Abstract
Background:
Undetected and untreated thyroid disorders are associated with adverse maternal and fetal outcomes. There are limited data on the prevalence of newly diagnosed thyroid disease during pregnancy from India. Therefore, this study was designed to evaluate the prevalence of thyroid dysfunction, especially hypothyroidism during the first trimester of pregnancy.
Materials and Methods:
The present cross-sectional study was conducted at Department of endocrinology and antenatal clinic in the Obstetrics and Gynecology Pt. B.D. Sharma PGIMS, Rohtak over a period of 1-year. The total sample population comprised of 461 pregnant women with uncomplicated intrauterine singleton pregnancies in the first trimester of gestation without any history of thyroid disease or intake of any thyroid medication. Morning blood samples from the participants were analyzed for thyroid function tests, which included FT3, FT4, thyroid-stimulating hormone (TSH) and anti-thyroid peroxidase antibodies (TPO).
Results:
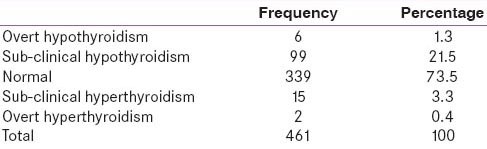
A total of 461 women were enrolled for this study. Mean maternal age was 23.79 ± 3.47 years. Median gestational age was 8 weeks 5 days. The median FT3, FT4 and TSH were 3.3 pg/mL, 1.25 ng/dL, and 1.40 mIU/L, respectively. Anti-TPO was elevated in 128 (27.8%) pregnant women. 99 (21.5%) women had sub-clinical hypothyroidism and 39 (39.4%) among them were positive for anti-TPO (P ≤ 0.001). 2 (0.4%) of women had overt hyperthyroidism, whereas 15 (3.3%) of the women had sub-clinical hyperthyroidism.
Conclusion:
Considering the immense impact that maternal thyroid dysfunction has on maternal and fetal outcomes, prompt identification of thyroid dysfunction and its timely treatment is essential. Thus, universal screening of pregnant women for thyroid dysfunction should be considered especially in a country like India due to the high prevalence of thyroid dysfunction.
Keywords: First trimester, pregnancy, thyroid dysfunction
INTRODUCTION
Evaluation of thyroid disease in pregnancy is important for gestational maternal health, obstetric outcome, and subsequent development of the child. The most frequent thyroid disorder in pregnancy is maternal hypothyroidism. It is associated with fetal loss, placental abruptions, preeclampsia, preterm delivery and reduced intellectual function in the offspring.[1] There is a wide geographic variation in prevalence of hypothyroidism during pregnancy. It varies from 2.5% from the west to 11% from India.[2,3,4,5,6,7,8,9,10] Prevalence of hypothyroidism was found to be more in Asian countries compared with the west. Before the onset of fetal thyroid function, that occurs about 12 weeks of gestation; the fetus is dependent on the placental transfer of maternal thyroid hormone for normal development.[11] Therefore, maternal hypothyroidism early in the pregnancy causes decreased availability of thyroid hormone during the initial phase of normal brain development and consequently is associated with increased rates of abortion and stillbirth, impaired neuropsychological development of fetus and congenital malformation and increase in perinatal mortality. Hyperthyroidism is much less common than hypothyroidism. It is seen in 0.5–2/1000 pregnancies and id remains untreated is associated with significantly higher frequency of obstetric complications such as preeclampsia, premature labor, low birth weight, fetal and perinatal loss. Sub-clinical hyperthyroidism (suppressed thyroid-stimulating hormone [TSH] alone) is seen in around 1.7% of pregnancies and is not associated with adverse outcomes.[10] Thus, prompt identification of thyroid disorder and timely initiation of therapy in pregnancy is essential. Therefore, the present study was carried out to study the prevalence of undetected thyroid dysfunction during the first trimester of pregnancy.
MATERIALS AND METHODS
The present cross-sectional study was conducted at Department of Endocrinology and antenatal clinic in the Obstetrics and Gynecology Department of Pt. B.D. Sharma PGIMS, Rohtak over a period of 1-year. The total sample population comprised of 461 pregnant women with uncomplicated intrauterine singleton pregnancies in the first trimester of gestation without any history of thyroid disease or intake of any thyroid medication. On the enrollment of participants, an informed written consent was taken, detailed history was enquired, participants were subjected to relevant general physical examination and findings were recorded on a predesigned proforma. 3 mL blood was sampled from the participants under aseptic conditions and analyzed for thyroid function tests, which included FT3, FT4, TSH and anti-thyroid peroxidase antibodies (TPO). Estimation for FT3, FT4 TSH, and anti-TPO was done using the electrochemiluminescence technique using commercially available kits by Advia Centaur CP analyzer system and immulite 1000. The analytical sensitivities for FT3, FT4, TSH and anti-TPO were 0.2 pg/mL, 0.1 ng/dL, 0.010 μIU/mL and 7 IU/mL respectively. Intra-assay coefficients of variation for FT3, FT4, TSH and anti-TPO were 3.8%, 2.20%, 5.2% and 5.6%, respectively. Laboratory reference range for FT3, FT4 and TSH were 2.3–4.2 pg/mL, 0.89–1.76 ng/dL and 0.35–5.5 mIU/L, respectively. Trimester specific cut-off values for TSH, that is, 0.1–2.5 mIU/L, 0.2–3.0 mIU/L and 0.3–3.0 mIU/L, respectively, in first, second and third trimester of pregnancy were used to diagnose hypothyroidism and hyperthyroidism.[11] Women with FT3, FT4 below the reference range along with elevated TSH were classified as having overt hypothyroidism while those having FT3, FT4 in normal range with TSH more than 2.5 mIU/L were diagnosed as having SCH. Women with FT3, FT4 above the reference range along with TSH value <0.1 mIU/L were classified as having overt hyperthyroidism while those having FT3, FT4 in normal range with TSH <0.1 mIU/L were diagnosed as having sub-clinical hyperthyroidism. Normal range for TPO antibody was <35 IU/mL and value greater than or equal to indicate elevated Anti-TPO in serum. Using these cut-offs thyroid dysfunction in pregnant women during the first trimester of pregnancy is shown in Table 1. The data were compiled and analyzed, using appropriate statistical tests in SPSS (Statistical Package for Social Sciences) version 21.
Table 1.
Thyroid dysfunction in the first trimester pregnant women (n=461)
RESULTS
A total of 461 women were enrolled for this study. Mean maternal age was 23.79 ± 3.47 years. Median gestational age was 8 weeks 5 days. 37.7% of the pregnant women enrolled in the study were primigravida and 62.3% were multigravida. Clinical examination showed goiter in 37 (8%) women. Anti-TPO was elevated in 128 (27.8%) pregnant women. History of thyroid illness in the family was present in 5 (1.08%) women. History of recurrent abortions (taken as more than and equal to three abortions) was present in 17 (3.68%) women. The median FT3, FT4, and TSH were 3.3 pg/mL, 1.25 ng/dL, and 1.40 mIU/L respectively. Table 1 shows thyroid dysfunction in pregnant first trimester women. Anti-TPO was elevated in 128 (27.8%) pregnant women and out of these 39 (30.5%), 6 (4.7%), were found to have sub-clinical hypothyroidism (SCH), hypothyroidism, respectively. 99 (21.5%) women had SCH and 39 (39.4%) among them were positive for Anti-TPO (P ≤ 0.001). 2 (0.4%) of women had overt hyperthyroidism while 15 (3.3%) of the women had sub-clinical hyperthyroidism.
DISCUSSION
The present study shows a high prevalence of undetected thyroid disorders during pregnancy during the first trimester of pregnant women. 99 (21.5%) women had SCH while 6 (1.3%) of women had hypothyroidism. 2 (0.4%) of women had overt hyperthyroidism while 15 (3.3%) of the women had sub-clinical hyperthyroidism. Prevalence of hypothyroidism was found to be more in Asian countries compared with west. In a large Chinese study, which included 2899 pregnant women, the prevalence of hypothyroidism was significantly higher in the high-risk group than in the nonhigh-risk group (10.9 vs. 7.0%, P = 0.008).[6] Dhanwal et al.[7] discovered that 14.3% women attending a tertiary public hospital in Delhi, India had hypothyroidism and majority of those women had SCH. Possible reasons for higher prevalence of hypothyroidism, both overt and sub-clinical, in Asian Countries include: increased iodine intake in diet as suggested by a Chinese study, presence of goitrogens in diet as reported from India and micronutrient deficiency such as selenium or iron deficiency that may cause hypothyroidism and goiter.[12,13,14] Thus, it is expected that the prevalence of hypothyroidism during pregnancy is higher in India and Asia. Moreover, prevalence of hypothyroidism in India is variable. Bandela et al.[8] from Andhra Pradesh reported 10% prevalence of SCH. Gayathri et al.[9] reported 2.8% prevalence of SCH. Possible reason for such variability could be the different upper limit cut-offs used for TSH.
Normal upper limit of TSH in pregnancy has been a subject of debate since a long time. In 2002, National Academy of Clinical Biochemistry (NACB) had laid down guidelines for the establishment of TSH reference intervals.[15] The guidelines said TSH reference intervals should be established from the 95% confidence limits of the log transformed values of at least 120 rigorously screened normal euthyroid volunteers with no detectable thyroid autoantibodies (TPOAb or TgAb), no personal or family history of thyroid dysfunction, no visible or palpable goiter, and no medications. NACB concluded that the upper limit of the serum TSH euthyroid reference range should be reduced to 2.5 mIU/L because >95% of rigorously screened normal euthyroid volunteers have serum TSH values between 0.4 and 2.5 mIU/L. This statement was supported by the 20 years follow-up study on Wickham cohort, which discovered increased risk of progression to overt hypothyroidism in individuals with serum TSH > 2.0 mIU/L, especially with positive TPO antibodies.[1] The recent Endocrine Society guidelines for thyroid dysfunction in pregnancy published in 2012 have again lowered the upper limit of reference range for normal TSH and suggested 0.1–2.5 mIU/L as the normal range for TSH values in the first trimester.[11] Using these recent trimester specific cut-offs for the diagnosis in the present study, we found a high prevalence (21.5%, 99/461) of SCH in first trimester pregnant women in contrast to various other studies from different parts of India where a higher cut-off using nonpregnant kit reference values had been used. Similar observations were made Dhnawal et al.[7] in their study where prevalence of SCH jumped to more than 50% using the first trimester specific TSH cut-off of 2.5 mIU/L rather than nonpregnant TSH cut-off of 4.5 mIU/L. Another possible reason for high prevalence of thyroid dysfunction in the present study is that we studied both high-risk individuals, that is, those with undiagnosed goiter, recurrent abortions, positive family history and low-risk individuals, that is, those without these risk factors together in this study. However, this universal screening is in line with observations made by Dave et al. who in their study regarding importance of universal screening for thyroid dysfunction in first trimester of pregnancy suggested that without adopting universal screening a large number of women with thyroid dysfunction will be missed.
Hyperthyroidism is much less common than hypothyroidism. The frequency of the disorder is relatively low, occurring in only 0.5–2/1000 pregnancies.[16] Untreated hyperthyroidism is associated with a significantly higher frequency of obstetric complications such as preeclampsia, premature labor, low birth weight, fetal and perinatal loss.[16] Mild or sub-clinical hyperthyroidism (suppressed TSH alone) is seen in 1.7% of pregnancies and is not associated with adverse outcomes. In the present study 15 (3.3%) and 2 (0.4%) of pregnant women were found to have newly diagnosed sub-clinical and overt hyperthyroidism, respectively. Possible reason for high prevalence of sub-clinical hyperthyroidism in our study population could be higher sensitivity of the thyroid gland to thyrotrophic molecules like human chorionic gonadotropin in our population leading to the gestational toxicosis. Price et al.[16] made similar observations in their study comparing thyroid function tests in Asian and western Caucasian pregnant nonpregnant women.
Prevalence of autoimmunity in euthyroid pregnant women was reported to be 10–15% in western literature.[17] According to data from the third National Health and Nutrition Examination Survey (NHANES-III) TPO positivity and anti-thyroglobulin antibodies were found in 12.6% and 13.6% of euthyroid women, respectively.[18] Anti-TPO were positive in 9.6% of 2899 first trimester pregnant Chinese women.[6] Dhanwal et al.[7] reported an anti-TPO prevalence of 6.82% in pregnant women. In the current study, anti-TPO was elevated in 128 (27.8%) women. Of these 39 (30.5%), 6 (4.7%), were found to have SCH, hypothyroidism, respectively. This was consistent with the findings of the study done by Marwah et al.[19] where median TSH was significantly higher among pregnant women with TPO antibody as compared to those with TPO negativity.
Considering the immense impact that maternal thyroid dysfunction has on maternal and fetal outcomes, prompt identification of thyroid dysfunction and timely initiation of treatment is essential. Thus, universal screening of pregnant women for thyroid dysfunction should be considered especially in a country like India due to the high prevalence of undiagnosed thyroid dysfunction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 2.Altomare M, La Vignera S, Asero P, Recupero D, Condorelli RA, Scollo P, et al. High prevalence of thyroid dysfunction in pregnant women. J Endocrinol Invest. 2013;36:407–11. doi: 10.3275/8658. [DOI] [PubMed] [Google Scholar]
- 3.Blatt AJ, Nakamoto JM, Kaufman HW. National status of testing for hypothyroidism during pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:777–84. doi: 10.1210/jc.2011-2038. [DOI] [PubMed] [Google Scholar]
- 4.Mosso L, Martínez A, Rojas MP, Margozzini P, Solari S, Lyng T, et al. Frequency of subclinical thyroid problems among women during the first trimester of pregnancy. Rev Med Chil. 2012;140:1401–8. doi: 10.4067/S0034-98872012001100004. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, et al. The prevalence of thyroid disorders during early pregnancy in China: The benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. 2011;164:263–8. doi: 10.1530/EJE-10-0660. [DOI] [PubMed] [Google Scholar]
- 6.Dhanwal DK, Prasad S, Agarwal AK, Dixit V, Banerjee AK. High prevalence of subclinical hypothyroidism during first trimester of pregnancy in North India. Indian J Endocrinol Metab. 2013;17:281–4. doi: 10.4103/2230-8210.109712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandela V, Havilah P, Hindumathi M, Prasad DK. Antenatal thyroid dysfunction in Rayalaseema region: A preliminary cross sectional study based on circulating serum thyrotropin levels. Int J Appl Biol Pharm Technol. 2013;4:74–8. [Google Scholar]
- 8.Gayathri R, Lavanya S, Raghavan K. Subclinical hypothyroidism and autoimmune thyroiditis in pregnancy – A study in south Indian subjects. J Assoc Physicians India. 2009;57:691–3. [PubMed] [Google Scholar]
- 9.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–52. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–41. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 11.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–65. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 12.Marwaha RK, Tandon N, Gupta N, Karak AK, Verma K, Kochupillai N. Residual goitre in the postiodization phase: Iodine status, thiocyanate exposure and autoimmunity. Clin Endocrinol (Oxf) 2003;59:672–81. doi: 10.1046/j.1365-2265.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 13.Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: A cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–50. doi: 10.1530/EJE-10-1041. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Bhansali A, Dutta P, Aggarwal A, Bansal MP, Garg D, et al. Persistence of goitre in the post-iodization phase: Micronutrient deficiency or thyroid autoimmunity? Indian J Med Res. 2011;133:103–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Demers LM, Spencer CA. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:33–44. doi: 10.1046/j.1365-2265.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 16.Price A, Obel O, Cresswell J, Catch I, Rutter S, Barik S, et al. Comparison of thyroid function in pregnant and non-pregnant Asian and western Caucasian women. Clin Chim Acta. 2001;308:91–8. doi: 10.1016/s0009-8981(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 17.Glinoer D, Rihai M, Grun JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab. 1994;79:197–204. doi: 10.1210/jcem.79.1.8027226. [DOI] [PubMed] [Google Scholar]
- 18.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T (4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 19.Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115:602–6. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]


