Abstract
Background:
High dose oral prednisolone (100 mg/day) in Graves’ orbitopathy (GO) is limited by lesser response, and greater side-effects compared to intravenous (iv) methylprednisolone. Low dose oral prednisolone has not been evaluated in GO. This study aimed to evaluate the safety and efficacy of low dose oral prednisolone in GO.
Materials and Methods:
A total of 114 consecutive GO patients were screened of which 65 patients with previously untreated moderate-severe GO, clinical activity score (CAS) >2, without co-morbid states were randomized into treatment Group-A (iv methylprednisolone 0.5 g for 3 days/month for 4 months) and Group-B (oral prednisolone 1 mg/kg/day for 6 weeks then tapered stopped), and followed-up. Thirty-one patients in each group with at least 1-year follow-up were analyzed. Responders were defined as improvement in ≥ 1 major response criteria or ≥ 2 minor response criteria. The trial is registered at ctri.nic.in (CTRI/2013/12/004264).
Results:
At 1-year, 27/31 (87.10%) patients were responders in Group-A compared to 17/31 (54.84%) in Group-B (P = 0.005). There was a greater improvement in CAS score in patients of Group-A as compared to Group-B (P < 0.001). Responders (n = 44) had significantly higher baseline intra-ocular pressures and left eye proptosis as compared to nonresponders. Cox-regression revealed baseline T4 levels, diplopia, and smoking history were predictive of remission. Low dose prednisolone was well tolerated, and the occurrence of adverse events were comparable in both groups.
Conclusions:
Low dose oral prednisolone is inferior to iv pulse methylprednisolone in managing GO, having a comparable side-effect profile. It can be a safe second line alternative in patients intolerant to pulse iv methylprednisolone.
Keywords: Graves’ orbitopathy, methylprednisolone, prednisolone
INTRODUCTION
Graves’ orbitopathy (GO) is an autoimmune disorder and the most common extra-thyroid manifestation of Graves’ disease with a prevalence of 2.9 cases per 100,000 population per year in men and 16.0 cases per 100,000 population per year in women, with a peak in the fifth and seventh decades.[1,2,3] Intravenous (iv)/oral steroids form the first line and mainstay of therapy in patients with moderately severe GO.[1,2,3,4,5,6,7,8,9]
It has been suggested that iv methylprednisolone is better cause of earlier onset of response, a higher response, along with lower side-effects compared to oral prednisolone.[9,10] Monthly iv methyl-prednisolone was found to be marginally better than oral prednisolone (88% vs. 63%; P ~ 0.02) in a study.[10] On patient self-assessment, there was no significant difference in the response between iv versus oral steroids (P = 0.27).[10] The dose of oral steroid used was high (starting with 100 mg/day).[10,11] It is a well-known that higher oral steroid doses are associated with more side-effects.[9,10,11] Use of iv methyl-prednisolone is limited by significant risk of fulminant hepatic failure with cumulative doses >8 g and lack of standardization of optimal regimen.[10,11,12]
Indians in general have lower body weight than Caucasians. Hence, there may be rationale for using lowers doses of oral prednisolone for managing GO in India. However, low dose oral prednisolone has not been evaluated in the management of GO. The lower dose may be expected to minimize the side-effects and allow for better recovery of the suppressed hypothalamic-pituitary adrenal axis. Hence, the aim of this study was to evaluate the safety and efficacy of low dose oral prednisolone in GO by comparing it with the current treatment standard, pulse iv methylprednisolone, in a randomized controlled trial (RCT).
MATERIALS AND METHODS
Consecutive patients of Graves’ disease with eye symptoms attending endocrine outpatient services of the department underwent GO severity and activity assessment. Proptosis was measured using Hertel's exopthalmometer. Lid aperture was measured with a ruler as the distance in the midline between the eyelids. Pinhole visual acuity was tested with a Snellen chart and expressed as a decimal. Assessment of field of vision was done using automated perimetry. Assessment of pupillary response and color vision was done. Indirect fundoscopy was done to check the status of fundus. In patients with suspicion of dysthyroid optic neuropathy (DON), visual evoked potentials were checked to rule out optic nerve involvement. Corneal staining was assessed with biomicroscope. Disease activity was assessed using clinical activity score (CAS).[13]
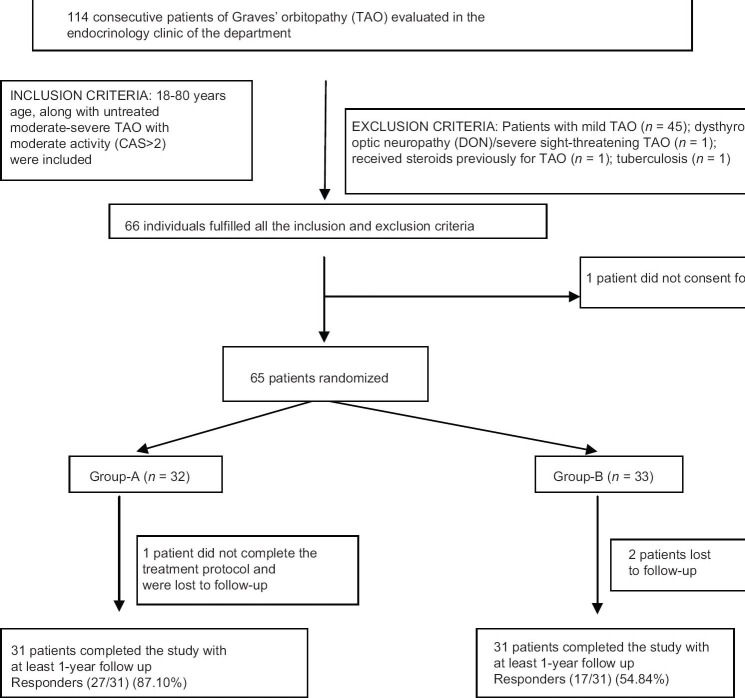
Moderate to severe GO was defined as marked soft tissue swelling (2c by NOSPECS), and/or proptosis (≥18 mm in females; ≥20 mm in males; 3a or more by NOSPECS), and/or inconstant/constant diplopia in primary or reading position (4b by NOSPECS), and/or punctuate staining of cornea, without any optic nerve involvement, as suggested by European group on GO [Table 1].[10,12] Patients with mild GO, DON and severe sight-threatening GO were excluded.[10,12] The inclusion and exclusion criteria have been elaborated in Table 2. Patients, 18–80 years age, along with untreated moderate-severe GO with moderate activity (CAS > 2) were included. Patients were excluded who had received steroids, radiotherapy, immunosuppressive therapy or surgery for GO. All patients underwent chest X-ray to rule out occult tuberculosis [Figure 1]. Patients with comorbidities making glucocorticoid administration difficult (uncontrolled hypertension, uncontrolled diabetes, tuberculosis, immune-compromised state, chronic liver disease, viral hepatitis) were excluded [Figure 1]. The study protocol was explained and only those who gave informed written consent were included. The trial is registered with clinical trials registry of India at ctri.nic.in (CTRI/2013/12/004264).
Table 1.
NOSPECS classification adapted from EUGOGO
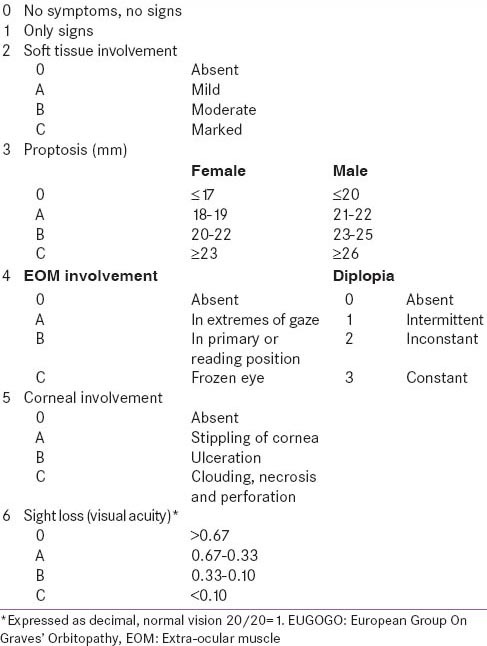
Table 2.
Inclusion and exclusion criteria adapted from EUGOGO
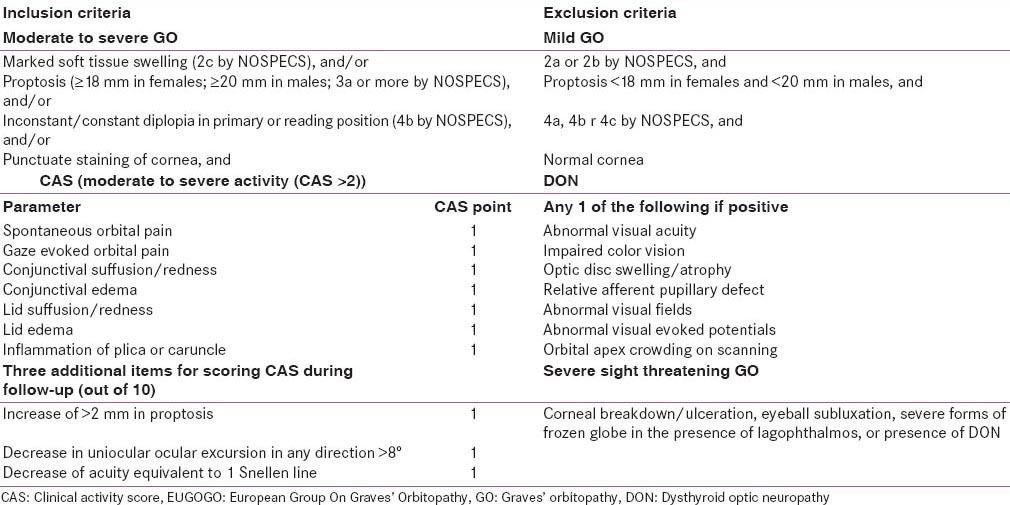
Figure 1.
Flowchart elaborating the study protocol. CAS: Clinical activity score, Group-A: Patients randomized to receive pulse intravenous methylprednisolone, Group-B: Patients randomized to receive oral prednisolone
The included patients attended outpatient department (OPD) services after 12 h fast, underwent clinical and anthropometric assessment. Blood samples were collected, serum separated and stored at −80°C. Biochemical investigations included estimation of fasting blood glucose (FBG), 2 h post 75 g glucose blood glucose (2hPGBG), liver function test, lipids, electrolytes, and renal function. Computerized tomography (CT) scan of orbits was done at baseline for assessment of extra-ocular muscle (EOM) thickness. The thickest section of the rectus muscle was measured perpendicular to the muscle axis in millimeters. Hyperthyroid or hypothyroid GO patients were made clinically and biochemically euthyroid for at least 3 months before randomization.
The patients were divided into 2 groups by a computer generated randomization table; Group-A: Patients receiving monthly iv methylprednisolone (0.5 g for 3 consecutive days in a month for 4 months; Solumedrol, Pharmacia and Upjohn, Gurgaon, India) and Group-B: Patients receiving oral prednisolone (starting with 1 mg/kg/day for 6 weeks then tapered gradually and stopped; Wysolone, Wyeth, Mumbai India). All patients received cholecalciferol sachet 60,000 U (DRISE, USV, Mumbai, India) initially weekly for 8 weeks and thereafter monthly, and 1250 mg of calcium carbonate equivalent to elemental calcium 500 mg (Shelcal-500, Elder, Mumbai, India) daily, for the duration of the study. All patients received injection zolindronic acid 5 mg (Natzold, Natco, India) infusion before the first dose of glucocorticoid to prevent glucocorticoid-induced osteoporosis.[14]
Treatment outcomes were assessed in terms of major response criteria (MRC) and minor response criteria (MiRC) adapted from previous studies.[11] MRC include decrease in CAS by ≥3 points or improvement in diplopia. MiRC includes decrease in lid retraction by ≥2 mm, decrease in proptosis by >2 mm, decrease in CAS by ≥2 points or improvement in grade of soft tissue swelling.[11] Responders (R) were defined as improvement in ≥1 major criteria or ≥2 minor criteria. Treatment failure (TF) was defined as presence of any one of the following even if the patient fulfilled the criteria for successful therapy: Worsening of one grade or more soft tissue involvement and/or diplopia, and/or an increase of lid aperture of ≥2 mm, and/or ≥2 mm increase of proptosis.[10,11] Patients who did not fulfill the response to therapy criteria, but without any features suggestive of TF were classified as nonresponders (NR). The eye with the worse features was used for assessment of response. The duration of the study was from May 2010 to April 2014. Institutional Ethics Committee approved the study protocol [Figure 1].
Follow-up
The patients received iv methylprednisolone on day care basis. Oral prednisolone was administered on an OPD basis. All patients were followed-up at least once monthly, when patients on oral prednisolone were enquired about drug compliance (checking of empty packs which were collected) and a fresh set of tablets issued. Patients were contacted telephonically monthly and by messaging services weekly to ensure compliance and enquired about problems. The patients were admitted only if there was some complication or illness necessitating admission. Detailed ophthalmologic evaluation for assessment of response to therapy was done at 1, 3, 6, 9 and 12 months after initiation of therapy. Treatment outcomes (responder, NR or TF) were assessed at 6-month and 12 months of follow-up. FBG, 2hPGBG, liver function test, lipids, electrolytes, and renal function were repeated during the above-mentioned follow-up visits.
Statistical analysis
All results were expressed as mean ± standard deviation. Independent sample t-test was used for calculation of P value. Chi-square test was used for categorical variables. P < 0.05 considered statistically significant. Data were analyzed according to the intention to treat principle. SPSS version 16 (Chicago, IL, USA) was used for data analysis. About 77% with moderate, severe GO responded to iv pulse methylprednisolone in contrast to 51% of patients receiving oral prednisolone.[10] Hence, it was calculated at least 53 patient need to be randomized into 2 groups, for alpha of 0.05 and power of 80%.
RESULTS
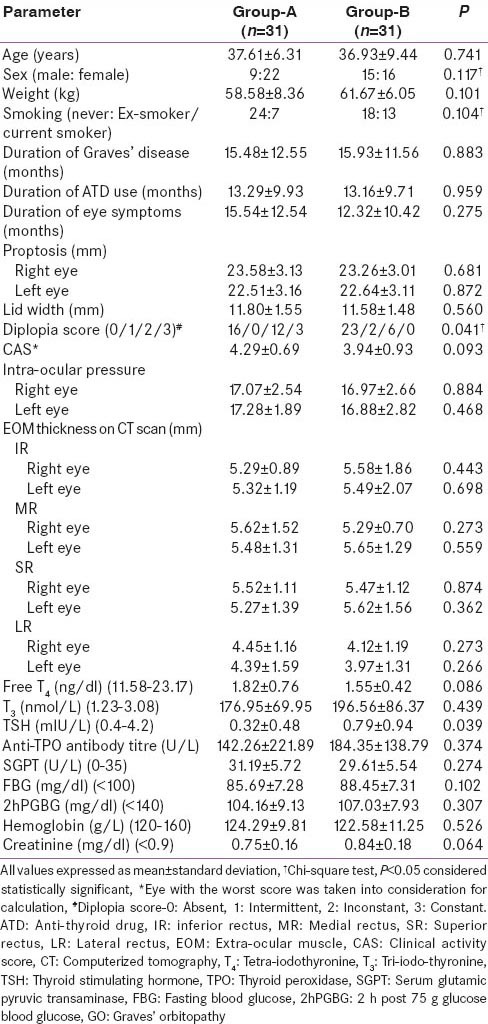
Sixty-five patients were randomized into Group-A and B [Figure 1]. The baseline clinical, ocular and biochemical parameters in Groups-A and B were comparable, except for, a higher diplopia score (P = 0.041) and lower thyroid stimulating hormone (TSH) (P = 0.039) in Group-A as compared to Group-B [Table 3]. The compliance rate of prednisolone tablets was 94%.
Table 3.
Comparison of baseline parameters of GO patients receiving intravenous methylprednisolone (Group-A) as compared to those receiving oral prednisolone (Group-B)
At 6-month, 25/31 (80.65%) patients in Group-A were responders in contrast to 12/31 (38.71%) patients in Group-B (P < 0.001). At 1-year follow-up, 27/31 (87.10%) patients were responders in Group-A and 17/31 (54.84%) were responders in Group-B (P = 0.005). TF was not observed in any patient of our study cohort [Table 4]. There was a significant reduction in lid-width, proptosis and CAS at 1-year, as compared to baseline, in both the groups [Table 4]. Diplopia scores improved both in patients of Group-A and B at 1-year, as compared to baseline, but was statistical significance only in Group-A (P = 0.009) [Table 4]. There was a greater improvement in CAS in patients of Group-A as compared to Group-B, which was statistically significant at 6-month (P < 0.001) and 1-year (P < 0.001) follow-up [Table 4]. Proptosis was significantly lower in Group-A as compared to Group-B at 6-month (P = 0.02) but not 1-year (P = 0.083) of follow-up. Lid width and diplopia were comparable between the two groups at 6-month and 1-year follow-up.
Table 4.
Comparison of ocular parameters of Group-A and Group-B at 6 and 12 months of follow-up
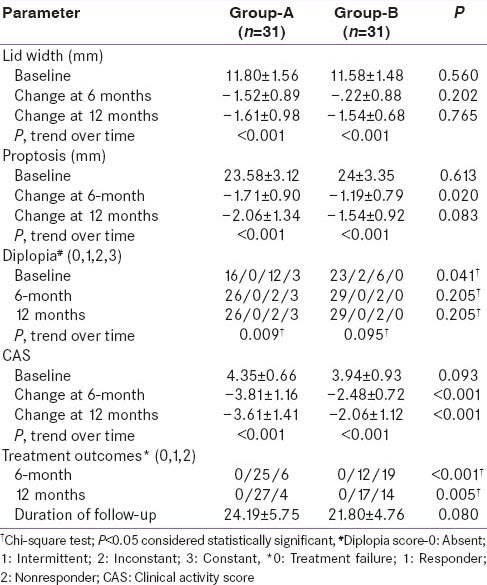
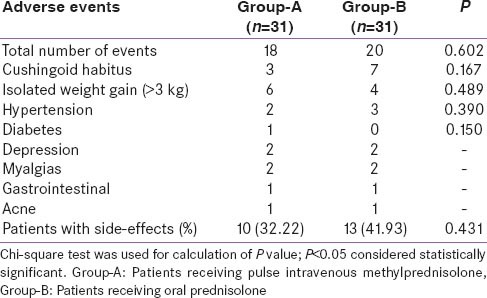
Responders (n = 44) had a significantly higher baseline bilateral intra-ocular pressure, and more severe left eye proptosis as compared to NR (n = 18) [Table 5]. Cox-regression analysis revealed that baseline T4 levels (P = 0.024), diplopia (P = 0.06) and smoking history (P = 0.08) were predictive of remission [Table 6]. Increased baseline T 4 and presence of smoking reduced the likelihood of remission; whereas presence of diplopia was associated with increased likelihood of remission [Table 6]. The occurrence of adverse events was comparable in both groups [Table 7]. No major adverse effects to steroid administration were noted. A mild transient and spontaneously reversible increase in liver enzymes were noted in 4 patients Group-A and none in Group-B.
Table 5.
Comparison of baseline parameters of GO patients who were responders as compared to those who were NRs at 1-year follow-up
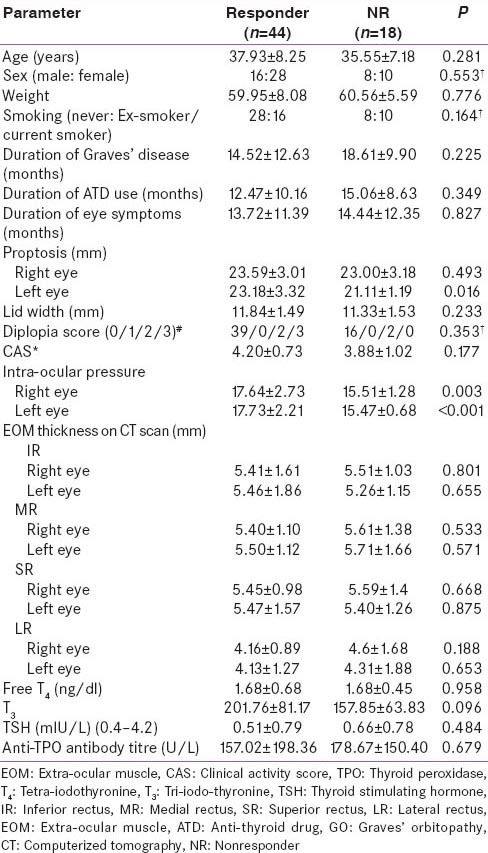
Table 6.
Cox-regression showing variables that independently predict remission of GO
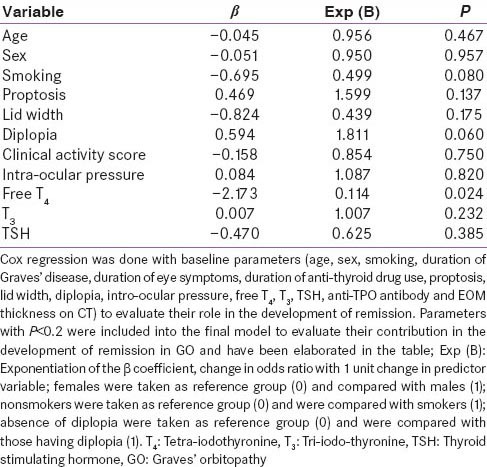
Table 7.
Comparison of adverse events in Group-A versus Group-B
DISCUSSION
Two recent RCT have shown that pulse iv glucocorticoids have a higher rate of favorable response (88% vs. 63% and 77% vs. 51% respectively) with fewer side effects than oral glucocorticoids.[9,10] It has been suggested that clinical response can be seen as early as 1–2 weeks following initiation of iv glucocorticoids.[9,10,11] In another randomized trial, iv methylprednisolone (four cycles at 500 mg for three consecutive days at 4-week intervals) was found to be effective in treating inflammatory changes and ocular movements in 83% of patients as compared to 11% in those who received placebo.[11] An alternative regimen that has been tried consists of 12 weekly infusions of methylprednisolone with a cumulative dose of 4.5 g (500 mg weekly for 6 weeks, then 250 mg weekly for 6 weeks).[10] The oral glucocorticoid dose used in these studies was high (prednisolone 100 mg/day) and was associated with greater side-effects, impairment of quality of life and increased recurrence following stoppage of glucocorticoids.[15,16] Indians in general have lower body weight than Caucasians. The median body weight of GO patients in our study was 58 kg in contrast to 70–74 kg in a study reported by Bartalena et al.[17] Use of high dose oral prednisolone (100 mg/day) for a short period in our patients was associated with frequent intolerable side effects (unpublished data). This was the rationale for evaluating a lower dose oral prednisolone at 1 mg/kg/day. The response rate to low dose oral prednisolone in our study was 54.84% which is comparable to that reported with high dose prednisolone among Caucasians (63.4% and 51%).[9,10] Glucocorticoids oral and iv both were well tolerated in our study, and the side effect profile was comparable between the groups. In our study, 41.93% of 31 patients on oral prednisolone complained of any adverse effect over a period of 1-year, as compared to 85.4% (35/41) patients over 1-year, and 51% (18/35) patients over 3 months in previously published studies among Caucasians.[9,10] The lower dose of prednisolone used in this study may explain this more favorable side-effect profile, as compared to previous studies. In a recent study evaluating the side-effects of glucocorticoid therapy observed by European thyroidologists in managing GO, it was reported that minor nonsevere adverse effects were more common with oral prednisolone, whereas major life-threatening adverse effects were more common with iv pulse methylprednisolone.[18] However, all but 1 patient with major life-threatening adverse effect with iv methylprednisolone had received a cumulative dose of the drug >8 g, which is higher than the current recommendations.[18]
In our study, pulse iv methyl-prednisolone was more effective in inducing a greater reduction of CAS and diplopia scores, along with a significantly higher response rate at 6-month and 1-year follow-up. This is in accordance with previous reports of a faster onset of action along with a greater response to iv glucocorticoids as compared to oral glucocorticoids. However, it is important to consider that response rates with oral prednisolone were significantly better at 1-year (54.84%) as compared to 6-month (38.71%). In one study, it was reported that on patient self-assessment there was no significant difference in the response between iv versus oral steroids (P = 0.27).[11] Patient self-assessment of outcomes was not done in our study and is a limitation. Limitations of this study include small number of patients evaluated, along with the relatively short duration of follow-up.
Glucocorticoids are believed to act by inhibition of leucocyte chemotaxis and cytokine release, reduction in glycosaminoglycan synthesis by orbital fibroblasts, and down regulation of adhesion molecules.[4] Glucocorticoids have been found to be most effective in patients with active ophthalmopathy, with significant inflammatory features, diplopia in primary gaze, and in whom vision is threatened.[5,19] This explains the higher left eye proptosis and intra-ocular pressures observed among responders in our study. Cox-regression also showed diplopia to be predictive of remission. Patients with a more clinically active GO have a better outcome with glucocorticoid therapy. Smoking had an adverse impact on remission of GO in our study, similar to previous reports.[1,2,3,4]
Our study showed that baseline EOM thickness was not predictive of response to glucocorticoid therapy. EOM thickness was comparable among responders versus NR. Repeat CT orbit was not done on follow-up and is also a limitation of this study. Also, lack of estimation of TSH receptor antibody titers was a limitation.
To the best of our knowledge, there is no study available from India, evaluating oral glucocorticoids in GO. The only study till date from India on GO showed that pulse iv dexamethasone is not inferior to pulse iv methylprednisolone in managing moderate severe GO in a cohort of 21 patients.[20] This is the first study ever evaluating the role of low dose oral prednisolone in the management of GO. There is only one study available till date on the use of low dose oral prednisolone in GO. That study showed that short-term low dose oral prednisolone for 6 weeks is effective in preventing exacerbation of initially mild or absent GO, when radio-iodine is used for managing Graves’ disease.[21] This study reiterates that iv methylprednisolone is superior to and should be the treatment of choice in managing GO. Oral prednisolone has been recommended as a second line agent in the management of GO, either alone or in combination with other investigative agents like cyclosporine or rituximab.[22] One of the limitations of this study is lack of third arm of patients who would have been treated with high dose oral prednisolone, allowing a direct comparison between low dose oral prednisolone versus high dose oral prednisolone groups. Paucity of patients prevented us from having this third arm of the study. In absence of this third arm, it can only be speculated that low dose oral prednisolone (used in this study) is as efficacious as high dose oral prednisolone (based on previous published western literature), with significantly better tolerability and side-effect profile. Low dose oral prednisolone can be considered as a safe second line alternative in Indian GO patients, not willing or intolerant to pulse iv methylprednisolone.
ACKNOWLEDGMENTS
The authors are grateful to the departments of Ophthalmology and Radiology for their assistance in the assessment of GO.
Footnotes
Source of Support: This study was funded by the Department of Endocrinology, Room Number-6, 4th floor Ronald Ross Building, Institute of Postgraduate Medical Education and Research (IPGMER), Seth Sukhlal Karnani Memorial (SSKM) Hospital, 244 AJC Bose Road, Kolkata - 700 020, India
Conflict of Interest: None declared.
REFERENCES
- 1.Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L. Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest. 2013;36:444–9. doi: 10.3275/8937. [DOI] [PubMed] [Google Scholar]
- 2.Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009;360:994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: Reality and perspectives. Endocr Rev. 2000;21:168–99. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 5.Melcescu E, Horton WB, Kim D, Vijayakumar V, Corbett JJ, Crowder KW, et al. Graves orbitopathy: Update on diagnosis and therapy. South Med J. 2014;107:34–43. doi: 10.1097/SMJ.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 6.Koshiyama H, Koh T, Fujiwara K, Hayakawa K, Shimbo S, Misaki T. Therapy of Graves’ ophthalmopathy with intravenous high-dose steroid followed by orbital irradiation. Thyroid. 1994;4:409–13. doi: 10.1089/thy.1994.4.409. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuka K, Sato A, Kawaguchi S, Hashimoto M, Suzuki Y. Effect of high-dose intravenous steroid pulse therapy followed by 3-month oral steroid therapy for Graves’ ophthalmopathy. Jpn J Ophthalmol. 2002;46:563–7. doi: 10.1016/s0021-5155(02)00544-0. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuka K, Sato A, Kawaguchi S, Hashimoto M, Suzuki Y. Effect of steroid pulse therapy with and without orbital radiotherapy on Graves’ ophthalmopathy. Am J Ophthalmol. 2003;135:285–90. doi: 10.1016/s0002-9394(02)01970-0. [DOI] [PubMed] [Google Scholar]
- 9.Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell’Unto E, Rocchi R, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: Results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–7. doi: 10.1210/jcem.86.8.7737. [DOI] [PubMed] [Google Scholar]
- 10.Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40. doi: 10.1210/jc.2005-0148. [DOI] [PubMed] [Google Scholar]
- 11.van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: A prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;158:229–37. doi: 10.1530/EJE-07-0558. [DOI] [PubMed] [Google Scholar]
- 12.Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, Kendall-Taylor P, et al. Multi-center study on the characteristics and treatment strategies of patients with Graves‘ orbitopathy: The first European Group on Graves’ Orbitopathy experience. Eur J Endocrinol. 2003;148:491–5. doi: 10.1530/eje.0.1480491. [DOI] [PubMed] [Google Scholar]
- 13.Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaag R, Koornneef L, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353–9. doi: 10.1056/NEJM198911163212002. [DOI] [PubMed] [Google Scholar]
- 14.Roux C, Reid DM, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Post hoc analysis of a single IV infusion of zoledronic acid versus daily oral risedronate on lumbar spine bone mineral density in different subgroups with glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23:1083–90. doi: 10.1007/s00198-011-1800-1. [DOI] [PubMed] [Google Scholar]
- 15.Wiersinga WM, Prummel MF, Terwee CB. Effects of Graves’ ophthalmopathy on quality of life. J Endocrinol Invest. 2004;27:259–64. doi: 10.1007/BF03345275. [DOI] [PubMed] [Google Scholar]
- 16.Lee PP, Spritzer K, Hays RD. The impact of blurred vision on functioning and well-being. Ophthalmology. 1997;104:390–6. doi: 10.1016/s0161-6420(97)30303-0. [DOI] [PubMed] [Google Scholar]
- 17.Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–63. doi: 10.1210/jc.2012-2389. [DOI] [PubMed] [Google Scholar]
- 18.Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, et al. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: A questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol. 2012;166:247–53. doi: 10.1530/EJE-11-0779. [DOI] [PubMed] [Google Scholar]
- 19.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 20.Philip R, Saran S, Gutch M, Agroyia P, Tyagi R, Gupta K. Pulse dexamethasone therapy versus pulse methylprednisolone therapy for treatment of Graves's ophthalmopathy. Indian J Endocrinol Metab. 2013;17:S157–9. doi: 10.4103/2230-8210.119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai A, Sassi L, Compri E, Marino F, Sivelli P, Piantanida E, et al. Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent graves’ orbitopathy: A retrospective cohort study. J Clin Endocrinol Metab. 2010;95:1333–7. doi: 10.1210/jc.2009-2130. [DOI] [PubMed] [Google Scholar]
- 22.Bartalena L. What to do for moderate-to-severe and active Graves’ orbitopathy if glucocorticoids fail? Clin Endocrinol (Oxf) 2010;73:149–52. doi: 10.1111/j.1365-2265.2010.03783.x. [DOI] [PubMed] [Google Scholar]