Abstract
Introduction:
Bilirubin as an antioxidant and malondialdehyde (MDA) as an oxidant have been shown to be associated with various complications of type 2 diabetes mellitus (DM).
Aims and Objectives:
The aim was to measure the levels of serum bilirubin and MDA in type 2 DM patients with and without diabetic retinopathy (DR) and to correlate them with severity of DR.
Materials and Methods:
A total number of 120 subjects out of which 40 were controls without type 2 DM and the rest 80 were type 2 DM patients were included in the study. Of those 80 diabetics, 44 patients did not have DR and 36 patients had DR.
Results:
The total bilirubin, direct bilirubin, indirect bilirubin were higher in controls as compared to cases (P = 0.017, 0.033, 0.024). Serum MDA levels were found to be higher in diabetics as compared to controls (P = 0.00). The values of all the three parameters, that is, total bilirubin, direct bilirubin and indirect bilirubin were lower in patients with retinopathy as compared to those without retinopathic changes (P = 0.00, 0.020, and 0.007). Subjects were assigned to quartiles based on serum total bilirubin concentration. The prevalence of DR was significantly lower among persons with the highest bilirubin quartile compared to those with the lowest quartile. The severity of DR was inversely proportional to the total bilirubin levels (P = 0.001). The multiple logistic regression analysis showed total bilirubin to be associated with prevalence of DR (P = 0.035).
Conclusions:
The levels of total bilirubin were significantly lower in patients with DR and also in the late stages of retinopathy as compared to those without retinopathy and in controls but MDA levels did not show any association with DR.
Keywords: Bilirubin, malondialdehyde, retinopathy, type 2 diabetes mellitus
INTRODUCTION
India faces a huge case load of type 2 diabetes mellitus (DM), which is projected to affect about 69.9 million Indians by the year 2025.[1] With limited access to quality health care, this population is prone to develop microvascular complications such as neuropathy, nephropathy, retinopathy and macrovascular problems like cardiovascular diseases. Diabetic retinopathy (DR) is a common microvascular complication, which is one of the leading causes of adult visual impairment and blindness.[2] Though all traditional risk factors such as hyperglycemia, dyslipidemia, hypertension and duration of diabetes are associated with development and progression of DR,[3] the altered milieu of oxidants and antioxidants in the sera of patients with type 2DM are additional contributors. In DM, the glucolipotoxicity leading to endothelial dysfunction and hyperglycemia is associated with free radical mediated lipid peroxidation.[4,5,6,7]
Bilirubin is a cytoprotectant with antioxidant and anti-inflammatory properties on the microvasculature.[8,9,10] Innate antioxidant shield is destroyed by increase of endogenous reactive oxygen species, which leads to oxidative stress.[11,12,13] Bilirubin was shown to reduce the oxidant levels in wounds of diabetic rats, which accelerated the wound healing.[14] Elevated bilirubin levels have been found to be a protective determinant for the development of type 2 DM.[15] The generation of free oxygen radicals in DM lead to accumulation of malondialdehyde (MDA) by peroxidative breakdown of phospholipids.[16] MDA is one of the indicators of oxidative stress. There are very few studies in the literature, which have shown the association between serum bilirubin and MDA levels with diabetic microvascular complications.[17,18,19,20,21,22,23,24,25] We hypothesized that there is a correlation between the levels of bilirubin and MDA with severity of retinopathy in patients with type 2 DM. There are no studies showing the association between DR and serum bilirubin in Indian population and only a few are available with MDA levels hence this study was undertaken.
AIMS AND OBJECTIVES
To measure serum bilirubin and MDA levels in type 2 DM patients with and without DR and to correlate the above values with severity of DR in type 2 DM.
MATERIALS AND METHODS
A cross-sectional case control study was carried out in the Department of Endocrinology, at a tertiary care center, after obtaining ethical clearance. Study was conducted under the participant's voluntary consent (oral and written) after explanation of the purpose, method, and course. Based on a study conducted by Turk et al.[22] on the relationship between DR and serum levels of ischemia modified albumin (IMA) and MDA levels in type 2 DM, the levels of MDA in patients with DR was 0.325 ± 0.172 and in patients without DR was 0.178 ± 0.131, which was statistically significant. In the present study expecting a mean difference of 0.007, MDA levels between cases with and without DR the confidence level of 95% and with the power of 80%, the sample size was estimated to be 75, which included 36 cases and 36 controls. A total number of 120 subjects were included in the present study out of which 40 were controls who were normal individuals without type 2 DM and the rest 80 were type 2 diabetes patients. Of these 80 diabetics, 44 patients without DR were selected, and 36 patients with DR were chosen.
The patients excluded were those with a history of chronic alcohol consumption, intake of hepatotoxic drugs within past 6 months, e.g. antitubercular treatment, antiepileptic drugs etc., history of preexisting hepatobiliary abnormalities or any other acute disease affecting hepatic functions within past 6 months or diagnosis of acute hepatic involvement at the time of enrollment into the study. The patients who were on anti-diabetic drugs like thiazolidinedione, α-1 glucosidase inhibitor as well as patients with hemolytic anemia were also excluded.
A detailed questionnaire was completed for all patients regarding age, gender, smoking history, history of alcohol intake or hepatobiliary disorders, duration of type 2 DM, history of hypertension, cardiovascular disease, neuropathy, nephropathy, stress. Screening for retinopathy was done by direct ophthalmoscopy, slit lamp with +90D and indirect ophthalmoscopy. A detailed anthropometry was done for all cases and controls.
The data for hemoglobin A1c (HbA1c) values, which were done within 3 months of recruitment as a routine standard of care were collected from the case records of the patients.
Three milliliters of venous blood was drawn from the patient on the day of examination and serum total, direct, indirect bilirubin level, and MDA levels were estimated using colorimetric kits.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 20 (IBM) was used to perform statistical analysis and the baseline characteristics of the participants were presented as the mean ± standard deviation. Median and inter quartile range was calculated wherever necessary. Chi-square test was used to see the comparison between the proportions. Data between the two groups were compared using Student's t-test for parametric variables and Mann–Whitney test for nonparametric data. Multiple groups were compared using Kruskal–Wallis test and analysis of variance test in the present study. Multiple logistic regression analysis was performed to assess independent associations between the presence of key risk factors in univariate analysis and suggested biomarkers such as serum total bilirubin and MDA levels. P < 0.05 was considered as statistically significant.
RESULTS
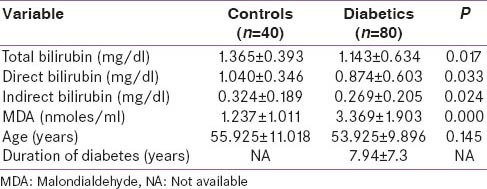
For the present study, subjects were taken after age and sex matching. We compared serum total bilirubin, direct bilirubin, indirect bilirubin between diabetics (n = 80) and controls (n = 40), all the three parameters were higher in controls as compared to cases (P = 0.017, 0.033, 0.024). Serum MDA levels were found to be higher in diabetics as compared to controls (P = 0.00) [Table 1].
Table 1.
Baseline characteristics (clinical and laboratory) of the controls and diabetics
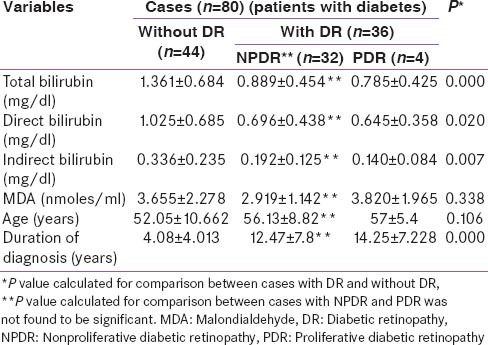
We compared for diabetics without retinopathy (n = 44) and diabetics having retinopathic changes (n = 36) the values of serum total bilirubin, direct bilirubin and indirect bilirubin and the values of all the three parameters were lower in patients with retinopathy as compared to those without retinopathic changes (P = 0.00, 0.020, 0.007). The duration of diabetes was higher in patients with retinopathy as compared to patients without DR (P = 0.00). Age and MDA levels did not show any significant correlation between the two groups (P = 0.106 and 0.338). In a subgroup analysis of patient with nonproliferative DR (NPDR) changes (n = 32) versus PDR changes (n = 4), there was no difference in serum total bilirubin, direct bilirubin, indirect bilirubin and MDA levels in the two groups. The duration of diagnosis of type 2 DM was also not found to be different in these two groups [Table 2].
Table 2.
Comparison of different relevant factors amongst type 2 diabetic patients with and without DR
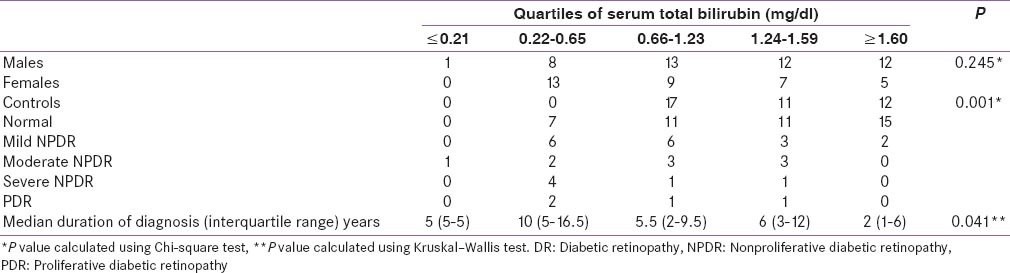
Subjects were assigned to quartiles based on serum total bilirubin concentration (Quartile - Q1≤0.21; Q2 0.22–0.65; Q3 0.66–1.23; Q4 1.24–1.59; Q5≥1.60). Patients with lower bilirubin values had higher median duration of diabetes as compared to patients with higher bilirubin value (P = 0.041). It was seen that bilirubin levels were not different between the two genders (P = 0.245). The prevalence of DR was significantly lower among persons with the highest bilirubin quartile compared to those with the lowest quartile. The severity of DR was inversely proportional to the total bilirubin levels (P = 0.001) [Table 3].
Table 3.
Correlation between serum total bilirubin and severity of DR
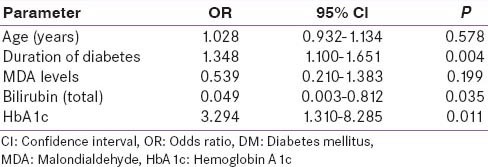
The multiple logistic regression analysis showed duration of diabetes and HbA1c (P = 0.004 and 0.011) to be predictors of development of DR and among MDA and total bilirubin the later was found to be a predictor of DR (P = 0.199 and 0.035). The direct and indirect bilirubin were not shown to be predictors of DR in the logistic regression analysis [Table 4].
Table 4.
Logistic regression analysis for association of bilirubin and MDA with retinopathy in type 2 DM
DISCUSSION
Hyperglycemia generates oxidative stress, which is exacerbated by metabolic stress. We investigated the association of serum bilirubin and MDA levels with severity of DR in the participants of this present study. Previously, several studies have been conducted on the association of serum bilirubin and vascular complications of type 2 DM.[17,18,19,20]
Bilirubin also exhibits strong antioxidant and anti-inflammatory property on vasculature. Thus, it may act as a cytoprotectant to the retinal vasculature.[8,9,10] Retina is a source of free radical production, and various retinal changes happen because of oxidative stress in humans with metabolic conditions like type 2 DM.[26] Neovascularization in proliferative retinopathy is due to disequilibrium between the pro and anti-angiogenic factors.[27] In patients, with type 2 DM serum bilirubin levels have also been proven to be associated with microalbuminuria and sub clinical atherosclerosis. Serum bilirubin concentration has been shown to be inversely related to and an independent determinant of log urinary albumin excretion.[19] Thus, increased level of serum bilirubin may be protective against DR amongst persons with diabetes.[20,21]
In the present study, controls were matched for age and sex because oxidant and antioxidant levels can change with age. The duration of diagnosis was not found to be significant between NPDR and PDR in the present study. The reason could be the small number of patients in PDR group (n = 4).
In our study, the total bilirubin levels showed an inverse relationship with the severity of DR (mild, moderate, severe NPDR and PDR), but the levels were not significantly different when the comparison was done between two groups, that is, NPDR and PDR. The multiple logistic regression analysis showed total bilirubin to be a predictor of the development of DR. Inoguchi et al.[18] reported that patients with Gilberts syndrome, which is associated with high bilirubin levels have a lower incidence of vascular complications. Another study done by Yasuda et al.[20] showed an indirect correlation between DR and bilirubin that is, a lower bilirubin levels were found in those patients with DR as compared to the diabetics without DR. Two other similar studies including one done by Cho[10] also showed bilirubin as a useful biomarker to predict risk of DR and another by Najam et al.[21] reported bilirubin levels to be inversely correlated to DR. Our study results are similar to the above mentioned studies. As expected the duration of DM was longer in patients who developed DR as compared to those who did not and also poor glycemic control was found to be associated with the development of DR.
In our study, we have included two additional parameters along with serum total bilirubin, that is, direct and indirect bilirubin. We found the levels to be higher in controls as compared to diabetics and in diabetics the levels were greater in patients with DR as compared to those who did not have retinopathy. However, in the multiple logistic regressions analysis, which was done to compare that out of total, direct and indirect bilirubin, which is the best predictor of the development of DR total bilirubin was shown to be the best predictor. Most of the other studies in the literature have compared only total bilirubin as the parameter for prediction of DR. Our study is the first of its kind to consider all the three parameters.
The duration of diagnosis of diabetes and glycemic control were confounding factors in our study, which were also found to predict the development of DR along with total bilirubin in the multivariate logistic regression analysis. The longer duration of diabetes and poorer glycemic controls are well known risk factors for the development of DR[2] but even after adjusting for these parameters lower total bilirubin levels still emerged as one of the important predictors of its development.
Another parameter analyzed in our study was serum MDA. MDA being an oxidant should be higher in DR. However, our study did not show the same trend. Its levels were higher in diabetics as compared to controls thus showing that the oxidant levels are higher in diabetics. It was not different in patients with and without DR, hence it cannot be used as a marker for prediction of development of DR, suggesting that the use of MDA as a biomarker to predict occurrence of DR is questionable. The MDA levels can vary on a day-to-day basis in smokers and alcoholics. Hence, in our study, participants with a history of smoking or alcohol consumption were excluded. Diurnal variation could, therefore, be one of the reasons for the difference in the levels in the samples and the further use of MDA as a marker to detect DR may be questionable. Our study has shown similar results to a study published by Turk et al.[22] who also showed that IMA is a better predictor of DR as compared to MDA. IMA levels were not measured in our patients. Our results are different from the study published by Vidya et al.,[23] Kumari et al.[24] and Bhutia et al.[25] who showed that MDA levels were predictors of severity of DR.
CONCLUSION
We demonstrated that elevated serum bilirubin levels are seen in diabetics without retinopathy and thus total bilirubin can have a protective effect on the development of DR but the role of serum MDA levels as a predictor or protective determinant of DR is questionable.
ACKNOWLEDGMENT
We are thankful to Dr Vasudha K C Professor of Biochemistry at M S Ramaiah medical college for her help and guidance for the completion of this project
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sicree R, Shaw J, Zimmet P. Diabetes and impaired glucose tolerance. In: Gan D, editor. Diabetes Atlas. International Diabetes Federation. 3rd ed. Belgium: International Diabetes Federation; 2006. pp. 15–103. [Google Scholar]
- 2.Marozas LM, Fort PE. Diabetic retinopathy-update on prevention techniques, present therapies, and new leads. US Ophthalmic Rev. 2014;7:54–58. doi: 10.17925/usor.2014.07.01.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen TT, Alibrahim E, Islam FM, Klein R, Klein BE, Cotch MF, et al. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: The multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32:1704–9. doi: 10.2337/dc09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai. 2010;93:682–93. [PubMed] [Google Scholar]
- 5.Su Y, Liu XM, Sun YM, Jin HB, Fu R, Wang YY, et al. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008;62:877–82. doi: 10.1111/j.1742-1241.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 6.Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–14. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Tang L, Chen B. Oxidative stress: Implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/752387. 752387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 9.Dekker D, Dorresteijn MJ, Pijnenburg M, Heemskerk S, Rasing-Hoogveld A, Burger DM, et al. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31:458–63. doi: 10.1161/ATVBAHA.110.211789. [DOI] [PubMed] [Google Scholar]
- 10.Cho HC. The relationship among homocysteine, bilirubin, and diabetic retinopathy. Diabetes Metab J. 2011;35:595–601. doi: 10.4093/dmj.2011.35.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DI, Park MJ, Choi JH, Lim SK, Choi HJ, Park SH. Hyperglycemia-induced GLP-1R downregulation causes RPE cell apoptosis. Int J Biochem Cell Biol. 2015;59:41–51. doi: 10.1016/j.biocel.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Xia H, Han Q, Chen B. Effects of antioxidant gene therapy on the development of diabetic retinopathy and the metabolic memory phenomenon. Graefes Arch Clin Exp Ophthalmol. 2015;253:249–59. doi: 10.1007/s00417-014-2827-8. [DOI] [PubMed] [Google Scholar]
- 13.Wautier MP, Tessier FJ, Wautier JL. Advanced glycation end products: A risk factor for human health. Ann Pharm Fr. 2014;72:400–8. doi: 10.1016/j.pharma.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Ram M, Singh V, Kumar D, Kumawat S, Gopalakrishnan A, Lingaraju MC, et al. Antioxidant potential of bilirubin-accelerated wound healing in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:955–61. doi: 10.1007/s00210-014-1011-3. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi A, Deetman PE, Corpeleijn E, Gansevoort RT, Gans RO, Hillege HL, et al. Bilirubin as a potential causal factor in type 2 diabetes risk: A Mendelian randomization study. Diabetes. 2014 doi: 10.2337/db14-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49:129–36. [PubMed] [Google Scholar]
- 17.Huang EJ, Kuo WW, Chen YJ, Chen TH, Chang MH, Lu MC, et al. Homocysteine and other biochemical parameters in Type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy. Clin Chim Acta. 2006;366:293–8. doi: 10.1016/j.cca.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007;298:1398–400. doi: 10.1001/jama.298.12.1398-b. [DOI] [PubMed] [Google Scholar]
- 19.Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, et al. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int. 2008;74:1197–201. doi: 10.1038/ki.2008.398. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda M, Kiyohara Y, Wang JJ, Arakawa S, Yonemoto K, Doi Y, et al. High serum bilirubin levels and diabetic retinopathy: The Hisayama Study. Ophthalmology. 2011;118:1423–8. doi: 10.1016/j.ophtha.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Najam SS, Sun J, Zhang J, Xu M, Lu J, Sun K, et al. Serum total bilirubin levels and prevalence of diabetic retinopathy in a Chinese population. J Diabetes. 2014;6:221–7. doi: 10.1111/1753-0407.12085. [DOI] [PubMed] [Google Scholar]
- 22.Turk A, Nuhoglu I, Mentese A, Karahan SC, Erdol H, Erem C. The relationship between diabetic retinopathy and serum levels of ischemia-modified albumin and malondialdehyde. Retina. 2011;31:602–8. doi: 10.1097/IAE.0b013e3181ed8cd1. [DOI] [PubMed] [Google Scholar]
- 23.Vidya D, Shekhar R, Prabodh S, Chowdary NV, Das MC, Reddy MJ. Oxidative stress in diabetic retinopathy. J Clin Diagn Res. 2011;5:994–7. [Google Scholar]
- 24.Kumari S, Panda S, Mangaraj M, Mandal MK, Mahapatra PC. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Clin Biochem. 2008;23:158–62. doi: 10.1007/s12291-008-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhutia Y, Ghosh A, Sherpa ML, Pal R, Mohanta PK. Serum malondialdehyde level: Surrogate stress marker in the Sikkimese diabetics. J Nat Sci Biol Med. 2011;2:107–12. doi: 10.4103/0976-9668.82309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grattagliano I, Vendemiale G, Boscia F, Micelli-Ferrari T, Cardia L, Altomare E. Oxidative retinal products and ocular damages in diabetic patients. Free Radic Biol Med. 1998;25:369–72. doi: 10.1016/s0891-5849(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 27.Zorena K, Raczynska D, Raczynska K. Biomarkers in diabetic retinopathy and the therapeutic implications. Mediators Inflamm. 2013;2013:193–604. doi: 10.1155/2013/193604. [DOI] [PMC free article] [PubMed] [Google Scholar]


