Abstract
Objective:
Diabetic foot ulcers and amputations are preventable. Aim of this study was to determine the distribution of categories of foot at risk in patients with diabetes, attending a tertiary care hospital and factors that affect it.
Materials and Methods:
Detail history and examination including neurological and vascular assessment were performed in 100 patients with diabetes attending a Tertiary Care Hospital. Foot at risk was classified according to the task force of foot care interest Group of American Diabetes Association. Category of foot at risk was correlated with demographic and clinical features.
Results:
Fifty-two percent patients had foot at risk-category 1 and 2. Loss of protective sensation (LOPS) was present in 33% (category 1). Peripheral arterial disease (PAD) was present in 19% (category 2). Both LOPS and PAD were present in 10% patients. 95% had never received foot care advice by health professionals, let alone prescriptive footwear or vascular consultation.
Conclusions:
This study brings forth that foot at risk of ulcer is rampant in patients with diabetes. There are lacunae in diabetic foot care at all levels of care. With the increase in diabetes, cost effective steps are required to improve foot care among diabetes in India. Considering the demographic profile of patients in our study, growing number of patients with diabetes, lack of time and staff allocated for foot care in our setup, audiovisual aids seems a good option to spread foot care awareness among diabetes.
Keywords: Diabetic foot, foot at risk, loss of protective sensation
INTRODUCTION
Many of the foot complications in diabetes are preventable.[1] Poor foot care in diabetes can lead to ulcer, amputation, sepsis and even death. Management of diabetic ulcer involves substantial cost.[2] Therefore, importance of foot health must be communicated to the patient at an early stage in diabetes.
Peripheral neuropathy, peripheral arterial disease (PAD), previous history of foot ulcer and foot deformity are risk factors. Foot care education, early detection, corrective prescriptive or accommodative footwear, active intervention and regular follow-up can prevent foot ulcers.
This study aims to assess presence and category of foot at risk in patients of diabetes mellitus who have not yet developed foot ulcer and thereby assess the requirement of foot care to be made available to prevent ulcer development.
We also studied the demographic and clinical profile so as to assess what strategies can be adopted for enhancing foot care in diabetes.
MATERIALS AND METHODS
A total of 100 patients with diabetes aged more than 30 years attending outpatient department or admitted to hospital wards of a tertiary care hospital from May 2013 to May 2014 were enrolled. This included both new and old follow-up patients. Patients who did not wish to complete the questionnaire and with cognitive/hearing impairment and previous history of foot ulcer or amputation were excluded.
Study design: Cross sectional study
Sample size: This is a preliminary study in which we recruited all patients with diabetes who met the inclusion and exclusion criteria presenting to our hospital between May 2013 and May 2014. This came to a total of 100 patients.
Approval of institute ethics committee was taken. Informed consent of the patient was taken. Detail of patient as per protocol designed based on Comprehensive Foot Examination and Risk Assessment[3] [Table 1] was done and noted.
Table 1.
Risk classification based on the comprehensive foot examination[3]

Examination included dermatologic, musculoskeletal, neurological assessment with 10-g monofilament (perception of pressure and identification of correct site) at first, third and fifth metatarsal heads and plantar surface of distal hallux.
Neurological assessment to determine loss of protective sensation (LOPS) that is perception of pressure and identification of correct site by 10-g monofilament at four different sites as suggested by Comprehensive Foot Examination and Risk Assessment Committee at first, third and fifth.
Metatarsal heads, and planter surface of distal hallux was done along with the assessment of other sensations. A volume of 10-g monofilaments pressure was applied over above mentioned sites with patients eyes closed. Patient was asked to mention the perception of pressure and recognition of a particular site for detection of LOPS. Sites, where there was callus, were avoided. Loss of protective sensation was defined when there was one or more abnormal test on neurological examination. This was based on American Diabetes Association (ADA) task force report for comprehensive foot examination.[3]
Vibration sensation was tested with128-Hz tuning forks by applying it over the tip of the great toe bilaterally. An abnormal response was considered when the patient lost vibratory sensation, and the examiner was able to perceive it while holding the fork on the tip of his own great toe.
Pinprick sensation was regarded abnormal when subjects could not perceive the pressure just enough to deform the skin applied by a pin just proximal to the toenail on the dorsal surface of the hallux.
Absence and presence of ankle reflexes were noted bilaterally.
Blood pressure was measured in the supine position with a standard aneroid (HEINE Gamma G5) Sphygmomanometer. Vascular assessment by manual assessment of foot pulses in both lower limbs by palpating both posterior tibial and dorsalis pedis pulses were done. Manual measurement of ankle brachial index (ABI) as well Doppler based analysis of ABI by hand held Doppler using 8 MHZ probe, (Life Dop; Summit Doppler, Diabetic Foot care India Madras Engineering services) was done by a single observer following standard procedure. Definition of peripheral vascular insufficiency was made on the basis ABI, and it was regarded as abnormal when ABI was either <0.9 or >1.4.
Based on above evaluation for diabetic neuropathy, peripheral vascular disease (PVD), foot deformity, presence of other micro vascular and macro vascular complications, analysis of risk factors were done.
Foot was categorized into (0–3) categories based on the comprehensive foot examination.[3] Category of foot was correlated with parameters like, previous foot care education, level of education, socioeconomic level, level of medical care, micro vascular and macro vascular complications.
RESULTS
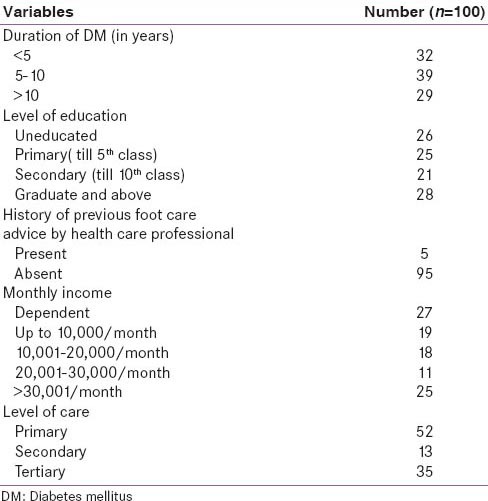
There were 46 male and 54 female. The median duration of diabetes was 7 years with a range of 0.05–25 years. The mean age was 53 ± 9.7 years with a range of 32–75 years. The mean BMI was 25.5 ± 4.5. Table 2 shows the demographic profile of patients.
Table 2.
Demographic parameters of study population
On neurological examination, it was found that perception of buckling of 10-g monofilament was the most abnormal test, and loss of protective sensation was found in 43%. This was followed by loss of vibration sense in 28%, loss of ankle reflex in 27% and loss of pin prick in 20%.
On detail vascular examination, it was found that the both posterior tibial and dorsalis pedis pulses were bilaterally absent in 4% and unilaterally absent in 22% subjects in the right foot and 19% in left foot. PAD was diagnosed in 19 in the right side and 18 on left side limbs by manual method of ABI. Whereas by Doppler method, PAD was diagnosed in 11 right and 15 left lower limbs. By manual method, bilateral PAD was found in nine patients. By Doppler method, bilateral disease was found in seven patients. ABI could not be measured in four patients by manual method as peripheral pulses were not palpable. By Doppler method, ABI could not be measured in one patient on the right side and one patient on the left side.
After completion of the examination, limbs were categorized based on task force of foot care interest Group ADA (3). 48%, 33%, 19% limbs were in category 0, 1, 2 respectively.
About 52% patients had foot at risk (category 1 and 2) among which 33 had LOPS with or without deformity (category 1). Nineteen patients had foot at risk category 2, which is 19 had PAD. Among them, 10 also had LOPS. Nine patients had PAD without LOPS [Table 3].
Table 3.
Category of foot of study population

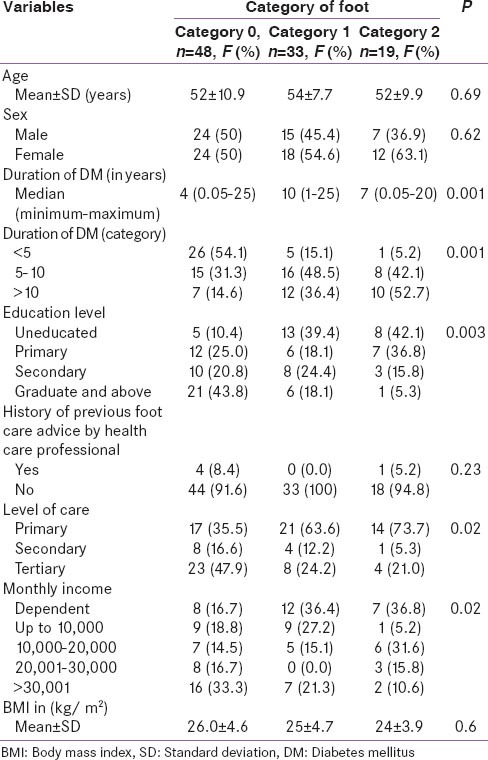
Category of foot at risk correlated with duration of diabetes, education level, level of health care and monthly income [Table 4].
Table 4.
Association of demographic variables with foot at risk (category of foot-0, 1, 2)
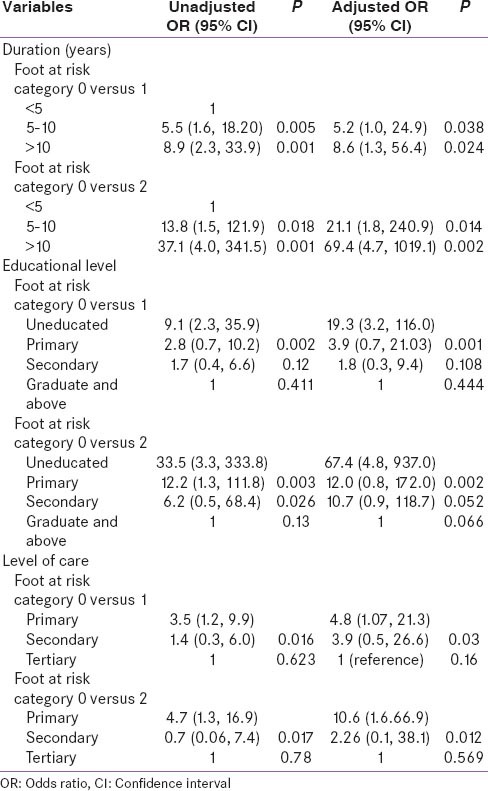
Multivariate analysis was done to find out independent risk factors for foot at risk. It was found that the duration of diabetes mellitus, formal education level and level of health care were independently associated with foot at risk [Table 5].
Table 5.
Association of demographic and clinical parameters with multivariate unadjusted OR and adjusted OR with foot at risk
DISCUSSION
People with diabetes have up to 46-fold greater risk of high-level lower extremity amputation than those without diabetes.[4,5]
Effectiveness of diabetic foot risk classification[3] is evident by the finding that patients in higher-risk groups had longer duration of diabetes, worse glycemic control, vascular and neuropathic variables, and more systemic complications of diabetes.[6] During three years of follow-up, ulceration occurred in 5.1%, 14.3%, 18.8%, and 55.8% of the patients in Groups 0, 1, 2, and 3, respectively.[6] All amputations were found in Groups 2 and 3. Thus foot risk classification of the International Working Group on the Diabetic Foot predicts ulceration and amputation and can function as a tool to guide prevention of lower extremity complications of diabetes.
Identification of foot at risk and adopting the further preventive measures is important. For screening and identification of peripheral neuropathy among persons with diabetes, various studies have shown that the 10-g Semmes–Weinstein monofilament is one of the most effective instruments. This instrument is inexpensive, noninvasive, reusable, and the test is easy to perform. Different studies have used different number of sites for detection of loss of protective sensation with the 10-g monofilament. Efficacy of use of 10-g monofilament for screening for LOPS is widespread and has been confirmed in a number of studies[7,9,10] including the Seattle Diabetic Foot Study.[11] The high predictive values of loss of pressure sensation (LOPS) for subsequent ulceration have been assessed using 10-g monofilament in multiple prospective studies.[7,8]
Different number of sites had been used for detection of LOPS. Totally, 10 sites for detection of LOPS had also been used in different studies. There is no difference in sensitivity and specificity of Monofilament test done at three and four points when compared to test done at 8 and 10 sites.[12]
Different risk factors play a cumulative role for development of ulceration in diabetes. Patients with neuropathy alone are at approximately 1.7 times greater risk for development of ulceration than patients without. This risk increases to 12.1 times when patients present with neuropathy and deformity. Patients with neuropathy, deformity and a history of ulcer or amputation are at approximately 36 times greater risk of developing another ulcer.[13]
Another important risk factor, which often results in amputation, is tissue ischemia due to atherosclerosis. Atherosclerosis manifests as PVD, which leads to chronic limb ischemia and poor wound healing.[14]
While ABI may not be sensitive enough to detect subtle signs of ischemia, it is useful as an initial measure prior to embarking on further investigations if there is a clinical index of suspicion.
Commonly PAD is only looked for once patient complaints of intermittent claudications or develops ulcer.[15]
Presence of PAD is defined by ABI value of <0.9 as well as >1.4.[16]
There is a wide variation in reported prevalence for foot at risk in a population from 5% to 80%.[17,18] Variation in population age group, geographical distribution and criteria used for diagnosis might explain this wide variation. In our study, 52% patients had foot at risk. Out of total 52 patients with foot at risk, 33 patients were in category 1 and 19 were in category 2. The reported prevalence of foot at risk from a centre in north India was 66.9%, and peripheral neuropathy was 34.9%.[19] In our study, prevalence rate of peripheral neuropathy was 43%.
In a multi-centric study from India, 15% prevalence of neuropathy was reported.[20]
For better foot care management, report of the task force of the foot care interest group of the ADA, with endorsement by the American association of clinical endocrinologists recommends that based on category of foot at risk, follow-up and treatment is planned. Category 0 should get their foot examined annually, and they should receive foot care education as well as footwear advice [Table 1]. None of the 48% patients in this category in our study gave history of ever receiving any foot care advice.
Patients with category 1 foot at risk should have follow-up every 3–6 monthly and should receive more attention on footwear and deformity. This group patient should be considered for prescriptive or accommodative footwear and surgery for deformity if required.
In category 2 foot at risk, follow-up by specialist should be more frequent that is every 2–3 monthly and vascular consultation should be sought. Footwear prescription should be similar to category 1 [Table 1].
Foot care education should be given to all categories of foot but in our study, a total of only 5% gave a history of ever receiving any foot care education and none among the grade 0 group. None of our patients had received prescriptive foot care or vascular consultation.
A relevant point noted in the demographic profile was that as 26% of our patients were illiterate, use of audiovisual aid in hospital as well as by mass media to spread foot care awareness among diabetes should be considered.
CONCLUSION
Foot at risk was present in 52% patients of diabetes attending our tertiary care center. Increasing duration of diabetes, lower educational, lower socioeconomic status and level of health care have significant correlation with foot at risk. Only 5% patients had previously received foot care education. None had received prescriptive footwear or vascular consultation. There are lacunae in standard practice to prevent foot ulcer and dissemination of diabetic foot care knowledge among patients. This needs to be addressed if we want to prevent foot ulcer. With diabetes rising to epidemic proportions and constraints of time and funds, cost effective steps to ensure foot care in diabetes needs to be taken if ulcers are to be prevented. Since demographic profile shows that 26% patients were uneducated, audio-visual aid to disseminate diabetic foot care education would be a cost effective option in our set-up.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–28. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med. 2014;31:1498–504. doi: 10.1111/dme.12545. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679–85. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavery LA, Ashry HR, van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care. 1996;19:48–52. doi: 10.2337/diacare.19.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DG, Lavery LA. Plantar pressures are higher in diabetic patients following partial foot amputation. Ostomy Wound Manage. 1998;44:30–2. 34, 36. [PubMed] [Google Scholar]
- 6.Peters EJ, Lavery LA International Working Group on the Diabetic FOot. Effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2001;24:1442–7. doi: 10.2337/diacare.24.8.1442. [DOI] [PubMed] [Google Scholar]
- 7.Mayfield JA, Sugarman JR. The use of the Semmes-Weinstein monofilament and other threshold tests for preventing foot ulceration and amputation in persons with diabetes. J Fam Pract. 2000;49:S17–29. [PubMed] [Google Scholar]
- 8.Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91:451–5. doi: 10.7547/87507315-91-9-451. [DOI] [PubMed] [Google Scholar]
- 9.Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North-West Diabetes Foot Care Study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377–84. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 10.Booth J, Young MJ. Differences in the performance of commercially available 10-g monofilaments. Diabetes Care. 2000;23:984–8. doi: 10.2337/diacare.23.7.984. [DOI] [PubMed] [Google Scholar]
- 11.Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: The Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202–7. doi: 10.2337/dc05-2031. [DOI] [PubMed] [Google Scholar]
- 12.Baraz S, Zarea K, Shahbazian HB, Latifi SM. Comparison of the accuracy of monofilament testing at various points of feet in peripheral diabetic neuropathy screening. J Diabetes Metab Disord. 2014;13:19. doi: 10.1186/2251-6581-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158:157–62. doi: 10.1001/archinte.158.2.157. [DOI] [PubMed] [Google Scholar]
- 14.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–21. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 16.Wu CK, Yang CY, Tsai CT, Chiu FC, Huang YT, Lee JK, et al. Association of low glomerular filtration rate and albuminuria with peripheral arterial disease: The National Health and Nutrition Examination Survey, 1999-2004. Atherosclerosis. 2010;209:230–4. doi: 10.1016/j.atherosclerosis.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 17.van Houtum WH. Amputations and ulceration; pitfalls in assessing incidence. Diabetes Metab Res Rev. 2008;24(Suppl 1):S14–8. doi: 10.1002/dmrr.826. [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK, Singh SK. Diabetic foot: A continuing challenge. Adv Exp Med Biol. 2012;771:123–38. [PubMed] [Google Scholar]
- 19.Jayaprakash P, Bhansali S, Bhansali A, Dutta P, Anantharaman R. Magnitude of foot problems in diabetes in the developing world: A study of 1044 patients. Diabet Med. 2009;26:939–42. doi: 10.1111/j.1464-5491.2009.02781.x. [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan V, Thomas N, Tandon N, Asirvatham A, Rajasekar S, Ramachandran A, et al. Profile of diabetic foot complications and its associated complications – A multicentric study from India. J Assoc Physicians India. 2005;53:933–6. [PubMed] [Google Scholar]


