information for all stakeholders connected with injectable therapy for diabetes. It is hoped that the FIT India recommendations will contribute to improved glycemic control in people with diabetes, in India, and across the world.
DUALITY OF INTEREST
The authors are members of FIT India advisory board, who have helped develop the Indian Insulin Injection Technique Recommendations 2015. FIT India is supported by Becton Dickinson India Private Limited (BD), a manufacturer of injecting devices. Members of the FIT advisory board have not received any honorarium from BD for their contribution to the recommendations. The Indian insulin injection technique recommendations 2.0 developed by FIT is a copyright of BD and shall be considered proprietary to BD India Private Limited, therefore, limited to be disclosed or published solely by BD India Pvt Ltd. FIT India is constituted to provide evidence-based information on best practices on injection technique, to all those using injectable therapies for diabetes care, in order to achieve best possible health outcomes, ensuring that the right dose is delivered at the right injection site, using the right technique, each time.
ACKNOWLEDGMENT
The authors acknowledge the significant contribution made by members of the South Asian Referee Group and the Indian Review Panel. An abridged version of the “FIT First Indian Insulin Injection Technique Recommendations” was published in Indian Journal of Endocrinology and Metabolism, November 2012 issue. Addenda have also been published in November 2013 and November 2014 issues of the journal.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 2.The IDF Atlas. 2014. Dec, [Last accessed on 2014 Dec 15]. Available from: http://www.idf.org/sites/default/files/DA-regional-factsheets-2014_FINAL.pdf .
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Crasto W, Jarvis J, Khunti K, Davies MJ. New insulins and new insulin regimens: A review of their role in improving glycaemic control in patients with diabetes. Postgrad Med J. 2009;85:257–67. doi: 10.1136/pgmj.2008.067926. [DOI] [PubMed] [Google Scholar]
- 5.Prasad R. Innovate for diabetes in India. [Last accessed on 2014 Dec 15]. Available from: http://www.blogs. novonordisk.com/graduates/2010/10/27/innovate-for-diabetes-in-india/
- 6.Funnell MM. Lessons from DAWN: Implementing effective insulin therapy. Int J Adv Nurs Pract. 2009;10:2. [Google Scholar]
- 7.Strauss K, De Gols H, Hannet I, Partanen TM, Frid A. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diabetes Int. 2002;19:71–6. [Google Scholar]
- 8.New UK recommendations for best practice in diabetes injection technique. [Last accessed on 2014 Dec 15]. Available from: http://www.primarycaretoday.co.uk/training/?pid=4216 and lsid=4268 and edname=29301.htm and ped=29301 .
- 9.Kamath SS. IAP workshop on safe injection practices: Recommendations and IAP plan of action. Indian Pediatr. 2005;42:155–61. [PubMed] [Google Scholar]
- 10.Injectable drug delivery 2011: Device focus. [Last accessed on 2014 Dec 15]. Available from: http://www.ondrugdelivery.com/publications/Injectable%20Devices%20 2011/Injection%20Devices%20June%202011%20Med%20Res.pdf .
- 11.Dolinar R. The importance of good insulin injection practices in diabetes management. US Endocrinol. 2009;5:49–52. [Google Scholar]
- 12.Grassi G, Scuntero P, Trepiccioni R, Marubbi F, Strayss K. Optimizing insulin injection technique and its effect on blood glucose control. Journal of Clinical Translational Endocrinology. 2014;148:145–50. doi: 10.1016/j.jcte.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson JA. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S2. doi: 10.1016/S1262-3636(10)70001-X. [DOI] [PubMed] [Google Scholar]
- 14.Frid A, Hirsch L, Gaspar R, Hicks D, Kreugel G, Liersch J, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S3–18. doi: 10.1016/S1262-3636(10)70002-1. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki K, Goto M, Yamamoto T, Tsujii S, Ishii H. Barriers and facilitators in relation to starting insulin therapy in type 2 diabetes. Diabetes. 1999;48(Suppl 1):A319. Abstract. [Google Scholar]
- 16.Hendra TJ. Starting insulin therapy in elderly patients. J R Soc Med. 2002;95:453–5. doi: 10.1258/jrsm.95.9.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korytkowski M, Bell D, Jacobsen C, Suwannasari R. Flex Pen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–48. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 18.Bohannon NJ, Jack DB. Type II diabetes: Tips for managing your older patients. Geriatrics. 1996;51:28–35. [PubMed] [Google Scholar]
- 19.Baruah MP. Insulin pens: The modern delivery devices. J Assoc Physicians India. 2011;59(Suppl):38–40. [PubMed] [Google Scholar]
- 20.Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28:173–7. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 21.Baruah MP, Kalra S, Unnikrishnan AG, Raza SA, Somasundaram N, John M, et al. Management of hyperglycemia in geriatric patients with diabetes mellitus: South Asian consensus guidelines. Indian J Endocrinol Metab. 2011;15:75–90. doi: 10.4103/2230-8210.81935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Insulin administration. Diabetes Care. 2004;27(Suppl 1):S106–9. doi: 10.2337/diacare.27.2007.s106. [DOI] [PubMed] [Google Scholar]
- 23.Travel and diabetes. [Last accessed on 2014 Dec 15]. Available from: http://www.sweet.org.au/docs/professionals/14_Travel_and_Diabetes.pdf .
- 24.Grassi G, Scuntero P, Trepiccioni R, Marubbi F, Strauss K. Insulin syringes. Optimizing insulin injection technique and its effect on blood glucose control. [Last accessed on 2014 Dec 15];J Clin Trans Endocrinol. 2014 148:145–50. doi: 10.1016/j.jcte.2014.07.006. Available from: http://www.bd.com/us/diabetes/page. aspx?cat=7001 & id=7251 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gitanjali B. A tale of too many strengths: Can we minimize prescribing errors and dispensing errors with so many formulations in the market? J Pharmacol Pharmacother. 2011;2:147–9. doi: 10.4103/0976-500X.83277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holleman F, Vermeijden JW, Kuck EM, Hoekstra JB, Erkelens DW. Compatibility of insulin pens and cartridges. Lancet. 1997;350:1601–2. doi: 10.1016/S0140-6736(05)64018-4. [DOI] [PubMed] [Google Scholar]
- 27.Insulin pens. [Last accessed on 2014 Dec 15]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 and id=7254 .
- 28.Syringe and pen needle sizes. [Last accessed on 2014 Dec 15]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 & id=7253 .
- 29.Skin thickness and needle size. [Last accessed on 2014 Dec 15]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 & id=32371 .
- 30.Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: Implications for needle length recommendations. Curr Med Res Opin. 2010;26:1519–30. doi: 10.1185/03007995.2010.481203. [DOI] [PubMed] [Google Scholar]
- 31.Lo Presti D, Ingegnosi C, Strauss K. Skin and subcutaneous thickness at injecting sites in children with diabetes: Ultrasound findings and recommendations for giving injection. Pediatr Diabetes. 2012;13:525–33. doi: 10.1111/j.1399-5448.2012.00865.x. [DOI] [PubMed] [Google Scholar]
- 32.Yadav S, Parakh A. Insulin therapy. Indian Pediatr. 2006;43:863–72. [PubMed] [Google Scholar]
- 33.Injection site selection. [Last accessed on 2014 Dec 15]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 and id=7261 .
- 34.Down S, Kirkland F. Injection technique in insulin therapy. Nurs Times. 2012;108(20):18–1. [PubMed] [Google Scholar]
- 35.Basi M, Hicks D, Kirkland F, Pledger J, Burmiston S. Improving diabetes injection technique. Clin Serv J. 2010 [Google Scholar]
- 36.Das AK. Rapid acting analogues in diabetes mellitus management. J Assoc Physician India. 2009. [Last accessed on 2014 Dec 15]. Available from: http://www.japi.org/february_2009/rapid_acting_analogue.html .
- 37.Deckert T. Intermediate-acting insulin preparations: NPH and lente. Diabetes Care. 1980;3:623–6. doi: 10.2337/diacare.3.5.623. [DOI] [PubMed] [Google Scholar]
- 38.How to inject an insulin syringe. [Last accessed on 2014 Dec 15]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 & id=7258 .
- 39.Bohannon NJ. Insulin delivery using pen devices. Simple-to-use tools may help young and old alike. Postgrad Med. 1999;106:57–8. doi: 10.3810/pgm.1999.10.15.751. 61-4, 68. [DOI] [PubMed] [Google Scholar]
- 40.Czupryniak L, Drzewoski J. Insulin injection mean time interval it is NOT its length that matters. Pract Diabetes Int. 2001;18:338. [Google Scholar]
- 41.Fowler MJ. Diabetes treatment, Part 3: Insulin and incretins. Clin Diabetes. 2008;26:35–9. [Google Scholar]
- 42.Lantus Product Monograph. [Last accessed on 2014 Dec 15]. Available from: http://www.products.sanofi.ca/en/lantus.pdf .
- 43.Workman B. Safe injection techniques. Nurs Stand. 1999;13:47–53. doi: 10.7748/ns1999.06.13.39.47.c2623. [DOI] [PubMed] [Google Scholar]
- 44.Kalra S, Balhara YP, Baruah MP, Chadha M, Chandalia HB, Chowdhury S, et al. Addendum 2: Forum for injection technique, India. Indian J Endocrinol Metab. 2014;18:800–3. doi: 10.4103/2230-8210.152762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–53. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Dische FE, Wernstedt C, Westermark GT, Westermark P, Pepys MB, Rennie JA, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158–61. doi: 10.1007/BF00276849. [DOI] [PubMed] [Google Scholar]
- 47.Swift B. Examination of insulin injection sites: An unexpected finding of localized amyloidosis. Diabet Med. 2002;19:881–2. doi: 10.1046/j.1464-5491.2002.07581.x. [DOI] [PubMed] [Google Scholar]
- 48.Albert SG, Obadiah J, Parseghian SA, Yadira Hurley M, Mooradian AD. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374–6. doi: 10.1016/j.diabres.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Westermark P, Araki S, Benson MD, Cohen AS, Frangione B, Masters CL, et al. Nomenclature of amyloid fibril proteins. Report from the Meeting of the International Nomenclature Committee on Amyloidosis, August 8.9, 1998. Part 1. Amyloid. 1999;6:63–6. doi: 10.3109/13506129908993290. [DOI] [PubMed] [Google Scholar]
- 50.Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, et al. Amyloid fibril protein nomenclature – 2002. Amyloid. 2002;9:197–200. doi: 10.3109/13506120209114823. [DOI] [PubMed] [Google Scholar]
- 51.Shikama Y, Kitazawa J, Yagihashi N, Uehara O, Murata Y, Yajima N, et al. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49:397–401. doi: 10.2169/internalmedicine.49.2633. [DOI] [PubMed] [Google Scholar]
- 52.Recommendations on Safe Disposal of Used Needles and Syringes in the Context of Targeted Intervention for Injecting Drug Users. [Last accessed on 2014 Dec 15]. Available from: http://www.nacoonline.org/upload/NGO%20 & %20Targeted/waste%20disposal%20recommendations%20for%20IDU%20TI.pdf .
- 53.Misnikova IV, Dreval AV, Gubkina VA, Rusanova EV. The risks of repeated use of insulin pen needles in patients with diabetes mellitus. J Diabetol. 2011;1:1–5. [Google Scholar]
- 54.WISE consensus group. WISE recommendations to ensure the safety of injections in diabetes. [Last accessed on 2014 Dec 15]. Available from: http://www.em-consulte.com/showarticlefile/689682/main.pdf . [DOI] [PubMed]
- 55.Palios J, Kadoglou NP, Lampropoulos S. The pathophysiology of HIV-/HAART-related metabolic syndrome leading to cardiovascular disorders: The emerging role of adipokines. Exp Diabetes Res 2012. 2012 doi: 10.1155/2012/103063. 103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalra S, Unnikrishnan AG, Raza SA, Bantwal G, Baruah MP, Latt TS, et al. South Asian consensus recommendations for the rational management of diabetes in human immunodeficiency virus/acquired immunodeficiency syndrome. Indian J Endocrinol Metab. 2011;15:242–50. doi: 10.4103/2230-8210.85573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalra S, Balhara YS, Baruah MP, Chadha M, Chandalia HB, Chowdhury S, et al. Addendum: First injection technique recommendations for patients with diabetes, forum for injection techniques India. Indian J Endocrinol Metab. 2013;17:1005–8. [Google Scholar]
- 58.Kesavadev J, Das AK, Unnikrishnan R, 1st, Joshi SR, Ramachandran A, Shamsudeen J, et al. Use of insulin pumps in India: Suggested guidelines based on experience and cultural differences. Diabetes Technol Ther. 2010;12:823–31. doi: 10.1089/dia.2010.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bode BW. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart, lispro, and glulisine. Endocr Pract. 2011;17:271–80. doi: 10.4158/EP10260.RA. [DOI] [PubMed] [Google Scholar]
- 60.Ponder SW, Skyler JS, Kruger DF, Matheson D, Brown BW. Unexplained hyperglycemia in continuous subcutaneous insulin infusion: Evaluation and treatment. Diabetes Educ. 2008;34:327–33. doi: 10.1177/0145721708315682. [DOI] [PubMed] [Google Scholar]
- 61.Beran D. Improving access to insulin: What can be done? Diabetes Manage. 2011;1:67–76. [Google Scholar]
- 62.Kalra S, Kalra B, Batra P. Patient motivation for insulin/injectable therapy: The Karnal model. Int J Clin Cases Invest. 2010;1:11–5. [Google Scholar]
- 63.Kalra S, Kalra B. A good diabetes counselor ‘Cares’: Soft skills in diabetes counseling. Int J Health. 2010;11:1–3. [Google Scholar]


