Abstract
Cannabinoid CB1 receptors (CB1Rs) mediate the presynaptic effects of endocannabinoids in the central nervous system (CNS) and most behavioral effects of exogenous cannabinoids. Cannabinoid receptor–interacting protein 1a (CRIP1a) binds to the CB1R C-terminus and can attenuate constitutive CB1R-mediated inhibition of Ca2+ channel activity. We now demonstrate cellular colocalization of CRIP1a at neuronal elements in the CNS and show that CRIP1a inhibits both constitutive and agonist-stimulated CB1R-mediated guanine nucleotide–binding regulatory protein (G-protein) activity. Stable overexpression of CRIP1a in human embryonic kidney (HEK)-293 cells stably expressing CB1Rs (CB1-HEK), or in N18TG2 cells endogenously expressing CB1Rs, decreased CB1R-mediated G-protein activation (measured by agonist-stimulated [35S]GTPγS (guanylyl-5′-[O-thio]-triphosphate) binding) in both cell lines and attenuated inverse agonism by rimonabant in CB1-HEK cells. Conversely, small-interfering RNA–mediated knockdown of CRIP1a in N18TG2 cells enhanced CB1R-mediated G-protein activation. These effects were not attributable to differences in CB1R expression or endocannabinoid tone because CB1R levels did not differ between cell lines varying in CRIP1a expression, and endocannabinoid levels were undetectable (CB1-HEK) or unchanged (N18TG2) by CRIP1a overexpression. In CB1-HEK cells, 4-hour pretreatment with cannabinoid agonists downregulated CB1Rs and desensitized agonist-stimulated [35S]GTPγS binding. CRIP1a overexpression attenuated CB1R downregulation without altering CB1R desensitization. Finally, in cultured autaptic hippocampal neurons, CRIP1a overexpression attenuated both depolarization-induced suppression of excitation and inhibition of excitatory synaptic activity induced by exogenous application of cannabinoid but not by adenosine A1 agonists. These results confirm that CRIP1a inhibits constitutive CB1R activity and demonstrate that CRIP1a can also inhibit agonist-stimulated CB1R signaling and downregulation of CB1Rs. Thus, CRIP1a appears to act as a broad negative regulator of CB1R function.
Introduction
Cannabinoid CB1 receptors (CB1Rs) mediate most central nervous system (CNS) effects of the phytocannabinoid Δ9-tetrahydrocannabinol (THC) and the endocannabinoids (Howlett et al., 2002). CB1Rs are guanine nucleotide–binding regulatory protein (G-protein)–coupled receptors (GPCRs) that primarily activate Gi/o proteins (Howlett et al., 2002) and are widely distributed throughout the CNS (Herkenham et al., 1991). CB1Rs mediate synaptic plasticity via inhibition of neurotransmitter release (Kano et al., 2009) and regulate memory/cognition, motor activity, motivation, anxiety, appetite, and energy balance (Howlett et al., 2002). Thus, in addition to mediating abuse-related effects of cannabinoids, CB1Rs are attractive, albeit challenging, targets for drug discovery for the treatment of multiple CNS disorders (Pacher et al., 2006). However, prolonged CB1R activation by direct agonists produces tolerance, dependence, perturbation of transcription factors, and CB1R adaptation (Smith et al., 2010; Lazenka et al., 2013). Therefore, there is a need to better understand the regulation of CB1R signaling.
CB1Rs also interact with regulatory proteins that modulate CB1R function and mediate downstream signaling (Howlett et al., 2010; Smith et al., 2010). These proteins include such ubiquitous GPCR regulators as GPCR kinase-3 (GRK3) and β-arrestin2, which mediate CB1R desensitization and intracellular trafficking (Jin et al., 1999; Nguyen et al., 2012). Proteins that interact with a limited subset of receptor types include GPCR-associated sorting protein-1 (GASP1) and AP-3, which mediate CB1R targeting to lysosomes (Martini et al., 2007; Rozenfeld and Devi, 2008). In addition, CB1Rs interact with specific cannabinoid receptor–interacting proteins CRIP1a and CRIP1b, which are not known to interact with any other GPCR (Niehaus et al., 2007).
CRIP1a/b interact with the last nine amino acids of the CB1R C-terminus, but not with the CB2R (Niehaus et al., 2007). Both CRIP1a/b proteins are encoded by the Cnrip1 gene, which contains four exons: 1, 2, 3a, and 3b. Alternative splicing produces transcripts comprising exons 1, 2, and 3a (CRIP1a) or 1, 2, and 3b (CRIP1b). CRIP1a homologs are found throughout vertebrates, whereas CRIP1b appears to be limited to primates (Niehaus et al., 2007). The search for CB1R C-terminal–interacting proteins was initiated because this region exhibited autoinhibition of constitutive (agonist-independent) CB1R activity, which was relieved by truncation of the distal C-terminus of the receptor (Nie and Lewis, 2001a,b). Indeed, electrophysiological recordings in superior cervical ganglion (SCG) neurons showed that expression of CRIP1a, but not CRIP1b, attenuated constitutive CB1-mediated inhibition of calcium channels, revealed by elimination of the inverse agonist activity of rimonabant (SR141716A). However, coexpression of CRIP1a and CB1Rs did not alter agonist-induced inhibition of calcium currents or CB1R expression levels (Niehaus et al., 2007), suggesting that CRIP1a inhibits constitutive CB1R activity.
CRIP1a is highly expressed in the brain (Niehaus et al., 2007), and some reports suggest that CRIP1a is regulated by seizure activity. Sclerotic hippocampi from epileptic patients exhibited reduced expression of mRNA for both CRIP1a and CB1R (Ludanyi et al., 2008). In contrast, CRIP1a mRNA was elevated in rat hippocampus and cortex following kainic acid–induced seizures (Bojnik et al., 2012). These findings suggest CRIP1a involvement in modulating CB1R function in the pathogenesis or neuroadaptive response to epilepsy. Furthermore, CRIP1a expression inhibited the neuroprotective effects of a cannabinoid agonist and conferred a neuroprotective effect on an antagonist, in a cultured neuronal model of glutamate excitotoxicity (Stauffer et al., 2011). To date, evidence supports functional interactions between CRIP1a and CB1R in striatal GABAergic medium spiny neurons (Blume et al., 2013), glutamatergic hippocampal neurons (Ludanyi et al., 2008), and retinal presynaptic terminals (Hu et al., 2010). In addition, the Cnrip1 gene is hypermethylated in certain colorectal cancers (Lind et al., 2011; Oster et al., 2011), further suggesting potentially important functions of CRIP1a in multiple physiologic systems.
Despite the potential significance of CRIP1a as a novel player in the endocannabinoid system, relatively little is known about its function. The present study determined the effects of CRIP1a on constitutive and agonist-stimulated G-protein activation in CB1R-expressing cells. Because CRIP1a binds to the CB1R C-terminus, which interacts with regulatory proteins that mediate CB1R desensitization and downregulation, the effects of CRIP1a on prolonged agonist-induced adaptation in CB1R expression and signaling were also examined. To examine colocalization of CRIP1a with CB1Rs in a defined neuronal population in the CNS, colabeling studies were conducted in the cerebellum because both proteins are highly expressed in this region (Herkenham et al., 1991; Niehaus et al., 2007) and it plays a major role in cannabinoid dependence (Tzavara et al., 2000). Finally, to investigate the effects of CRIP1a on endocannabinoid function, its influence on depolarization-induced suppression of excitation (DSE) was examined in autaptic hippocampal neurons.
Materials and Methods
Chemicals
[35S]GTPγS (guanylyl-5′-[O-thio]-triphosphate; 1150–1300 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). [3H]SR141716A (44.0 Ci/mmol) was purchased from GE Healthcare (Buckinghamshire, UK). WIN55,212-2 [[(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone] (dissolved in ethanol), GDP, pertussis toxin, phenylmethanesulfonyl fluoride, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). THC, CP55,940 [(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol], levonantradol [[(6S,6αR,9R,10αR)-9-hydroxy-6-methyl-3-[(2R)-5-phenylpentan-2-yl]oxy-5,6,6a,7,8,9,10,10α-octahydrophenanthridin-1-yl] acetate], HU-210 [3-(1,1′-dimethylheptyl)-6αR,7,10,10αR-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[β,δ]pyran-9-methanol], noladin ether, and SR141716A [5-(4-chlorophenyl)-1 (2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide] were provided as solutions in ethanol by the Drug Supply Program of the National Institute on Drug Abuse (NIDA, Rockville, MD). Methanandamide was purchased from Cayman Chemical (Ann Arbor, MI). LI-COR Odyssey infrared dye secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE). α-Tubulin antibody was purchased from Santa Cruz Biotechnology (Dallas, TX). All other reagent-grade chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Stable Transfection and Treatment of Cultured Cells.
Human embryonic kidney (HEK) 293 cells stably expressing the human CB1R subcloned into pcDNA3 vector (hCB1-HEK) (Abood et al., 1997) were cultured in Dulbecco’s modified Eagle’s medium (DMEM), 1× high glucose containing 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 100 μg/ml streptomycin (P/S), 0.25 mg/ml geneticin (G418), and 15 mM HEPES. hCB1-HEK cells stably cotransfected with CRIP1a subcloned into the pcDNA3.1zeo vector (hCB1-HEK-CRIP1a) (Niehaus et al., 2007) were cultured in the same media with the addition of 0.1 mg/ml zeocin.
Stable CRIP1a-overexpression and -knockdown N18TG2 cell clones were generated by transfecting (Lipofectamine 2000; Invitrogen, Carlsbad, CA) N18TG2 cells with either a pcDNA3.1-CRIP1a mouse cDNA plasmid for overexpression, or two different pRNATin-H1.2 small-interfering RNA (siRNA)-CRIP1a vectors for knockdown. The GenScript siRNA target finder program (GenScript, Piscataway, NJ) was used to select CRIP1a siRNA-target sequences. CRIP1a N18TG2 cell lines were generated by isolating and expanding G418-resistent single colonies in selection media containing 600 μg/ml G418 (Gibco/Life Technologies, Grand Island, NY). Cells were maintained in DMEM/F12 media with 10% heat-inactivated bovine serum, GlutaMax, and P/S, with 0.25 mg/ml geneticin.
For ligand pretreatments, appropriate concentrations of drugs were added to treatment media (DMEM, 1% FBS, P/S) and sterile-filtered, and drug treatment media was added to cells for the appropriate time period. To terminate drug treatments, cells were rinsed twice for 2 minutes with warm rinse media (DMEM, 1% FBS), and harvested for assays.
Membrane Homogenate Preparation
Cells were harvested in phosphate-buffered saline with 0.4% (w/v) EDTA or by gentle scraping and centrifuged at 1000g for 10 minutes to remove media. Cells were homogenized in ice-cold 50 mM Tris-HCl, 3 mM MgCl2, and 1 mM EGTA, pH 7.4 (membrane buffer), and centrifuged at 50,000g for 10 minutes. The resulting pellets were homogenized in 50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, pH 7.4 (TME buffer) with 100 mM NaCl, and protein content was determined.
Cerebella were obtained from adult male Sprague-Dawley rats (Harlan, Indianapolis, IN). Rats were sacrificed by rapid decapitation, brains were removed, and cerebella were dissected on ice. Cerebellum samples were homogenized in membrane buffer and membranes were isolated by centrifugation as described above. Experiments were performed with the approval of the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 7th Edition.
CRIP1a Generation, Purification, and Determination of Stoichiometry
A CRIP1a cDNA insert was subcloned into the BamHI and XhoI sites of the pGEX-4T-1 vector (GE Healthcare, Piscataway, NJ) to generate a glutathione S-transferase (GST)–tagged CRIP1a (GST tag–thrombin cleavage site–CRIP1a) construct. Plasmid DNA encoding GST-tagged CRIP1a was transformed into Escherichia coli BL21-DE3–competent cells. E. coli were grown to optical density 600 = 0.6 from a single colony and then GST-tagged CRIP1a expression was induced via addition of isopropyl thiogalactoside (1 mM) for 6 hours. E. coli were collected via centrifugation (1000g, 10 minutes, 4°C) and a bacterial lysate produced via sonication with lysozyme (25 μg/ml). CRIP1a induction and solubility tests were performed by polyacrylamide gel electrophoresis on harvested lysates using 10% polyacrylamide gels, which were stained with Coomassie blue to verify protein expression. Crude lysate was then separated into soluble and insoluble lysates. GST-tagged CRIP1a was isolated from bacterial lysate using a GSTrap FF column (Amersham Biosciences, Piscataway, NJ) as follows. The column was equilibrated with binding buffer [0.1 M phosphate buffered saline (PBS)], bacterial lysate was added to allow GST-CRIP binding, the column was washed (PBS), and the GST tag was cleaved via thrombin (500 units in 0.5 ml PBS). Following elution with PBS, CRIP1a was purified by the subsequent removal of thrombin using HiTrap benzamidine column purification. Briefly, the column was equilibrated with binding buffer (0.05 M Tris-HCl, 0.5 M NaCl, pH 7.4) and then the sample was added to the column followed by elution with binding buffer. CRIP1a eluates were collected and pooled, and CRIP1a pools and a BSA protein standard curve were subjected to polyacrylamide gel electrophoresis using 15% polyacrylamide gels, and visualized by Coomassie blue stain. Stained gel images were captured via ImageJ, and CRIP1a concentration was determined by subsequent linear regression analysis (Windows Excel). Purified CRIP1a concentration curves were then generated in tandem with hCB1-HEK (±CRIP1a) cell membrane preparations or rat cerebellar membranes to determine CRIP1a concentration via immunoblot analysis on 15% polyacrylamide gels. From these data, the stoichiometric relationship between CRIP1a concentration in cell membranes and CB1R levels, determined by [3H]SR141716A Bmax values, was calculated.
Immunoblotting
Samples (70 μg) of cell membrane homogenates or purified CRIP1a standards were added to sample buffer (1 M Tris-HCl, 20% SDS, 1 M dithiothreitol, 60% sucrose, bromophenol blue) and boiled for 10 minutes. Samples were loaded into 15% SDS polyacrylamide gels, and electrophoresis was conducted at 120 V for 1.5 hours. Proteins were transferred by electrophoresis onto polyvinylidene difluoride membranes at 70 V for 70 minutes. Blots were blocked for 1 hour at room temperature with 5% (w/v) nonfat dry milk and then rinsed with Tris-buffered saline with 0.1% (v/v) Tween-20 (TBST). Primary antibody (rabbit anti-CRIP1a antiserum 077.4; 1:500; Niehaus et al., 2007) was incubated overnight at 4°C, followed by TBST rinse. Secondary antibody (LI-COR goat anti-rabbit 800 CW IR dye, 1:5,000) was then incubated at room temperature for 1 hour, followed by TBST rinse. Blots were visualized with the LI-COR Odyssey system.
[3H]SR141716A Binding
Saturation analysis of [3H]SR141716A binding was performed by incubating 30 μg of membrane protein with 0.5–10 nM [3H]SR141716A in TME with 0.5% (w/v) BSA, in a total volume of 0.5 ml with and without 5 μM unlabeled SR141716A to determine nonspecific binding. The assay was incubated for 90 minutes at 30°C and terminated by vacuum filtration through GF/B glass fiber filters that were presoaked in Tris buffer containing 0.5% (w/v) BSA. Bound radioactivity was determined using liquid scintillation spectrophotometry at 45% efficiency for [3H].
[3H]CP55,940 Binding
Saturation analysis of [3H]CP55,940 binding was performed by incubating 100 μg of membrane protein with 0.2–8 nM [3H]CP55,940 in TME (without NaCl) with 0.5% (w/v) BSA, in a total volume of 0.5 ml with and without 5 μM unlabeled SR141716A to determine nonspecific binding. The assay was incubated for 90 minutes at 30°C and terminated by vacuum filtration through GF/B glass fiber filters that were presoaked in Tris buffer containing 0.5% (w/v) BSA. Bound radioactivity was determined using liquid scintillation spectrophotometry at 45% efficiency for [3H].
[35S]GTPγS Binding
Cell membrane preparations (10 μg of protein) were incubated with various drugs, 100 mM NaCl, 0.1% BSA, 10 μM (CB1-HEK), or 20 μM (N18TG2) GDP and 0.1 nM [35S]GTPγS in TME in 0.5-ml total volume, for 2 hours at 30°C. In some experimental conditions, 100 mM NaCl was omitted to increase constitutive receptor activity. Basal binding was assessed in the absence of agonist, and nonspecific binding was measured with 10-μM unlabeled GTPγS. The reaction was terminated by vacuum filtration through GF/B glass fiber filters. Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency for [35S].
Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry Analysis of Endocannabinoids
Arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) were measured using a method modified from Di Marzo et al. (2000). Briefly, 2 pmol of AEA-d8 and 2 nmol 2AG-d8 as deuterated internal standards were added to each sample. The endocannabinoids were extracted from the samples with 3 volumes chloroform/methanol (2:1, v/v containing 34.8 mg phenylmethanesulfonyl fluoride/ml) and a 0.73% (w/v) sodium chloride mixture. The organic phases from the three extractions were pooled and the organic solvents were evaporated to dryness with nitrogen. Dried samples were reconstituted in 100 μl of chloroform and mixed with 1 ml of cold acetone to precipitate proteins. The mixtures were centrifuged and the upper layers were collected and evaporated to dryness with nitrogen. The extracts were reconstituted with 100 μl of methanol and placed in autosample vials for liquid chromatography–electrospray ionization–tandem mass spectrometry analysis. The AEA and 2-AG were separated and detected using a Shimadzu SCL HPLC system (Kyoto, Japan) with a Discovery HS C18 Column 15 cm × 2.1 mm, 3 μm (Supelco/Sigma-Aldrich, Bellefonte, PA) kept at 40°C and an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray (Ontario, Canada) run in multiple reaction monitoring mode. The mobile phase consisted of 10:90 water/methanol with 0.1% (w/v) ammonium acetate and 0.1% (v/v) formic acid. The flow rate was 0.3 ml/min and total run time was 10.00 minutes. The injection volume was 20 μl and the autosampler temperature was set at 5°C. The mass spectrometer was run in electrospray ionization positive mode. Ions were analyzed in multiple reaction monitoring mode and the following transitions were monitored: (348 > 62) and (348 > 91) for AEA; (356 > 62) for AEA-d8; (379 > 287) and (379 > 269) for 2-AG; (387 > 96) for 2AG-d8. The standard curves for the samples were 0.039–1.25 pmol AEA and 0.06–2.0 nmol 2-AG. The limit of detection and limit of quantification were set at 0.039 pmol for AEA and 0.063 nmol for 2-AG.
Hippocampal Culture Preparation
All procedures used in this study were approved by the Animal Care Committee of Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Animals. Mouse (CD1 strain) hippocampal neurons isolated from the CA1–CA3 region were cultured on microislands as described previously (Furshpan et al., 1976; Bekkers and Stevens, 1991). Neurons were obtained from animals (age postnatal day 0–2) and plated onto a feeder layer of hippocampal astrocytes that had been laid down previously (Levison and McCarthy, 1991). Cultures were grown in high glucose (20 mM) DMEM containing 10% horse serum, without mitotic inhibitors and used for recordings after 8 days in culture and for no more than 3 hours after removal from culture medium.
Electrophysiology
When a single neuron is grown on a small island of permissive substrate, it forms synapses—or “autapses”—onto itself. All experiments were performed on isolated autaptic neurons. Whole cell voltage-clamp recordings from autaptic neurons were carried out at room temperature using an Axopatch 200A amplifier (Axon Instruments, Burlingame, CA). The extracellular solution contained (in mM) 119 NaCl, 5 KCl, 2.5 CaCl2, 1.5 MgCl2, 30 glucose, and 20 HEPES. Continuous flow of solution through the bath chamber (∼2 ml/min) ensured rapid drug application and clearance. Drugs were typically prepared as stocks, and then diluted into extracellular solution at their final concentration and used on the same day.
Recording pipettes of 1.8–3 MΩ were filled with (in mM) 121.5 potassium gluconate, 17.5 KCl, 9 NaCl, 1 MgCl2, 10 HEPES, 0.2 EGTA, 2 MgATP, and 0.5 LiGTP. Access resistance and holding current were monitored and only cells with both stable access resistance and holding current were included for data analysis. Conventional stimulus protocol: The membrane potential was held at –70 mV and excitatory postsynaptic currents (EPSCs) were evoked every 20 seconds by triggering an unclamped action current with a 1.0-millisecond depolarizing step. The resultant evoked waveform consisted of a brief stimulus artifact and a large downward spike representing inward sodium currents, followed by the slower EPSC. The size of the recorded EPSCs was calculated by integrating the evoked current to yield a charge value (in pC). Calculating the charge value in this manner yields an indirect measure of the amount of neurotransmitter released, at the same time minimizing the effects of cable distortion on currents generated far from the site of the recording electrode (the soma). Data were acquired at a sampling rate of 5 kHz.
DSE Stimuli.
After establishing a 10- to 20-second 0.5 Hz baseline, DSE was evoked by depolarizing to 0 mV for 50 milliseconds, 100 milliseconds, 300 milliseconds, 500 milliseconds, 1 second, 3 seconds, and 10 seconds, followed in each case by resumption of a 0.5-Hz stimulus protocol for 20–80+ seconds, allowing EPSCs to recover to baseline values. This approach allowed us to determine the sensitivity of the synapses to DSE induction.
Transfection of Autaptic Cultures
Neurons were transfected using a modified calcium phosphate–based method (Jiang et al., 2004). Briefly, plasmids for hemagglutinin (HA)-tagged CRIP1a and for the fluorescent marker mCherry (2 μg/well) were combined with 2 M CaCl2 and gradually added to HEPES-buffered saline; the mixture was added to the serum-free neuronal media. Coverslips were incubated with this mixture in a separate well for 2.5 hours and extra media was placed in a 10% CO2 incubator to induce equilibration. At the end of 2.5 hours, the reaction mixture was replaced with acidified serum-free media for 20 minutes. After this, cells were returned to their home wells.
Immunostaining of Autaptic Cultures
Autaptic neurons cultured on coverslips were transfected and prepared as described previously (Straiker et al., 2009). Briefly, paraformaldehyde-fixed neurons were incubated with an HA11 antibody overnight at 4°C and then washed six times with 0.1 M PBS. Cells were next incubated with fluorescein isothiocyanate–conjugated donkey secondary antibody (anti-mouse, 1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1.5 hours at room temperature. Finally coverslips were washed, dried, and mounted. Images were acquired with a Leica TCS SP5 confocal microscope (Leica Microsystems) using Leica LAS AF software and a 63× oil objective. Images were processed using ImageJ (available at http://rsbweb.nih.gov/ij/) and/or Photoshop (Adobe Inc., San Jose, CA).
Immunostaining of Rat Brain Sections
Tissue preparation and immunofluorescence labeling were conducted as described (Falenski et al., 2007) with minor modification. Adult male Sprague-Dawley rats (200–250 g) (Harlan) were housed on a 12-hour light/dark cycle in single cages and were provided with food and water ad libitum. Rats were injected with ketamine/xylazine (75 mg/kg, i.p.), flushed transcardially with saline, and perfused with 4% paraformaldehyde in 100 mM sodium phosphate buffer (pH 7.4). Brains were removed and postfixed in the same fixative overnight, and then placed in sodium phosphate buffer plus 30% sucrose for cryoprotection. Coronal sections of the cerebellum (20 μm) were cut on a cryostat maintained at –20°C and thaw-mounted onto gelatin-subbed slides. Sections were incubated for 30 minutes in PBS containing 0.1% Triton × 100 and then for 1 hour in SuperBlock Blocking Buffer (Pierce, Rockford, IL) with 0.1% Triton × 100. Sections were then incubated with rabbit anti-CRIP1a-Ct (antiserum 077.4, 1:1000) and guinea pig anti-CB1R-Ct (against CB1R residues 401–473, 1:1000) in SuperBlock Blocking Buffer for 72 hours, followed by incubation in appropriate secondary antibodies (CRIP1a, Alexa-488; CB1, Alexa-595; 1:200) for 1 hour. Slides were washed, and coverslips were mounted using Vectashield (Vector, Burlingame, CA) and sealed with clear nail polish. Images were captured at 60× magnification on a Zeiss 700 laser scanning confocal microscope.
For immunolabeling of CRIP1a with visualization by immunohistochemistry, brains were removed and postfixed in Bouin’s fixative for 3 days at room temperature before being embedded in paraffin wax. Coronal sections (10 μm) were cut and mounted on glass slides. Dewaxed sections were blocked with 5% normal goat serum/PBS with 0.2% Triton X-100 (PBST) and then incubated with 077.4 CRIP1a antiserum diluted 1:1000 in 5% normal goat serum in PBST. Bound antibodies were revealed by using the avidin-biotin complex, peroxidase method (Vector Laboratories). The specificity of immunostaining was established by testing antisera preabsorbed with the KPNETRSLMWVNKESFL peptide antigen (20 μM), which comprises the C-terminal region of the rat CRIP1a protein.
Immunostaining of Mouse Brain Sections
GAD67-GFP mice were generated by Dr. Yuchio Yanagawa [Gunman University, Gunma, Japan (Tamamaki et al., 2003)]. Brain sections were prepared from mice perfused with 4% paraformaldehyde. Brains were removed and immersed in 30% sucrose for 24–72 hours at 4°C. Tissue was then frozen in a freezing compound (Tissue-Tek O.C.T., VWR, Radnor, PA) and sectioned (15–30 μm) using a Leica CM1850 cryostat (Leica Microsystems). Tissue sections were mounted onto Superfrost Plus slides, washed in PBS, then treated with SEA BLOCK blocking buffer (Thermo Scientific, Rockford, IL). Cells were treated overnight at 4°C with antibodies prepared in PBS and detergent (saponin, 0.1%). Secondary antibodies (Alexa 488, 495, or 647, anti-mouse, anti-rabbit, or anti-guinea pig as appropriate; Invitrogen, Carlsbad, CA) were subsequently applied overnight at 4°C. Monoclonal synaptic vesicle 2 (SV2) and GAD65 antibodies were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA) and used at 1:500. The guinea pig CB1 and CRIP1a antibodies were developed in house and used at 1:300 and have been described previously (Berghuis et al., 2007; Hu et al., 2010). Images were acquired with a Leica TCS SP5 confocal microscope (Leica Microsystems) using Leica LAS AF software and a 63× oil objective. Images were processed using ImageJ (available at http://rsbweb.nih.gov/ij/) and/or Photoshop (Adobe Inc., San Jose, CA). Images were modified only in terms of brightness and contrast.
Data Analysis
Unless otherwise noted, all binding data are reported as mean values ± standard error of the mean (S.E.M.) of at least three independent experiments performed in duplicate ([3H]SR141716A and [3H]CP55,940) or triplicate ([35S]GTPγS). Data were analyzed using GraphPad/Prism v5.0 software (GraphPad Software, La Jolla, CA). Bmax, KD, Emax, and EC50 values were determined by nonlinear regression analysis. Nonlinear regression was used to fit the data to the following equation: B = (Bmax)(L)/(KD + L), where B is the amount of 3H-ligand bound at each ligand concentration L, Bmax is the maximal predicted amount of 3H-ligand bound, and KD is the equilibrium dissociation constant for 3H-ligand binding. Saturation curve-fitting analysis for [3H]SR141716A and [3H]CP55,940 were weighted by 1/x (1/3H-ligand concentration), because nonspecific binding is relatively high for these cannabinoid ligands and increases linearly with 3H-ligand concentration. For studies of G-protein activation, Emax and log EC50 were likewise determined from log concentration-effect curves, where E is the percentage change in [35S]GTPγS binding relative to basal binding at any given concentration of receptor ligand, Emax is the maximal percentage change from basal [35S]GTPγS binding observed at maximally effective concentrations of ligand, and log EC50 is the log10 of the molar concentration of receptor ligand producing half-maximal modulation of [35S]GTPγS binding. Statistical comparison was performed on log EC50 values, which were then transformed and reported as EC50 values. Basal [35S]GTPγS binding is determined in the absence of receptor ligand. Net stimulated [35S]GTPγS binding is defined as agonist-stimulated minus basal binding. Percentage stimulation is defined as (net stimulated binding/basal binding) × 100%.
Significance was determined using analysis of variance (ANOVA) and the posthoc Newman-Keuls multiple comparison test for comparison of three or more conditions or by Student’s t test for comparison of two conditions. In the few instances where unequal variance between groups was detected by F test, Welch’s correction for unequal variance was applied. Two-way ANOVA and the posthoc Bonferroni test were used in experiments comparing two or more sets of independent variables. Results were considered statistically significant when the P value was ≤0.05. All inferential statistics were performed using GraphPad Prism v5.0d software.
Results
Stoichiometry of CB1R and CRIP1a Expression in Stably Transfected HEK-293 Cells and Rat Cerebellum.
Niehaus et al. (2007) previously showed that CRIP1a localizes to the cell membrane and interacts with the C-terminal tail of CB1Rs without affecting CB1R expression levels. To confirm that stable coexpression of CRIP1a did not affect CB1R expression and to determine CRIP1a/CB1 expression ratios, CB1R Bmax values were obtained using [3H]SR141716A saturation binding analysis (Supplemental Fig. 1) in HEK-293 cells stably expressing CB1Rs (CB1-HEK) and the same CB1-HEK cell line was then stably transfected with CRIP1a (CB1-HEK-CRIP1a). Results showed no significant difference in CB1R expression as determined by [3H]SR141716A Bmax values between CB1-HEK cells with and without stable coexpression of CRIP1a (Table 1). Likewise, no difference in the KD value of [3H]SR141716A was observed between CB1-HEK and CB1-HEK-CRIP1a cell lines (Table 1). These results confirm that stable CRIP1a overexpression did not affect CB1R expression or affinity for [3H]SR141716A in CB1-HEK cells.
TABLE 1.
Stoichiometry of CRIP1a and CB1 receptor expression in CB1-HEK and CB1-HEK–CRIP1a cells compared with rat cerebellum
Membranes prepared from the indicated tissue sources were incubated with varying concentrations of [3H]SR141716A, as described in Materials and Methods. Bmax and KD values were derived from nonlinear regression analysis of the saturation binding curves. CRIP1a protein values were determined by quantitative immunoblot of the indicated tissue source, using purified CRIP1a as an internal standard, as described in Materials and Methods. Data are mean values ± S.E.M. (n = 4–6)
| Tissue Source | CB1 Bmax | CB1 KD | CRIP1a | Molar Ratio CRIP1a/CB1 |
|---|---|---|---|---|
| pmol/mg | nM | pmol/mg | ||
| CB1-HEK | 1.34 ± 0.12 | 1.45 ± 0.19 | 0.56 ± 0.13 | 0.42 ± 0.09 |
| CB1-HEK-CRIP1a | 1.17 ± 0.13 | 1.63 ± 0.35 | 8.20 ± 0.64** | 7.01 ± 0.55** |
| Rat cerebellum | 3.76 ± 0.31 | 0.49 ± 0.04 | 115 ± 12.2 | 32.02 ± 4.39 |
P < 0.01 different from corresponding value in CB1-HEK cells by Student’s t test with Welch’s correction (note: values from cerebellum were not included in the analysis).
The effect of CRIP1a on CB1R function can probably be determined in part by the molar ratio of CRIP1a to CB1R. To determine the stoichiometric relationship of CRIP1a to CB1R expression, quantitative immunoblot analysis of CRIP1a was performed. CRIP1a was purified using GST-pulldown methodology and CRIP1a concentration curves were included in immunoblots to determine unknown CRIP1a concentrations (Fig. 1A). The C-terminally directed CRIP1a antibody (Niehaus et al., 2007) produced relatively clean blots, with few or no extraneous labeling other than the 18-kDa band corresponding to CRIP1a (Supplemental Fig. 2). Experimentally determined CRIP1a concentrations were then compared with CB1R Bmax values to determine the molar stoichiometric relationship of CRIP1a/CB1Rs (Table 1). In CB1-HEK cells, the molar ratio of CRIP1a/CB1 was less than 1 (0.34 ± 0.08), indicating that the CB1R is in molar excess relative to CRIP1a natively expressed in CB1-HEK cells. In CB1-HEK-CRIP1a cells, CRIP1a was in molar excess to the CB1R, with a CRIP1a/CB1R ratio of 5.44 ± 0.42, which was significantly different from CB1-HEK cells. For comparison of expression ratios of CRIP1a/CB1R in a native tissue, [3H]SR141716A saturation analysis and quantitative immunoblotting of CRIP1a were also conducted in rat cerebellar membranes (Table 1). Interestingly, rat cerebellum had a CRIP1a/CB1R molar ratio of 32.02 ± 4.39, indicating a greater molar excess of CRIP1a relative to the CB1R than in the CB1-HEK-CRIP1a cells. These results demonstrate that membranes from CB1-HEK-CRIP1a cells express a significantly greater molar ratio of CRIP1a/CB1R than CB1-HEK cells, but not greater than the ratio obtained in membranes from rat cerebellar homogenates.
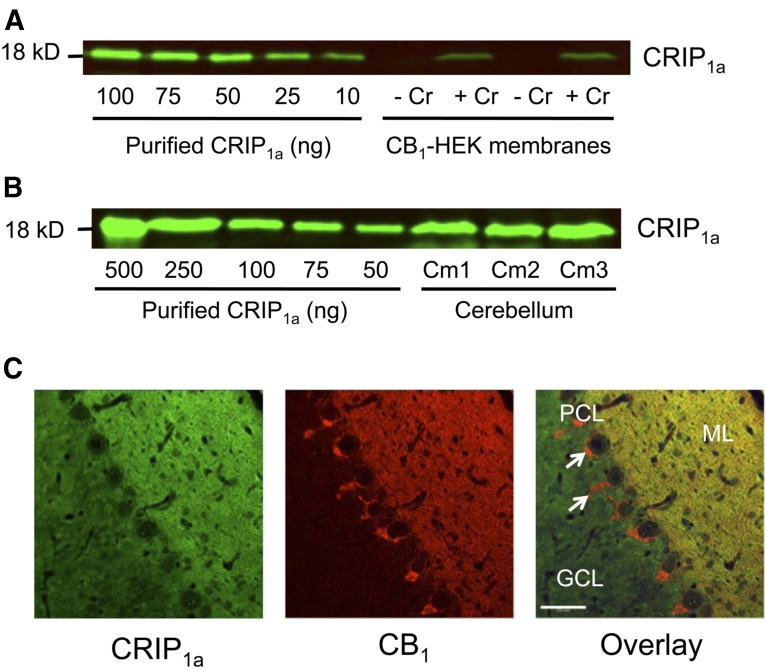
Fig. 1.
Quantitative CRIP1a immunoblots and immunohistochemical localization of CRIP1a in rat cerebellum. CRIP1a was immunologically identified by (A and B) immunoblotting or (C) immunofluorescence staining. Quantitative immunoblotting was conducted by comparison of a standard curve of purified CRIP1a with CRIP1a immunoreactivity in membrane homogenates from (A) CB1-HEK cells with and without stable cotransfection of CRIP1a or (B) rat cerebellum. (C) Immunofluorescence staining of CRIP1a (green) and CB1 receptor (red), or overlay (yellow) indicates areas of colocalization in rat cerebellum as determined by confocal microscopy. Note the characteristic scarcity of CB1R-ir in the granule cell layer (GCL) and colocalization of CB1R and CRIP1a-ir in the molecular layer (ML). Arrows indicate CB1R-ir in perisomatic basket cell axon terminals, which are devoid of CRIP1a-ir. PCL, Purkinje cell layer. Scale bar, 200 μm.
The stoichiometry result in rat cerebellum is complicated by the question of whether CRIP1a is colocalized in the same cells with CB1Rs in this tissue. Therefore, to determine whether CB1Rs and CRIP1a are colocalized in rat cerebellum, brain sections were colabeled with rabbit anti-CRIP1a (green) and guinea pig anti-CB1R-Ct (red) antibodies (Fig. 1C). Colocalization at this level of resolution of CRIP1a-immunoreactivity (ir) with CB1-ir is evident in the molecular layer. CRIP1a-ir is also evident at lower levels in the granule cell layer, where CB1-ir is seldom detected. In the Purkinje cell layer, intense CB1R-ir can be seen in putative axon terminals, possibly from basket cells that are presynaptic to the unstained somata of Purkinje cells; little or no CRIP1a-ir is evident in the axon terminals of basket cells. The widespread but heterogeneous distribution of CRIP1a among rat cerebellar layers was confirmed by immunohistochemical staining visualized with peroxidase labeling, which was blocked by coincubation with an antigen peptide corresponding to the C-terminal region of CRIP1a (Supplemental Fig. 3). These results indicate that in the cerebellum CRIP1a is putatively colocalized with CB1Rs in axonal fibers arising from the glutamatergic granule cells that project throughout the molecular layer, and the granule cell layer also contains CRIP1a at lower levels.
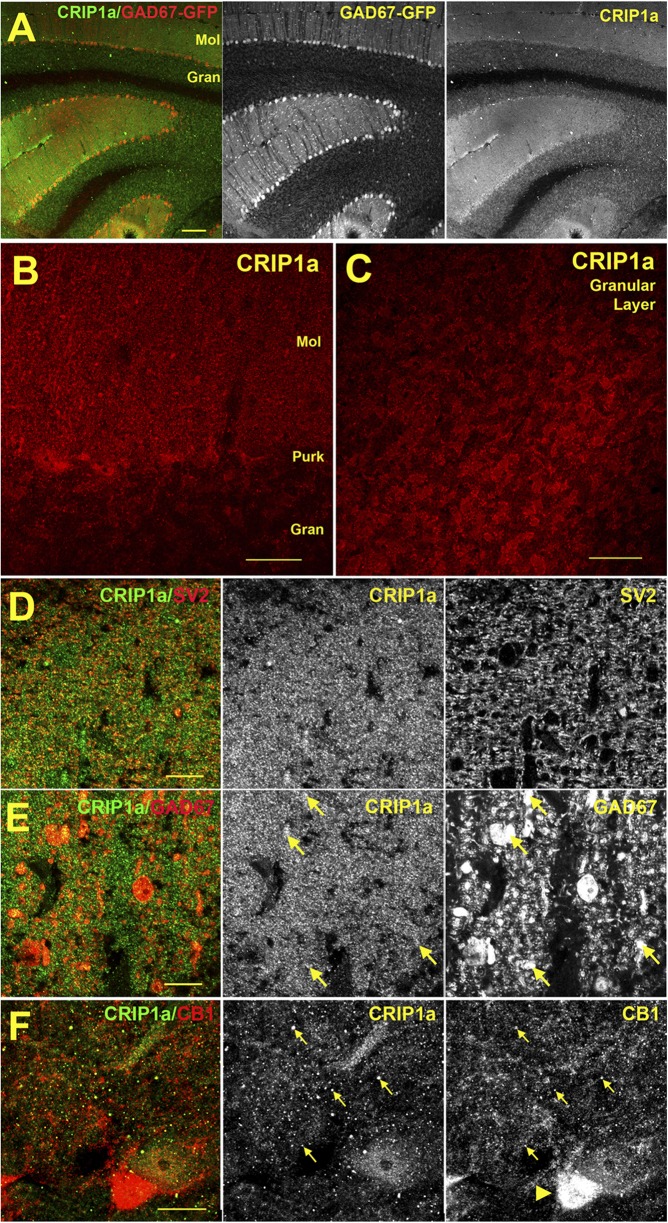
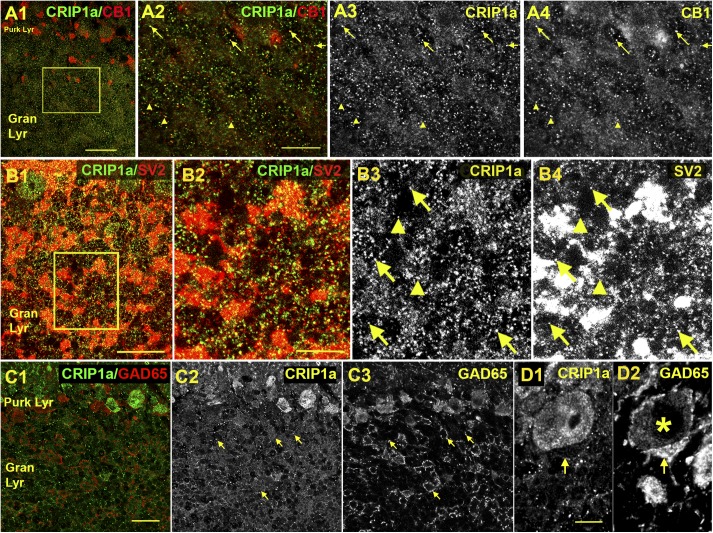
To determine the localization of CRIP1a and its colocalization with CB1Rs in specific cellular elements of the cerebellum, immunofluorescent labeling was performed in the GAD67-GFP mouse, which expresses green fluorescent protein in GABAergic neurons (Tamamaki et al., 2003), with costaining of multiple subcellular markers. Using an antibody developed against CRIP1a (Hu et al., 2010), we examined the distribution of this protein in multiple cerebellar subregions. CRIP1a is widely distributed in the cerebellum, abundant in both the molecular and granular layers (Fig. 2A-C), similar to what was detected in the rat using an independent antibody in Fig. 1. In the molecular layer the staining substantially overlaps with SV2, a presynaptic marker (Fig. 2D), consistent with a presynaptic localization. However, we also observed costaining with GAD67-GFP positive processes, perhaps belonging to Purkinje cells (Fig. 2E). CRIP1a was widely colocalized with CB1R throughout the molecular layer (Fig. 2F), but not in the pinceau region near Purkinje cells (e.g., arrowhead Fig. 2F) where the most intense CB1R expression is seen. Higher magnification images in the granule cell layer showed that CRIP1a is commonly colocalized with CB1Rs (Fig. 3A). Indeed most CRIP1a puncta overlapped with CB1R-ir, although there were some CB1R-positive puncta that were not positive for CRIP1a. The diffuse CRIP1a staining appeared to correspond to mossy terminal staining as shown by overlap with the brighter SV2 staining (Fig. 3B). However, the most punctate CRIP1a staining, seen when mossy terminal staining was allowed to saturate (Fig. 3, B2–B4), also partially overlapped with SV2. CRIP1a staining in the granule cell layer did not colocalize with GAD65-ir (Fig. 3C) or GAD67-GFP (data not shown), suggesting that expression is restricted to excitatory cells in the granule layer (Meyer et al., 2002). It is notable that CRIP1a is not detected in the pinceau region of basket cell inputs to the Purkinje neurons (Fig. 3D), an area that is associated with strong CB1R expression (e.g., Fig. 2F). These results indicate widespread colocalization of CRIP1a with CB1Rs in multiple cellular elements of the cerebellum, although CRIP1a and CB1R expression did not completely overlap in all cerebellar subregions examined.
Fig. 2.
Immunohistochemical localization of CRIP1a in the cerebellum of GAD67-GFP transgenic mice. (A) Overview of CRIP1a staining versus GAD67-GFP in murine cerebellum shows a broad distribution in both the molecular (Mol) and granular (Gran) layers. (B) CRIP1a distribution near Purkinje cells. (C) CRIP1a protein in granular layer. (D) CRIP1a overlaps substantially with presynaptic marker SV2 in the molecular layer. In the adjacent image (E), CRIP1a also partially overlaps with GAD67-GFP neurons (arrows), including probable Purkinje cell processes. (F) Sample of CB1R colocalization with CRIP1a (arrows) in the molecular layer near Purkinje cells. Purkinje pinceau region is dense with CB1 (arrowhead). Scale bars, (A) 100 μm; (B) 25 μm; (C) 30 μm; and (D–F) 10 μm.
Fig. 3.
Colocalization of CRIP1a with CB1R, SV2, GAD65, and parvalbumin in the murine cerebellar granule cell layer. (A) Staining of CRIP1a (green) versus CB1R (red) shows numerous points of overlap in the granular layer of the murine cerebellum (arrows). But there are also clear cases where CB1R expression does not overlap with CRIP1a (arrowheads). Even punctate CRIP1a staining that appears to be green is often accompanied by CB1R staining (e.g., left arrow). Note larger CB1R-positive structures correspond to pinceau staining. (B) CRIP1a (green) is commonly associated with the presynaptic marker SV2 (red), both in the bright staining corresponding to mossy terminals but also, if the image is allowed to saturate as in this case, with a subset of isolated puncta (arrows). However nonoverlap also occurs (arrowheads). (C) CRIP1a (green) does not overlap with GAD65 (red) staining (arrows). (D) CRIP1a is absent in the pinceau region as identified by GAD65 staining (D1). Purkinje cell is marked by an asterisk. Scale bars, (A1) 35 μm; (A2–A4) 15 μm ; (B1) 25 μm; (B2–4) 10 μm; (C) 20 μm; (D) 5 μm. Gran Lyr, granular layer; Purk Lyr, Purkinje layer.
Effects of CRIP1a on CB1R-Mediated G-Protein Activation in Stably Transfected HEK-293 Cells.
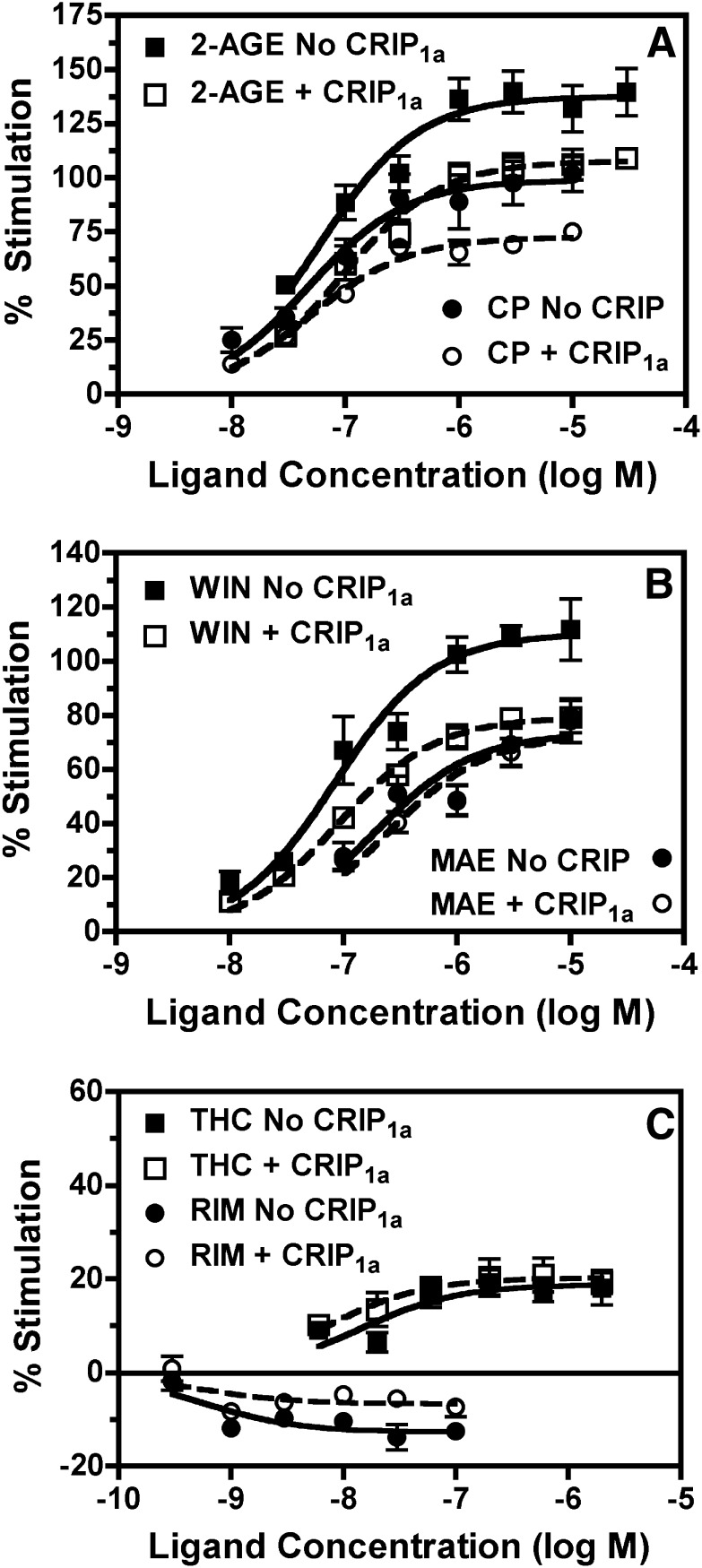
To determine the effects of CRIP1a on CB1R mediated G-protein activity, basal and ligand-modulated [35S]GTPγS binding was conducted in CB1-HEK (±CRIP1a) cells (Fig. 4). Concentration-effect curves for a variety of cannabinoid ligands were examined, including the classic phytocannabinoid THC, and its synthetic analogs HU210 and levonantradol; the aminoalkylindole WIN55,212-2; the bicyclic cannabinoid CP55,940; the eicosanoids noladin ether [2-arachidonoylglycerol ether (2-AGE)], a stable analog of 2-AG that is also a putative endocannabinoid, and methanandamide (MAEA), a stable analog of anandamide; and the diarylpyrazole inverse agonist rimonabant, also known as SR141716A. Nonlinear regression fitting of the concentration-effect curves revealed maximal stimulation (Emax) and EC50 values for each ligand (Table 2). Results in CB1-HEK cells showed that noladin ether, WIN55,212-2 and HU210 appeared to act as full agonists (Fig. 4; Table 2). CP55,212-2 acted as a high efficacy partial agonist relative to 2-AGE, and MAEA and levonantradol were moderate efficacy partial agonists. THC acted as a low efficacy partial agonist and rimonabant acted as an inverse agonist (Fig. 4; Table 2).
Fig. 4.
Effects of CRIP1a overexpression on concentration-effect curves of ligand-modulated [35S]GTPγS binding in CB1-HEK cell membranes. Membranes from CB1-HEK cells with (open symbols) and without (closed symbols) stable cotransfection of CRIP1a were incubated (as described in Materials and Methods) with 100 mM NaCl, 10 μM GDP, 0.1 nM [35S]GTPγS, and varying concentrations of the indicated ligands: (A) noladin ether (2-AGE) and CP55,940 (CP); (B) WIN55,212-2 (WIN) or methanandamide (MAE); or (C) ∆9-tetrahydrocannabinol or rimonabant (RIM). Data are mean percentage stimulation ± S.E.M. (n = 3–6). No CRIP1a, no transfection of CRIP1a; CRIP1a, stable transfection of CRIP1a.
TABLE 2.
Emax and EC50 values of ligand-modulated [35S]GTPγS binding in CB1-HEK and CB1-HEK–CRIP1a cells
Varying concentrations of the indicated ligands were incubated with membranes prepared from the indicated cell lines in the presence of 10 μM GDP and 0.1 nM [35S]GTPγS, as described in Materials and Methods. Emax and EC50 values were derived from nonlinear regression analysis of ligand concentration-effect curves. Data are mean values ± S.E.M. (n = 3–6). The P values in the rightmost column denote significance of the effect of CRIP1a overexpression, derived from two-way ANOVA (ligand concentration × cell line) of the concentration effect curves. Values <0.05 are considered significant. Significance of Emax and EC50 values between cell lines were determined by Student’s t test.
| CB1-HEK | CB1-HEK-CRIP1a | ||||
|---|---|---|---|---|---|
| Ligand |
Emax |
EC50 |
Emax |
EC50
|
P value |
| % Stim | nM | % Stim | nM | ||
| 2-AGE | 137.7 ± 9.7a | 59.9 ± 3.8 | 108.2 ± 3.7a,* | 104 ± 16 | <0.0001 |
| HU210 | 114.6 ± 5.9a,b | 0.04 ± 0.01 | 96.0 ± 6.1b,§ | 0.06 ± 0.01 | <0.0001 |
| WIN55,212-2 | 112.7 ± 8.8a,b,c | 110 ± 41 | 79.5 ± 1.7c,* | 90.6 ± 6.2 | <0.0001 |
| CP55,940 | 100.0 ± 8.7b,c | 48.8 ± 2.2 | 72.0 ± 2.7c,* | 51.4 ± 6.3 | <0.0001 |
| MAEA | 73.8 ± 6.1c | 202 ± 37 | 73.0 ± 6.0c | 255 ± 40 | 0.4928 |
| Levonantradol | 73.7 ± 11.3c | 34.2 ± 8.6 | 72.8 ± 0.5c | 19.0 ± 5.2 | 0.2360 |
| THC | 19.6 ± 2.7d | 11.5 ± 2.4 | 20.4 ± 3.1d | 8.1 ± 1.2 | 0.1740 |
| Rimonabant | −12.9 ± 1.5 | 1.0 ± 0.4 | −6.5 ± 1.5* | 0.5 ± 0.1 | 0.0002 |
P < 0.05 different from CB1-HEK cells. §P = 0.05 different from CB1-HEK cells. a–dSignificance of Emax and EC50 values between cell lines were determined by Student's t test. Significant differences between ligand Emax values within each cell line are denoted as follows: ligands without any similar letter designations are P < 0.05 different from each other as determined by one-way ANOVA with posthoc Newman-Keuls test.
In agreement with our previous findings (Niehaus et al., 2007), stable CRIP1a expression reduced the apparent inverse agonism of rimonabant compared with CB1-HEK cells without CRIP1a transfection (Fig. 4), as indicated by a main effect of cell line in two-way ANOVA (cell line × ligand concentration) of the concentration-effect curves (Table 2). This reduction in inverse agonism was attributable to a lesser maximal inhibition of basal G-protein activation by rimonabant in CB1-HEK-CRIP1a than in CB1-HEK cells (Table 2). However, [35S]GTPγS binding measured in the absence of cannabinoid ligand (basal) did not differ between cell types. Basal binding in CB1-HEK cells was 89.8 ± 6.1 fmol/mg and in CB1-HEK-CRIP1a cells was 86.4 ± 5.5 fmol/mg. Stable expression of CRIP1a also reduced stimulation of G-protein activation by the high efficacy agonists 2-AGE, WIN55,212-2, HU210, and CP55,940 (Fig. 4; Table 2), as indicated by an effect of cell line in two-way ANOVA. This decrease in agonist-stimulated activity was attributable to a reduction in maximal stimulation (Emax) in CB1-HEK-CRIP1a relative to CB1-HEK cells, without any significant differences in EC50 values between the cell lines (Table 2).
Interestingly, concentration-effect curves for G-protein activation by the partial agonists MAEA, levonantradol, and THC were unaffected by CRIP1a overexpression (Fig. 4; Table 2). There was no significant effect of cell line in the presence of any of these partial agonists, and neither the Emax or EC50 values of these ligands differed between CB1-HEK and CB1-HEK-CRIP1a cells. Because of the differential effect of CRIP1a on G-protein activation by ligands of different intrinsic efficacies, the relative efficacy relationship among some ligands differed between cell types. In CB1-HEK cells the order of descending relative efficacy, on the basis of Emax values, was 2-AGE ≥ HU210 = WIN55,212-2 ≥ CP55,940 ≥ MAEA = levonantradol > THC > > rimonabant, whereas in CB1-HEK-CRIP1a cells it was 2-AGE > HU210 > WIN55,212-2 = CP55,940 = MAEA = levonantradol > THC > > rimonabant.
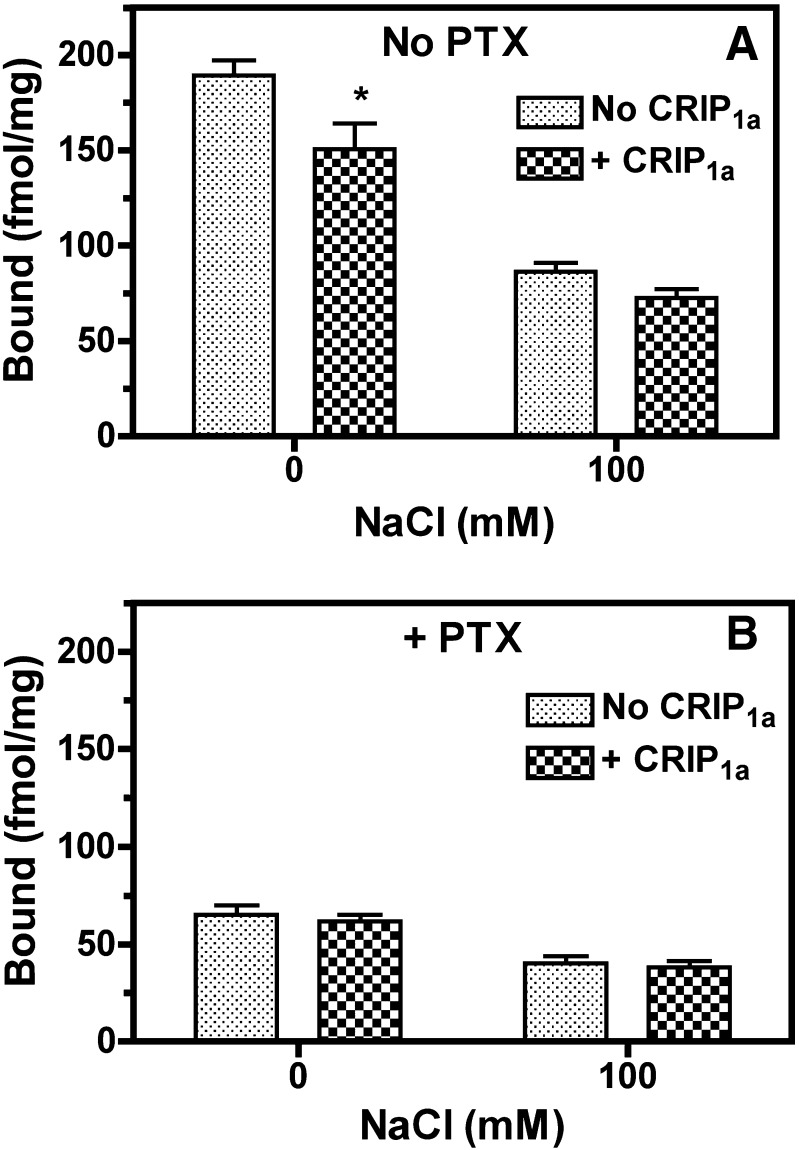
The results described above indicate that stable CRIP1a expression in CB1-HEK-CRIP1a cells did not inhibit basal [35S]GTPγS binding or [3H]SR141716A binding. However, CRIP1a inhibited the inverse agonist effects of rimonabant under conditions of high [Na+], which reduces constitutive GPCR activity (Seifert and Wenzel-Seifert, 2002). We therefore determined whether CRIP1a inhibits constitutive receptor activity that is unrestricted by sodium. Results showed that 100 mM NaCl reduced basal [35S]GTPγS binding in both CB1-HEK and CB1-HEK-CRIP1a cells by >50% relative to the absence of Na+ (Fig. 5A), as confirmed by two-way ANOVA (effect of Na+, P < 0.0001). To distinguish receptor-mediated from receptor-independent G-protein activity, cells were pretreated with or without pertussis toxin (PTX), which uncouples Gi/o-proteins from receptor-stimulated guanine nucleotide exchange (Sunyer et al., 1989). Na+ also inhibited basal [35S]GTPγS binding by 38% in both cell lines after treatment with PTX (effect of sodium, P < 0.0001), but the effect was diminished relative to untreated cells (Fig. 5B). Importantly, CRIP1a inhibited basal [35S]GTPγS binding in untreated cells (effect of CRIP1a, P = 0.009), but not in cells pretreated with PTX (no effect of CRIP1a, P = 0.496). The inhibitory effect of CRIP1a on basal [35S]GTPγS binding was significant only in untreated cells in the absence of Na+, as confirmed by Bonferroni posthoc analysis. These results indicate that CRIP1a inhibits spontaneous receptor-mediated G-protein activity, particularly in the absence of sodium, but does not affect receptor-independent [35S]GTPγS binding.
Fig. 5.
CRIP1a overexpression in CB1-HEK cells suppressed basal [35S]GTPγS binding in the absence of sodium in a PTX-sensitive manner. Membranes from CB1-HEK cells with and without stable cotransfection of CRIP1a were pretreated for 24 hours with and without 50 ng/ml PTX and were then incubated with 10 μM GDP and 0.1 nM [35S]GTPγS in the presence and absence of 100 mM NaCl (as described in Materials and Methods). Data are mean fmol/mg bound ± S.E.M. (n = 4). *P < 0.05 different from cells without CRIP1a cotransfection under the corresponding conditions, as determined by two-way ANOVA with Bonferroni posthoc test. No CRIP1a, no transfection of CRIP1a; CRIP1a, stable transfection of CRIP1a.
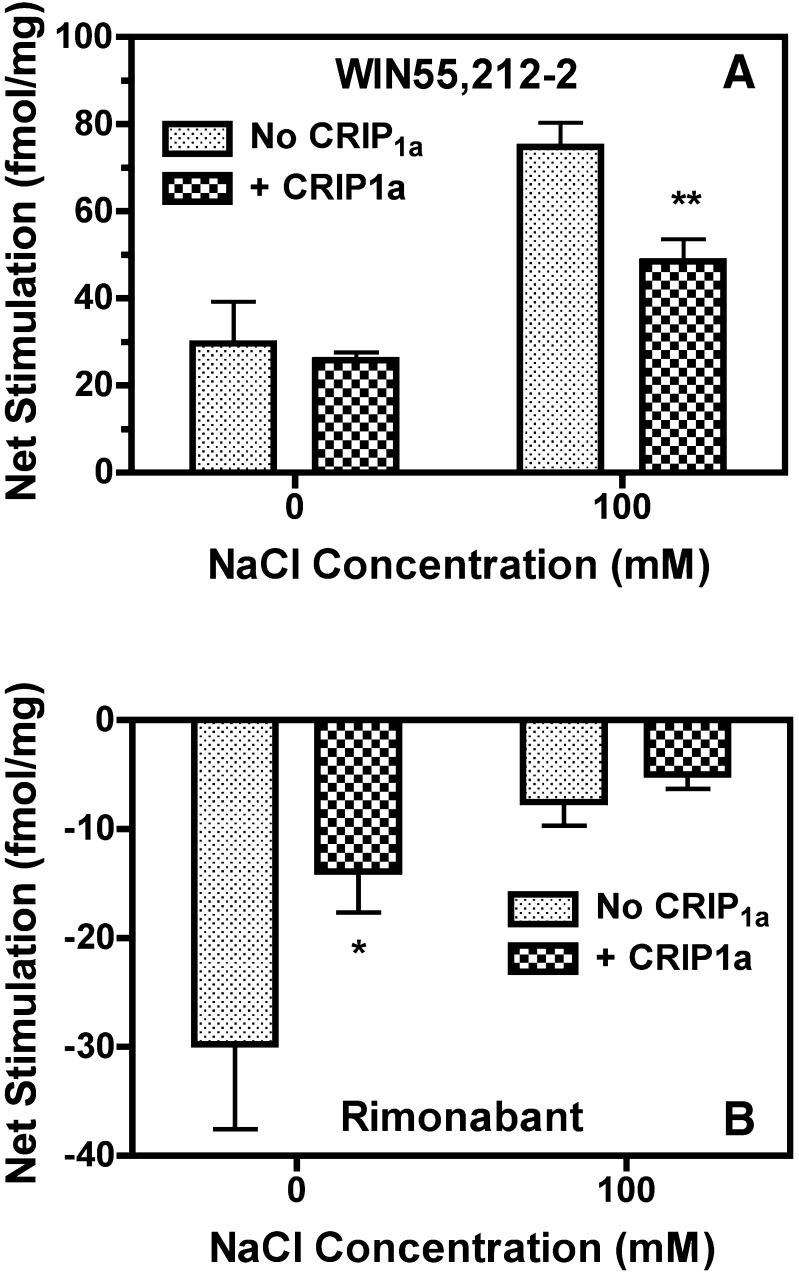
To determine the effects of Na+ on CB1R ligand–modulated G-protein activity, net [35S]GTPγS binding was determined in the presence of a maximally effective concentration of WIN55,212-2 or rimonabant with and without 100 mM NaCl in CB1-HEK and CB1-HEK-CRIP1a cells. Na+ enhanced net stimulated [35S]GTPγS binding by WIN55,212-2 (effect of Na+, P < 0.0001), and there was a significant interaction between Na+ and CRIP1a (P = 0.0346), as indicated by two-way ANOVA (Fig. 6A). In the absence of Na+, WIN55,212-2 had no effect on [35S]GTPγS binding. In contrast, WIN55,212-2 stimulated [35S]GTPγS binding in the presence of 100 mM Na+ (P < 0.05 by Bonferroni posthoc test). The opposite effect of Na+ was seen with rimonabant (Fig. 6B). There was a significant effect of both Na+ (P = 0.0024) and CRIP1a (P = 0.0466) on net rimonabant-inhibited [35S]GTPγS binding by two-way ANOVA. However, posthoc analysis revealed that rimonabant only significantly inhibited [35S]GTPγS binding in the absence of Na+ (P < 0.05 by Bonferroni test). These results indicate that CRIP1a maximally attenuates agonist-stimulated [35S]GTPγS binding in the presence of high [Na+] and maximally attenuates inverse agonist-inhibited [35S]GTPγS binding in the absence of Na+, suggesting that CRIP1a attenuates both agonist-stimulated and constitutive CB1R-mediated G-protein activation in a manner consistent with the effects of Na+ on basal receptor activity seen in Fig. 5.
Fig. 6.
CRIP1a overexpression in CB1-HEK cells affects net ligand-modulated [35S]GTPγS binding in a sodium-dependent manner. Membranes from CB1-HEK cells with and without stable cotransfection of CRIP1a were incubated with 10 μM GDP and 0.1 nM [35S]GTPγS in the presence and absence of 100 mM NaCl (as described in Materials and Methods). Data are mean fmol/mg bound ± S.E.M. (n = 4–5). *P < 0.05, **P < 0.01 different from cells without CRIP1a cotransfection under the corresponding conditions, as determined by two-way ANOVA with Bonferroni posthoc test. No CRIP1a, no transfection of CRIP1a; CRIP1a, stable transfection of CRIP1a.
A potential confounding factor in the interpretation of the effects of CRIP1a on basal CB1R-mediated G-protein activity is the possible presence of endocannabinoids in the membrane preparation. To determine whether endocannabinoids could have contributed to basal G-protein activity, mass spectrometric analysis of CB1-HEK and CB1-HEK-CRIP1a cells and isolated cell membranes was performed to quantify the two major endocannabinoids, 2-AG and AEA. However, endocannabinoid levels were below the limit of detection for both 2-AG (>0.063 pmol) and AEA (>0.039 nmol) relative to deuterated standards, in extracts of both intact cells and isolated membranes, as determined in analysis of three independent samples. These results indicate that endocannabinoids are unlikely to have contributed to apparent basal CB1R-mediated G-protein activity in either cell line.
Effect of CRIP1a on CB1R Desensitization and Downregulation in Stably Transfected HEK-293 Cells.
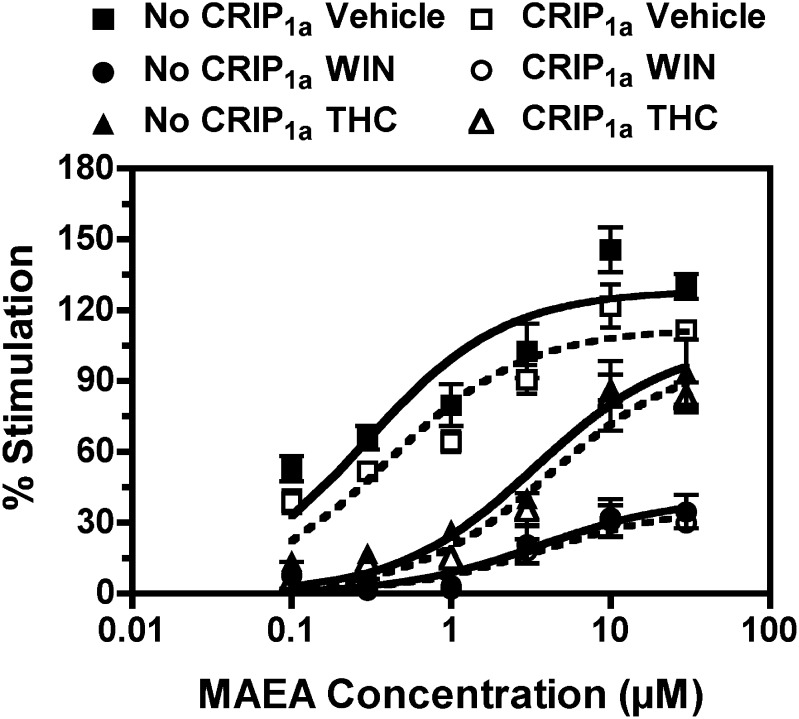
To determine whether CRIP1a affected the regulation of CB1R function by prolonged agonist occupancy, CB1-HEK cells with and without stable CRIP1a cotransfection were pretreated with WIN55,212-2 (10 μM), THC (6 μM), or vehicle for 4 hours, followed by MAEA-stimulated [35S]GTPγS binding to assess CB1R function. MAEA was used to assess CB1R activation after prolonged ligand pretreatment because acute stimulation of [35S]GTPγS binding by this ligand was unaffected by CRIP1a (Fig. 2B; Table 2). Pretreatment of the cells with WIN55,212-2 or THC decreased CB1R-mediated G-protein activation in membranes prepared from either cell line (Fig. 7). Significantly lower MAEA Emax values were seen in WIN55,212-2–pretreated compared with vehicle-pretreated cells with or without CRIP1a cotransfection (Table 3). In contrast, THC pretreatment did not affect MAEA Emax values in either cell line. However, pretreatment with either drug significantly increased MAEA EC50 values (Table 3). Thus, pretreatment with either WIN55,212-2 or THC apparently desensitized MAEA-stimulated G-protein activity in both cell lines. However, there were no apparent differences in the level of desensitization produced by either ligand between CB1-HEK cells with and without stable CRIP1a cotransfection. Indeed, two-way ANOVA of Emax values indicated a significant effect of drug pretreatment (P < 0.0001), but there was no effect of CRIP1a (P = 0.306). Likewise, two-way ANOVA of EC50 values revealed a significant effect of drug pretreatment (P = 0.0036), but there was no effect of CRIP1a (P = 0.848). Subsequent one-way ANOVA with posthoc Newman-Keuls multiple comparison test revealed that neither Emax nor EC50 values of MAEA differed significantly between CB1-HEK cells with and without stable CRIP1a cotransfection after WIN55,212-2 or THC pretreatment. These results indicate that CRIP1a did not affect cannabinoid-induced CB1R desensitization under these conditions.
Fig. 7.
CRIP1a does not affect ligand-induced desensitization of CB1R-mediated G-protein activation in CB1-HEK cells. Cells were pretreated for 4 hours with 10 μM WIN55,212-2 (WIN), 6 μM THC, or vehicle prior to harvesting and preparation of membranes. Varying concentrations of MAEA were incubated with membranes prepared from the indicated cell lines in the presence of 100 mM NaCl, 10 μM GDP, and 0.1 nM [35S]GTPγS, as described in Materials and Methods. Data are mean percentage stimulation ± S.E.M. (n = 4). No CRIP1a, no transfection of CRIP1a; CRIP1a, stable transfection of CRIP1a.
TABLE 3.
Curve-fit values of MAEA-stimulated [35S]GTPγS and [3H]SR141716A binding in CB1-HEK and CB1-HEK-CRIP1a cells pretreated with vehicle, WIN55,212-2, or THC
Cells were pretreated for 4 hours with 10 μM WIN55,212-2, 6 μM THC, or vehicle. Varying concentrations of MAEA were incubated with membranes prepared from the indicated cell lines in the presence of 10 μM GDP and 0.1 nM [35S]GTPγS, as described in Materials and Methods. Varying concentrations of [3H]SR141716A were incubated with membranes prepared from the indicated cell lines, as described in Materials and Methods. Emax, EC50, Bmax, and KD values were derived from nonlinear regression analysis of the concentration-effect or saturation binding curves. Data are mean values ± S.E.M. (n = 4–5).
| Cell Line Pretreatment |
|||
|---|---|---|---|
| Vehicle | WIN55,212-2 | THC | |
| MAEA-stimulated [35S]GTPγS binding | |||
| CB1-HEK | |||
| Emax (% Stim) | 128.5 ± 8.0 | 42.7 ± 6.9** | 100.5 ± 8.5 |
| EC50 (nM) | 308 ± 71 | 5412 ± 1870§ | 4609 ± 2137§ |
| CB1-HEK-CRIP1a | |||
| Emax (% Stim) | 112.6 ± 5.1 | 36.9 ± 4.5** | 104.3 ± 7.3 |
| EC50 (nM) | 423 ± 82 | 4112 ± 633§§ | 5175 ± 1316§§ |
| [3H]SR141716A binding | |||
| CB1-HEK | |||
| Bmax (pmol/mg) | 1.19 ± 0.11 | 0.56 ± 0.10** | 0.31 ± 0.04** |
| KD (nM) | 1.78 ± 0.23 | 1.37 ± 0.34 | 1.78 ± 0.59 |
| CB1-HEK-CRIP1a | |||
| Bmax (pmol/mg) | 0.97 ± 0.15 | 0.86 ± 0.14 | 0.62 ± 0.12 |
| KD (nM) | 1.72 ± 0.46 | 2.18 ± 0.69 | 2.40 ± 1.07 |
P < 0.01, different from vehicle-treated cells of the same type, as determined by one-way ANOVA with posthoc Dunnett’s test; §P = 0.05, §§P < 0.01 different from vehicle-treated cells of the same type, as determined by one-way ANOVA of square root transformed data with posthoc Dunnett’s test.
Basal [35S]GTPγS binding was not significantly affected by ligand pretreatment in either cell line (Supplemental Fig. 4). Basal [35S]GTPγS binding in vehicle-pretreated cells was 48.9 ± 9.2 and 43.9 ± 4.2 fmol/mg in cells without and with CRIP1a coexpression, respectively. There was no significant effect of pretreatment (P = 0.584) or CRIP1a expression (P = 0.547) with regard to basal [35S]GTPγS binding, according to two-way ANOVA. Likewise, one-way ANOVA revealed no significant effect of pretreatment in either cell line. These results indicate that the pretreatment ligand was sufficiently removed prior to assay, because residual agonist that might have remained in the membrane preparation from pretreated cells would be predicted to elevate the apparent “basal” level of [35S]GTPγS binding.
In contrast, CRIP1a overexpression attenuated cannabinoid ligand–induced CB1R downregulation, as determined using the identical pretreatment protocol that was used to examine effects on G-protein activation. Two-way ANOVA of [3H]SR141716A Bmax values revealed significant effects of ligand pretreatment (P = 0.0001) but no effect of CRIP1a expression (P = 0.199). However, there was a trend toward an interaction between these two factors (P = 0.06). With regard to [3H]SR141716A KD values, there was no effect of ligand pretreatment (P = 0.812) or CRIP1a expression (P = 0.345), nor was there an interaction (P = 0.736). In CB1-HEK cells, pretreatment with either 10 μM WIN55,212-2 or 6 μM THC decreased the Bmax value of [3H]SR141716A binding by 53 and 74%, respectively (Table 3), as determined by one-way ANOVA with posthoc Dunnett’s test. However, analysis of Bmax values in CB1-HEK-CRIP1a cells showed no significant effect of ligand pretreatment by one-way ANOVA (P = 0.274). Likewise, one-way ANOVA revealed no significant effects of pretreatment on KD values in either CB1-HEK (P = 0.734) or CB1-HEK-CRIP1a (P = 0.798) cells. These results indicate that pretreatment with either WIN55,212-2 or THC significantly downregulated CB1Rs in CB1-HEK cells, but not in CB1-HEK-CRIP1a cells. Moreover, the lack of effect of ligand pretreatment on [3H]SR141716A KD values in either cell line further indicates that the effects of these pretreatments on CB1R levels or activation of G-proteins were not attributable to insufficient removal of the pretreatment ligand prior to assay.
Effects of CRIP1a on CB1R-Mediated G-Protein Activation in Stably Transfected N18TG2 Cells.
Results in CB1-HEK cells with and without stable cotransfection of CRIP1a indicated that CRIP1a negatively modulates constitutive and high efficacy agonist-stimulated G-protein activation by CB1Rs. To determine whether similar effects could be demonstrated in a neural cell type, mouse neuroblastoma N18TG2 cells, which endogenously express CB1Rs, were stably transfected with CRIP1a. In addition, because N18TG2 cells endogenously express CRIP1a at moderate levels that are in excess of CB1R levels (Supplemental Fig. 5, Table 4), cell lines with stable transfection of siRNA against CRIP1a were also generated. Two cloned cell lines (OX1 and OX5) were isolated that stably overexpressed CRIP1a mRNA without any alteration in CB1R mRNA (Supplemental Fig. 5B). CRIP1a clones expressed 8:1 and 7:1 (CRIP1a/CB1R) cDNA ratios, respectively, as compared with a 1:7 ratio in untransfected N18TG2 cells (determined by real-time polymerase chain reaction using eno2 as a standard; Supplemental Fig. 5A). Comparative immunoblots also indicated greater relative expression of CRIP1a protein in CRIP1a-overexpressing clones compared with untransfected N18TG2 cells (Supplemental Fig. 5C). Likewise, two clones (KD2C and KD2F) were isolated that exhibited siRNA-mediated knockdown of CRIP1a mRNA relative to untransfected N18TG2 cells, whereas cells transfected with empty siRNA vector did not exhibit CRIP1a knockdown (Supplemental Fig. 5A). Immunoblot analysis showed that CRIP1a protein levels were reduced by 50–60% in siRNA knockdown clones relative to untransfected or vector control–transfected N18TG2 cells (Supplemental Fig. 5C), but CB1R protein levels did not differ between any of these cell models (Supplemental Fig. 5D).
TABLE 4.
Stoichiometry of CRIP1a and CB1 receptor expression in N18TG2 and N18TG2-CRIP1a cells
Membranes prepared from the indicated cell line were incubated with varying concentrations of [3H]CP55,940, as described in Materials and Methods. Bmax and KD values were derived from nonlinear regression analysis of the saturation binding curves. CRIP1a protein values were determined by quantitative immunoblot using purified CRIP1a as an internal standard, as described in Materials and Methods. Data are mean values ± S.E.M. (n = 4–8).
| Tissue Source | CB1 Bmax | CB1 KD | CRIP1a | Molar Ratio CRIP1a/CB1 |
|---|---|---|---|---|
| pmol/mg | nM | pmol/mg | ||
| Control N18TG2 | 0.298 ± 0.039 | 2.35 ± 0.33 | 0.56 ± 0.07 | 1.87 ± 0.23 |
| N18-CRIP1a-OX1 | 0.277 ± 0.033 | 3.02 ± 0.71 | 1.35 ± 0.16** | 4.87 ± 0.59** |
| N18-CRIP1a-OX5 | 0.270 ± 0.050 | 4.22 ± 0.96 | 1.28 ± 0.09** | 4.74 ± 0.34** |
| N18-CRIP1a-KD | 0.320 ± 0.045 | 2.49 ± 0.38 | N.D. | N.D. |
P < 0.01 different from corresponding value in control N18TG2 cells by one-way ANOVA with posthoc Dunnett’s test. N.D., not determined.
To determine the precise level of membrane-delimited CRIP1a protein expression in CRIP1a-OX N18TG2 clones for comparison with results in CRIP1a-overexpressing CB1-HEK cells, quantitative immunoblots using purified CRIP1a standards were then conducted in isolated membranes prepared from the two CRIP1a-transfected clones and untransfected N18TG2 cells. Untransfected N18TG2 cells expressed 0.56 ± 0.06 pmol CRIP1a per mg of membrane protein, whereas clones OX1 and OX5 expressed 1.35 ± 0.16 pmol/mg and 1.28 ± 0.09 pmol/mg, respectively. The results of CRIP1a quantitative immunoblots were then compared with Bmax values derived from cannabinoid radioligand binding assays in control and CRIP1a-overexpressing N18TG2 cell lines to determine the stoichiometric ratio of CRIP1a/CB1 and the effect of CRIP1a overexpression on CB1R Bmax values. Owing to low CB1R expression levels in N18TG2 cells, [3H]CP55,940 was used as the radioligand because it yielded greater specific/nonspecific binding ratios than [3H]SR141716A. Quantitative immunoblot and [3H]CP55,940 binding analysis indicated that CRIP1a-overexpressing N18TG2 clones have approximately a 2.3- to 2.5-fold increase in both CRIP1a protein expression and the CRIP1a/CB1 expression ratio, in comparison with untransfected N18TG2 cells (Table 4).
In line with findings from stable CB1-HEK-CRIP1a cells, CRIP1a overexpression (clones OX1 and OX5) or knockdown (clone KD2C) did not alter the Bmax values of [3H]CP55,940 binding (Table 4), as determined by one-way ANOVA (P = 0.899). Likewise, [3H]CP55,940 KD values were not significantly affected by CRIP1a overexpression or knockdown (P = 0.145 by one-way ANOVA; Table 4). These results demonstrate altered expression of CRIP1a protein without altering CB1R density in both CRIP1a-overexpressing and knockdown cells compared with control N18TG2 cells. It is noteworthy to mention that the relative increase in CRIP1a/CB1R ratios in CRIP1a-overexpressing N18TG2 cells was less than that in the CB1-HEK cell models, where CRIP1a-overexpressing cells showed a 16-fold increase over control cells, as compared with ∼2.5-fold increase in N18TG2-CRIP1a cells.
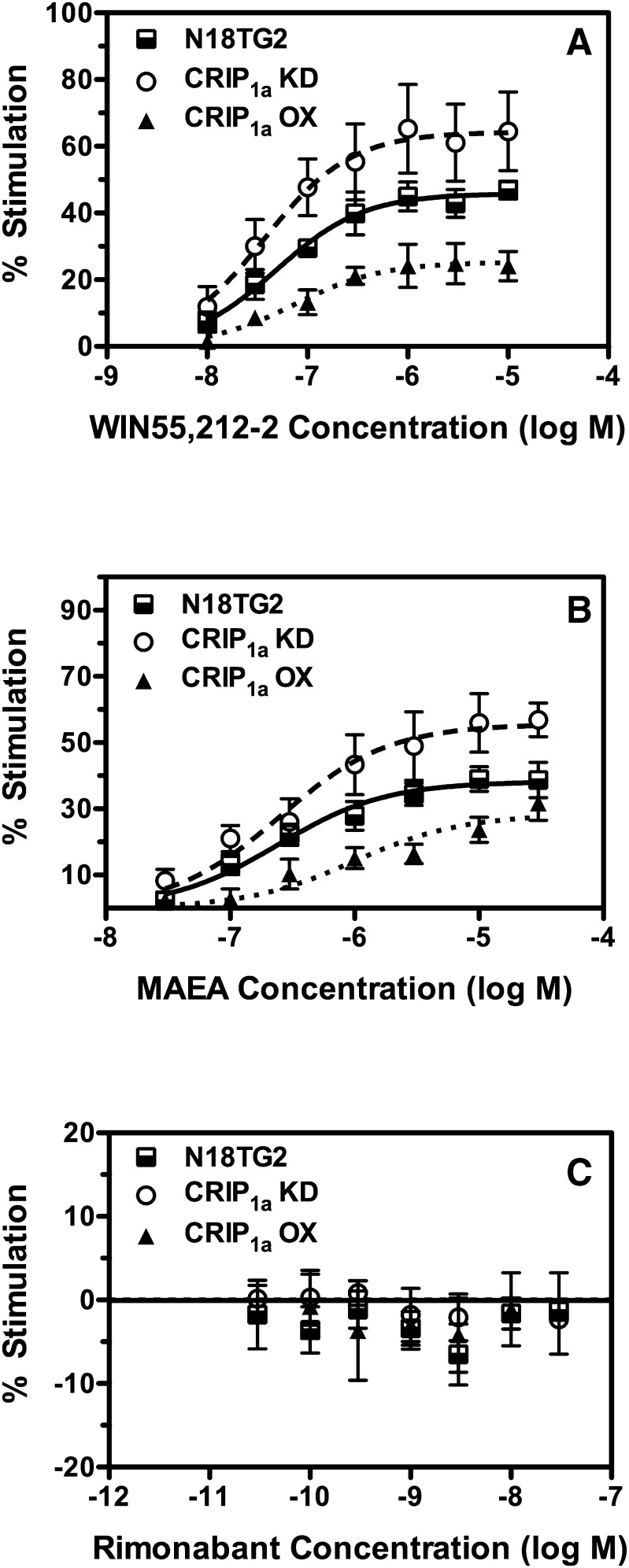
To determine whether CRIP1a overexpression in N18TG2 cells affected constitutive and agonist-stimulated G-protein activation by CB1Rs, ligand concentration-effect curves for modulation of [35S]GTPγS binding were examined using WIN55,212-2, MAEA, and rimonabant in CRIP1a knockdown clone KD2C (N18-CRIP1a-KD), and overexpressing clone OX1 (N18-CRIP1a-OX) compared with untransfected N18TG2 cells. Basal [35S]GTPγS binding did not differ significantly between N18TG2 (68.1 ± 6.0 fmol/mg), N18-CRIP1a-KD (66.6 ± 16.6), and N18-CRIP1a-OX cells (70.5 ± 5.9 fmol/mg). Results of ligand concentration-effect curves showed that CRIP1a overexpression decreased WIN55,212-2– and MAEA-stimulated [35S]GTPγS binding relative to control N18TG2 cells (Fig. 8, A and B). There was a significant effect of CRIP1a knockdown on [35S]GTPγS binding stimulated by either WIN55,212-2 (P < 0.0001) or MAEA (P < 0.0001) in N18-CRIP1a-KD (clone KD2C) relative to control N18TG2 cells, as revealed by two-way ANOVA of the concentration-effect curves. Likewise, two-way ANOVA revealed a significant effect of CRIP1a overexpression on [35S]GTPγS binding stimulated by either WIN55,212-2 (P < 0.0001) or MAEA (P < 0.0001) in N18-CRIP1a-OX (clone OX1) relative to control N18TG2 cells. In contrast, rimonabant did not reliably inhibit basal [35S]GTPγS binding in a concentration-dependent manner in any of the three N18TG2 cell lines examined under these experimental conditions (Fig. 8C), in contrast to results obtained in the CB1-HEK cell models. Accordingly, there were no significant effects of either rimonabant concentration or CRIP1a expression levels in comparing the rimonabant concentration-effect curves among these three N18TG2 cell lines using two-way ANOVA. Similar results were obtained with all three ligands when comparing ligand-modulated [35S]GTPγS binding in the other CRIP1a-overexpressing (OX5) and knockdown (KD2F) clones to control N18TG2 cells (Supplemental Fig. 6 and Fig. 8).
Fig. 8.
Effect of CRIP1a knockdown and overexpression on concentration-effect curves of ligand-modulated [35S]GTPγS binding in N18TG2 neuroblastoma cell membranes. Membranes from wild-type N18TG2 cells or N18TG2 cells with siRNA-mediated knockdown (CRIP1a-KD; clone 2C) or overexpression (CRIP1a-OX; clone 1) of CRIP1a were incubated as described in Materials and Methods with 100 mM NaCl, 20 μM GDP, 0.1 nM [35S]GTPγS, and varying concentrations of WIN55,212-2, methanandamide, or rimonabant (RIM). Data are mean percentage stimulation ± S.E.M. (n = 5–9).
Nonlinear regression analysis of the ligand concentration-effect curves showed that the Emax values of both WIN55,212-2 and MAEA were significantly higher in N18-CRIP1a-KD relative to control N18TG2 cells (Table 5). Conversely, the WIN55,212-2 Emax value was lower in N18-CRIP1a-OX cells compared with control N18TG2 cells. However, the MAEA Emax value did not differ significantly between N18-CRIP1a-OX cells and control N18TG2 cells, but both WIN55212-2 and MAEA Emax values in N18-CRIP1a-OX cells were significantly lesser than in N18-CRIP1a-KD cells. In contrast, neither WIN55212-2 nor MAEA EC50 values were significantly altered by manipulation of CRIP1a expression levels in these N18TG2 cells lines. Moreover, because two-way ANOVA showed no significant effect of rimonabant concentration in these N18TG2 cell models, curve-fitting analysis was not performed with this ligand. These results indicate that CRIP1a knockdown enhances whereas CRIP1a overexpression attenuates agonist-stimulated G-protein activation mediated by CB1Rs that are endogenously expressed in N18TG2 neuroblastoma cells.
TABLE 5.
Emax and EC50 values of ligand-stimulated [35S]GTPγS binding in N18TG2 cells with and without stable overexpression or knockdown of CRIP1a
Varying concentrations of WIN55,212-2 or MAEA were incubated with membranes prepared from the indicated cell lines in the presence of 20 μM GDP and 0.1 nM [35S]GTPγS, as described in Materials and Methods. Emax and EC50 values were derived from nonlinear regression analysis of ligand concentration-effect curves. Data are mean values ± S.E.M. (n = 5–9). Significance of Emax and EC50 values between cell types were determined by one-way ANOVA with posthoc Newman-Keuls test.
| WIN55,212-2 | MAEA | |||
|---|---|---|---|---|
| Cell line | Emax | EC50 | Emax | EC50 |
| % Stim | nM | % Stim | nM | |
| WT-N18TG2 | 45.1 ± 5.5 | 53.8 ± 12.2 | 40.1 ± 5.3 | 497 ± 197 |
| N18-CRIP1a-OX | 26.0 ± 3.5* | 82.5 ± 8.9 | 30.2 ± 3.5 | 594 ± 178 |
| N18-CRIP1a-KD | 64.0 ± 8.5*,§§ | 40.9 ± 8.3 | 56.2 ± 5.3*,§ | 322 ± 71 |
P < 0.05 different from WT-N18TG2 cells. §P < 0.05, §§P < 0.01 different from N18-CRIP1a-OX cells.
Key: N18-CRIP1a-OX, N18TG2 cells overexpressing CRIP1a (clone 1); N18-CRIP1a-KD, N18TG2 cells with siRNA-mediated knockdown of CRIP1a (clone 2C).
Potential effects of CRIP1a on constitutive CB1R-mediated G-protein activation could not be determined in the N18TG2 cell models because rimonabant did not significantly inhibit basal [35S]GTPγS binding. It is possible, however, that endocannabinoids present in N18TG2 cells could have obscured the inhibitory effects of rimonabant on basal G-protein activity, perhaps by competing with rimonabant. To address this question, lipid fractions from N18TG2 and N18-CRIP1a-OX cells, or membranes isolated from each cell line, were analyzed by mass spectrometry to determine the content of 2-AG and AEA. Results showed detectable levels of 2-AG in both intact cells and membrane preparations, although levels on a per-cell basis were approximately 7.5-fold greater in intact cells than isolated membranes (Supplemental Fig. 7). Importantly, 2-AG levels did not differ between N18TG2 and N18-CRIP1a-OX cells in fractions prepared from either intact cells or isolated membranes. To determine whether the higher 2-AG levels in intact cells compared with membranes were attributable to greater lipase activity in cells, the cells were incubated in the presence and absence of the diacylglycerol lipase inhibitor tetrahydrolipstatin (orlistat). Results showed that orlistat treatment significantly decreased 2-AG levels in intact cell preparations but not in membranes (Supplemental Fig. 6). In intact cells, two-way ANOVA revealed a significant effect of orlistat (P = 0.032) but not CRIP1a overexpression (P = 0.696), and there was no significant interaction between the two factors (P = 0.768). In isolated membranes, there was only a nonsignificant trend toward an effect of orlistat (P = 0.105), and there was no effect of CRIP1a overexpression (P = 0.574) and no interaction between the two factors (P = 0.860). AEA levels were detectable in intact cells but were approximately 0.02% of the levels detected for 2-AG, or approximately 0.01 pmol/107 cells (data not shown). Two-way ANOVA revealed no effects of either CRIP1a overexpression (P = 0.540) or orlistat (P = 0.452), and there was no significant interaction (0.791). AEA levels in membrane preparations were below the limit of detection. These results suggest that differences in endocannabinoid levels were not responsible for the lack of inhibitory effects of rimonabant in membrane preparations from N18TG2 cells with and without overexpression of CRIP1a.
Effects of CRIP1a on Endocannabinoid Signaling in Hippocampal Neuronal Cultures.
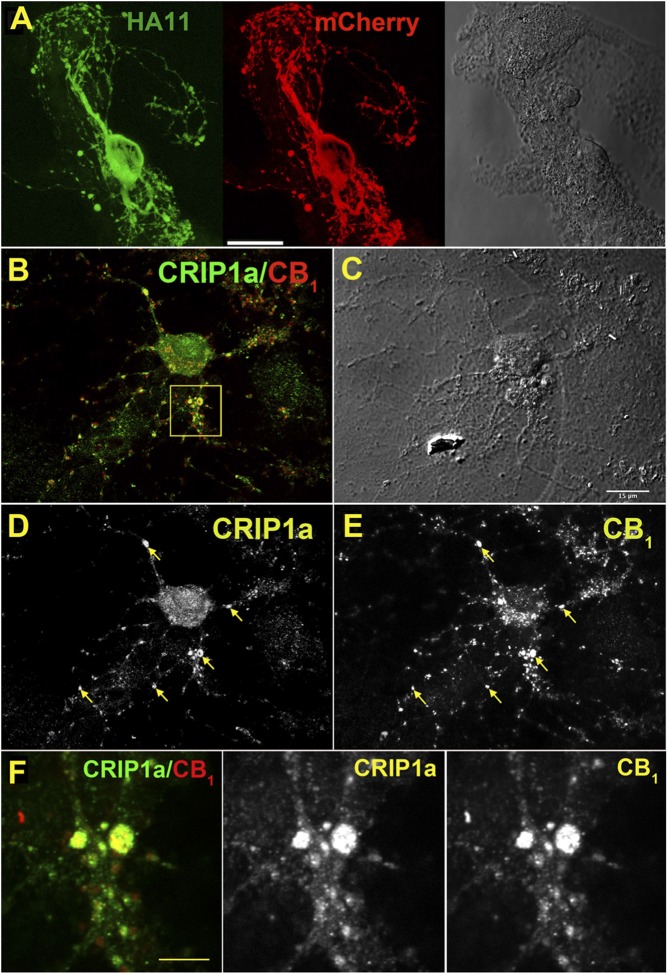
Results from both HEK-293 and N18TG2 cell models indicate that CRIP1a can negatively modulate agonist-stimulated CB1R activity at the level of G-protein activation. However, our previous results from isolated SCG neurons indicated that although CRIP1a overexpression attenuated constitutive CB1R-mediated Ca2+ channel inhibition, it did not alter agonist-inhibited Ca2+ channel activity (Niehaus et al., 2007). Therefore, the effects of CRIP1a overexpression on synaptic function of CB1Rs were examined in a more CNS-relevant model. Autaptic hippocampal neurons express all the components of a functional cannabinoid signaling system, including presynaptic CB1Rs, depolarization-dependent production of endocannabinoids, probably 2-AG (Straiker and Mackie, 2005), and monacylglycerol lipase, which hydrolyzes 2-AG and thereby controls the duration of cannabinoid signaling (Straiker et al., 2009). To assess whether CRIP1a can functionally interact with this endogenous cannabinoid signaling system, CRIP1a was overexpressed in autaptic neurons. The distribution of CRIP1a protein was determined in transfected neurons using an HA11 antibody against the HA tag on the CRIP1a protein, and results showed that CRIP1a was widely expressed throughout the transfected neuron (Fig. 9A). This widespread cellular localization was similar to that of endogenous CRIP1a (Fig. 9, B and D; compare with Figs. 1, 2, and 3). Although endogenously expressed CB1R appeared to be primarily limited to neuronal processes and putative autaptic terminals (Fig. 9, B and E), endogenous CRIP1a was widely colocalized in these cellular elements with CB1Rs (Fig. 9, B and F). These results suggest that CRIP1a is spatially positioned in a manner such that it could modulate CB1R function.
Fig. 9.
CRIP1a partially colocalizes with CB1R in autaptic hippocampal neurons. (A) Micrograph in transfected autaptic hippocampal neuron shows HA11 staining of transfected HA-CRIP1a (green) in a mCherry-labeled (red) neuron, indicating that CRIP1a is expressed throughout the transfected neuron. Right panel: Nomarski image of the island is shown for reference. Scale bar, 20 μm. (B) Endogenous CRIP1a (green) and CB1R (red) staining in an untransfected autaptic hippocampal neuron (overlap in yellow). (C) Differential interference contrast image corresponding to (B). Scale bar, 15 μm. (D) CRIP1a and (E) CB1R staining from (B). Arrows indicate overlap. (F) Zoom from inset box in (B). Scale bar, 3 μm.
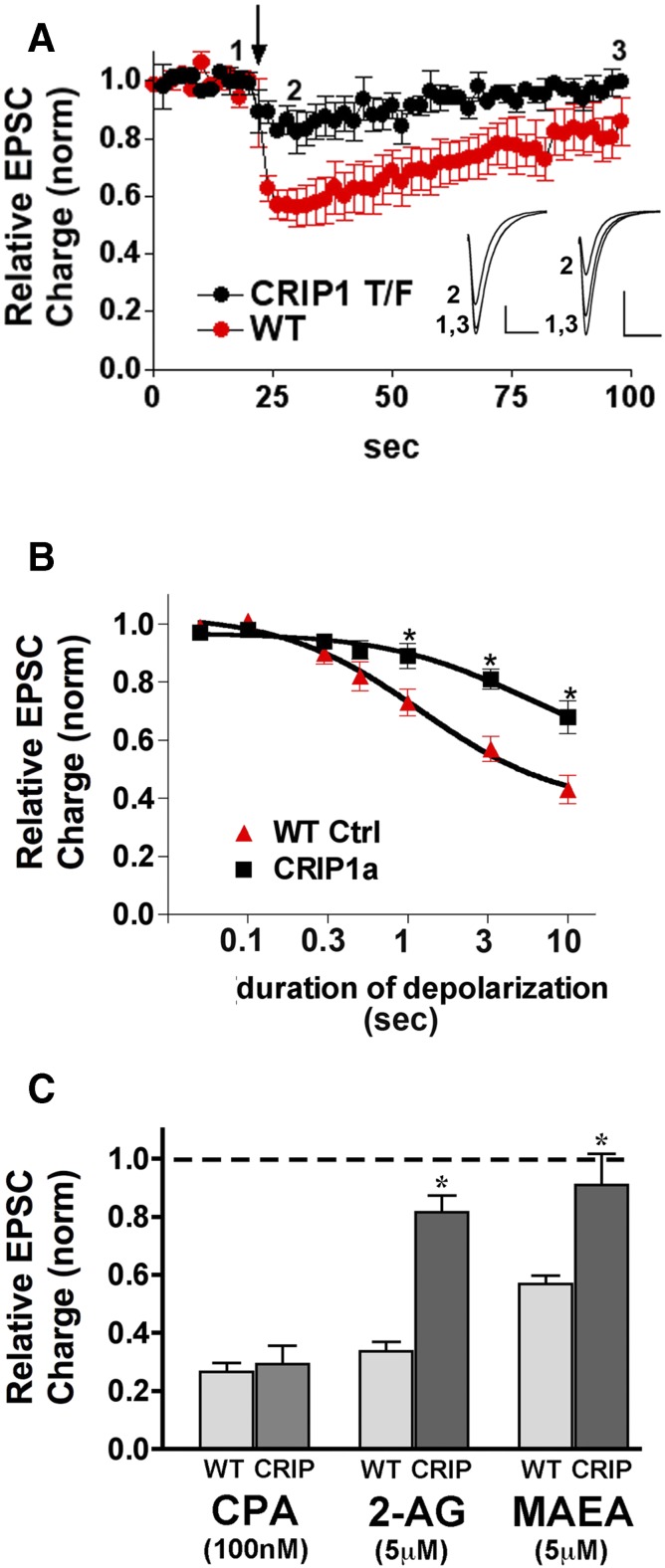
To determine whether CRIP1a overexpression altered the sensitivity of DSE induction in these autaptic hippocampal neurons, depolarization duration-response curves were obtained. Neurons were depolarized for progressively longer durations (50 milliseconds, 100 milliseconds, 300 milliseconds, 500 milliseconds, 1 second, 3 seconds, and 10 seconds) and the resulting CB1R-dependent inhibition was measured. Results showed that the depolarization duration-response curve in CRIP1a-overexpressing neurons differed substantially from that of control conditions, with a diminished inhibition at 1-, 3- and 10-second depolarizations (Fig. 10, A and B; P < 0.05 by Bonferroni posthoc test after two-way ANOVA).
Fig. 10.
Overexpression of CRIP1a diminishes CB1-mediated DSE in autaptic hippocampal neurons. (A) DSE time-courses for wild-type (WT; red) versus CRIP1a transfected neurons (black) in response to 3-second depolarization (arrow). Insets show sample EPSCs from control (1), maximal DSE inhibition (2), and recovery (3) for CRIP1a-transfected (left) and WT (right) neurons. (B) “Dose” response for DSE using a range of depolarizations from 50 milliseconds to 10 seconds. The wild-type DSE dose response is shown for comparison. *P < 0.05 Bonferroni posthoc test, two-way ANOVA. (C) Bar graph shows relative EPSC charge (1.0 = baseline, no inhibition) after treatment with three drugs under WT and CRIP1a-transfected conditions: CPA, 100 nM; 2-AG (5 μM); MetAEA (5 μM). *P < 0.05, unpaired t test.
2-AG is a strong candidate to serve as the endocannabinoid-mediating DSE at autaptic hippocampal synapses (Straiker and Mackie, 2005, 2009; Jain et al., 2013). Therefore, the response in CRIP1a-transfected neurons to 2-AG was examined to determine whether sensitivity to exogenously added 2-AG was diminished to the same degree as was DSE. Figure 10C shows that inhibition of EPSCs by 2-AG (5 μM) was substantially attenuated in CRIP1a-overexpressing neurons relative to controls [relative EPSC charge with 2-AG (5 μM) treatment in wild-type neurons: 0.34 ± 0.03, n = 4; in CRIP1a-transfected neurons: 0.82 ± 0.06, n = 7; P < 0.05 by unpaired t test]. We have previously reported that anandamide activates CB1Rs to inhibit neurotransmitter release in excitatory and inhibitory autaptic neurons (Straiker and Mackie, 2005, 2009). Thus, the hydrolysis-resistant analog MAEA was tested under control and CRIP1a-transfected conditions. As with 2-AG, CRIP1a overexpression also diminished MAEA signaling [Fig. 10C; relative EPSC charge with MAEA (5 μM) treatment in wild-type neurons: 0.57 ± 0.03, n = 4; in CRIP1a-transfected neurons: 0.91 ± 0.11, n = 5; P < 0.05 by unpaired t test]. To determine whether CRIP1a over expression would suppress constitutive inhibition of EPSCs by CB1Rs, the effects of rimonabant (100 nM) were examined. However, no effect of this inverse agonist was detected regardless of whether CRIP1a was overexpressed (data not shown), as previously reported for nontransfected hippocampal autaptic cultures (Straiker et al., 2012).
To ascertain whether CRIP1a transfection interfered more generally with Gi/o-mediated modulation of neurotransmission, inhibition by the adenosine A1 receptor agonist cyclopentyladenosine (CPA) was examined, because it was previously found to robustly inhibit EPSCs in autaptic hippocampal neurons (Straiker et al., 2002). Figure 10C shows that CRIP1a overexpression did not interfere with CPA responses [relative EPSC charge after treatment with CPA (100 nM) in control neurons: 0.27 ± 0.03, n = 10; in CRIP1a-transfected neurons: 0.29 ± 0.06, n = 4]. Together, these results indicate that CRIP1a attenuates the inhibition of excitatory synaptic transmission by endocannabinoids, and that this action of CRIP1a is selective for modulation of synaptic transmission by CB1Rs.
Discussion
This study extends the findings that CRIP1a attenuates constitutive inhibition of Ca2+ channels by CB1Rs in cotransfected SCG neurons (Niehaus et al., 2007). Here we showed that CRIP1a attenuated rimonabant-mediated inhibition of basal [35S]GTPγS binding in CRIP1a-overexpressing CB1-HEK cells, suggesting that CRIP1a overexpression disrupts constitutive CB1R-mediated G-protein activation. CRIP1a overexpression also inhibited basal [35S]GTPγS binding in CB1-HEK cells in the absence of Na+ but not in the presence of Na+ or in PTX-pretreated cells, further indicating that CRIP1a inhibits CB1R constitutive activity. There was an opposing effect of Na+ on agonist- versus inverse agonist–modulated G-protein activity such that inverse agonism was maximized in the absence of Na+, a condition under which PTX-sensitive basal G-protein activity was highest. Conversely, agonist-stimulated G-protein activation was maximized by 100 mM Na+, a condition under which PTX-sensitive basal G-protein activation was minimal. CRIP1a overexpression suppressed the inverse agonist activity of rimonabant in the absence of Na+, whereas CRIP1a only suppressed agonist-stimulated G-protein activation with Na+ present. The absence of endocannabinoids in CB1-HEK membranes suggests that CRIP1a effects did not result from dampening endocannabinoid tone. Altogether, these results indicate that CRIP1a overexpression inhibits constitutive and agonist-stimulated G-protein activity in CB1-HEK cells in a manner consistent with the behavior of receptor G-protein complexes under the allosteric modulatory influence of Na+ (Seifert and Wenzel-Seifert, 2002).
If CRIP1a regulates constitutive activity of CB1Rs in vivo, then it can be hypothesized to modulate both cellular trafficking of CB1Rs and pharmacological responses to inverse agonists. Constitutive activity may be required for CB1R internalization and targeting to presynaptic terminals (Leterrier et al., 2004, 2006), although this has been disputed (McDonald et al., 2007). However, confirmation of constitutive CB1R activity in the CNS has been elusive. For example, rimonabant concentrations necessary to depress basal G-protein activity in brain are greater than those required to antagonize CB1 agonist–stimulated activity (Sim-Selley et al., 2001), and high rimonabant concentrations decrease basal G-protein activity in CB1R knockout mice (Breivogel et al., 2001). CRIP1a overexpression inhibits constitutive CB1R activity in CB1-HEK cells, so it is possible that CRIP1a contributes to the low level of constitutive activity in the brain. This would be consistent with our finding that inverse agonism by rimonabant was not detected in N18TG2 cells, where endogenous CRIP1a might suppress constitutive CB1R activity. Although siRNA-mediated knockdown of CRIP1a did not significantly enhance inverse agonism by rimonabant in N18TG2 cells, this finding could result from the low CB1R expression level and the fact that rimonabant was only examined in the presence of 100 mM Na+ in these cells. Inhibition of basal [35S]GTPγS binding by rimonabant in N18TG2 cells is minimal with Na+ present but detectable after replacement of Na+ with K+ (Meschler et al., 2000). Thus, these neural cell models will be useful to investigate CB1R inverse agonism and the role of endogenous CRIP1a using varying cation concentrations in future studies.
The present study also confirmed that differences in CRIP1a expression do not influence total CB1R expression levels or ligand binding affinity (Niehaus et al., 2007) in both HEK-293 and N18TG2 cell models, despite the use of different radioligands in each cell line. The finding of similar results with both antagonist/inverse agonist ([3H]SR141716A) and agonist ([3H]CP55,940) radioligands suggests that CRIP1a might influence CB1R signaling efficacy without affecting the formation of high-affinity receptor G-protein complexes, which will be addressed in future studies.
The effect of CRIP1a on CB1R-mediated G-protein activation was dependent on the stoichiometric relationship between CRIP1a and CB1Rs. In CB1-HEK cells, stable CRIP1a transfection increased the CRIP1a/CB1R expression ratio from <0.5 to approximately 7. In N18TG2 cells, which endogenously express both proteins, CRIP1a transfection increased the ratio of CRIP1a/CB1R from approximately 2 to 5. Importantly, CRIP1a expression levels in both overexpressed cell lines were lower than in rat cerebellum, suggesting that these cells do not express supraphysiological CRIP1a levels relative to native tissues. Whether the ratio of overexpressed CRIP1a/CB1R is supraphysiological is difficult to ascertain because CRIP1a was more widely distributed throughout rat and mouse cerebellum than CB1Rs, although colocalization was observed throughout the molecular and granule cell layers.
A major finding of the present study is that CRIP1a overexpression attenuated cannabinoid agonist–stimulated G-protein activation in both CB1-HEK and N18TG2 cells. This was probably not the result of unnaturally overexpressed levels of CRIP1a because the opposite effect—enhancement of agonist-stimulated G-protein activity—was observed with siRNA-mediated knockdown of CRIP1a in N18TG2 cells. CRIP1a knockdown was not examined in CB1-HEK cells because CB1Rs were more highly expressed than CRIP1a in this model. Although the inhibitory effect of CRIP1a overexpression on maximal agonist-stimulated G-protein activation was moderate (∼20–40%) in either cell line, a robust effect of CRIP1a was observed when comparing N18TG2 cells with CRIP1a knocked down versus overexpressed, whereby up to a 2.5-fold difference in agonist Emax values was observed. These results indicate that CRIP1a exerts a dramatic effect on CB1R-mediated G-protein signaling under appropriate stoichiometric conditions. Moreover, CRIP1a overexpression in autaptic hippocampal neurons attenuated both DSE and inhibition of excitatory synaptic currents by 2-AG and MAEA, demonstrating that CRIP1a can regulate one of the most critical functions of CB1Rs in neurons. In addition, CRIP1a overexpression did not alter synaptic inhibition by an adenosine A1 agonist, suggesting that CRIP1a selectively modulates CB1R activity without altering activity of other GPCRs. Altogether, these results suggest that CRIP1a could be an important modulator of endocannabinoid signaling in the CNS.
The effects of CRIP1a on CB1R-mediated G-protein activation were also found to be ligand-dependent in CB1-HEK cells. CRIP1a overexpression inhibited G-protein activation by agonists of high intrinsic efficacy, including 2-AGE, WIN55,212-2, HU-210, and CP55,940, but not ligands of lower intrinsic efficacy, including MAEA, levonantradol, and THC. This effect was probably not related solely to chemotype because HU-210 and THC are classic cannabinoids, whereas 2-AGE and MAEA are eicosanoids, yet CRIP1a only affected the ligand with highest intrinsic efficacy in each class. It is possible that association of CRIP1a with CB1Rs is modulated by the presence of bound ligand in an efficacy-dependent manner. This ligand-selective action of CRIP1a was also dependent on cell type, because CRIP1a attenuated signaling induced by MAEA in both N18TG2 cells and hippocampal neurons but not CB1-HEK cells. Thus, the effects of CRIP1a on CB1R–G-protein interactions could be dependent on G-protein subtype, which varies among cell types (Atwood et al., 2011). Previous work demonstrated differential association of distinct Gαi/o subtypes with CB1Rs occupied by different ligands (Mukhopadhyay and Howlett, 2005) and differential abilities of different ligands to activate purified Gi versus Go (Glass and Northup, 1999). In addition, different Gαi/o subtypes interact selectively with either the C-terminus or third intracellular loop of the CB1R (Mukhopadhyay and Howlett, 2001; Anavi-Goffer et al., 2007), so CRIP1a association with the CB1R C-terminus (Niehaus et al., 2007) might differentially interfere with G-protein association with these distinct intracellular domains of the CB1R. Because the C-terminus serves as a docking site for multiple protein-protein interactions (Howlett et al., 2010; Smith et al., 2010), the effects of CRIP1a on CB1R–G-protein interactions might depend on both ligand occupancy of the receptor and the presence of additional interacting proteins.
The CB1R C-terminus interacts with proteins that mediate desensitization and downregulation. For example, probable G-protein–coupled receptor kinase phosphorylation sites have been identified (Hsieh et al., 1999; Jin et al., 1999; Daigle et al., 2008) and isolated fragments of the CB1R C-terminus can bind to β-arrestins (Singh et al., 2011; Bakshi et al., 2007) and GASP1 (Martini et al., 2007). Prolonged agonist exposure downregulates CB1Rs expressed in HEK-293 (Shapira et al., 2003) but not N18TG2 cells (McIntosh et al., 1998). Therefore, we examined the effects of prolonged agonist exposure in CB1-HEK cells, with and without CRIP1a overexpression, on CB1R levels and agonist-induced activation of G-proteins. Interestingly, our results showed that CRIP1a overexpression interfered with CB1R downregulation (degradation) but not desensitization (uncoupling from G-protein activation). These findings suggest that CRIP1a might interfere with GASP1 association with CB1Rs (Martini et al., 2007, 2010;) or with C-terminal CB1R phosphorylation at sites that mediate internalization (Jin et al., 1999; Daigle et al., 2008;) but not at sites that mediate desensitization (Jin et al., 1999; Morgan et al., 2014). Regional differences in CB1R downregulation after repeated cannabinoid administration have been observed in the CNS of rodents (Sim-Selley, 2003; Sim-Selley et al., 2006) and humans (Villares, 2007; Hirvonen et al., 2012). Thus, differential colocalization of CB1Rs with CRIP1a could contribute to regional differences in CB1R downregulation, and might thereby influence the development of differential tolerance to distinct pharmacological effects of cannabinoids.
The present study provides evidence that CRIP1a attenuates constitutive and agonist-induced G-protein activation by CB1Rs in two distinct cell lines, HEK-293 and N18TG2. Additionally, CRIP1a inhibits DSE in autaptic hippocampal neurons, suggesting that CRIP1a modulates the physiologic actions of endocannabinoids in the CNS. These results indicate that CRIP1a is a negative regulator of acute CB1R-mediated G-protein signaling. CRIP1a also attenuated agonist-induced CB1R downregulation, suggesting that it might oppose the development of cannabinoid tolerance. Thus, CRIP1a could play an important regulatory role in the endocannabinoid system by differentially modulating acute versus chronic activation of CB1Rs.
Note Added in Proof
A recently published article by some of the coauthors of this paper showed that manipulation of CRIP1a expression in N18TG2 cells modulated constitutive and agonist-stimulated extracellular signal-regulated kinase 1/2 phosphorylation by the CB1R, and that CB1R coupling to Gαo and Gαi3 was attenuated, whereas coupling to Gαi1 and 2 was enhanced, by overexpression of CRIP1a (Blume et al., 2015).
Supplementary Material
Acknowledgments
The authors thank Hengjun He and Elizabeth Krahn for technical assistance, and Dr. Laura J. Sim-Selley and Erica C. Lyons for critical review of the manuscript.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 2-AGE
2-arachidonoylglycerol ether
- AEA
arachidonoylethanolamide
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CB1R
cannabinoid CB1 receptor
- CNS
central nervous system
- CP55,940
(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- CPA
cyclopentyladenosine
- DMEM
Dulbecco’s modified Eagle’s medium
- DSE
depolarization-induced suppression of excitation
- EPSC
excitatory postsynaptic current
- FBS
fetal bovine serum
- G418
geneticin
- GPCR
G-protein–coupled receptor
- GST
glutathione S-transferase
- GTPγS
guanylyl-5′-[O-thio]-triphosphate
- HA
hemagglutinin
- HEK
human embryonic kidney
- HU-210
3-(1,1′-dimethylheptyl)-6αR,7,10,10αR-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[β,δ]pyran-9-methanol
- ir
immunoreactivity
- levonantradol
[(6S,6αR,9R,10αR)-9-hydroxy-6-methyl-3-[(2R)-5-phenylpentan-2-yl]oxy-5,6,6a,7,8,9,10,10α-octahydrophenanthridin-1-yl] acetate
- MAEA
methanandamide
- PBS
phosphate-buffered saline
- P/S
penicillin/streptomycin
- PTX
pertussis toxin
- SCG
superior cervical ganglion
- siRNA
small-interfering RNA
- SR141716A
rimonabant, 5-(4-chlorophenyl)-1 (2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide
- SV2
synaptic vesicle 2
- THC
Δ9-tetrahydrocannabinol
- WIN55,212-2
[(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone
Authorship Contributions:
Participated in research design: Smith, Blume, Straiker, Sayers, Poklis, Abdullah, Chen, Mackie, Howlett, Selley.
Conducted experiments: Smith, Blume, Straiker, David, Secor McVoy, Sayers, Poklis, Abdullah, Cox, Egertová.
Contributed new reagents or analytic tools: Blume, Chen, Elphick, Mackie, Howlett.
Performed data analysis: Smith, Blume, Straiker, David, Secor McVoy, Poklis, Cox, Selley.
Wrote or contributed to the writing of the manuscript: Smith, Blume, Straiker, Sayers, Abdullah, Elphick, Mackie, Howlett, Selley.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [R21-DA025321, R01-DA003690, R03-DA035424, F31-DA023747, F31-DA032215, R01-DA011322, K05-DA021696, and T32-DA007027], the National Institute on Neurologic Disorders and Stroke [T32-NS007288], the National Eye Institute [R21-EY021831], the Biotechnology and Biological Sciences Research Council [S19916], and the National Center for Advancing Translational Sciences [UL1-TR000058] in support of the A.D. Williams Fund of Virginia Commonwealth University. Mass spectrometry was supported in part with funding from the National Institute on Drug Abuse [P30-DA033934]. Rat microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported in part with funding from the National Institute on Neurologic Disorders and Stroke [P30-NS047463].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abood ME, Ditto KE, Noel MA, Showalter VM, Tao Q. (1997) Isolation and expression of a mouse CB1 cannabinoid receptor gene. Comparison of binding properties with those of native CB1 receptors in mouse brain and N18TG2 neuroblastoma cells. Biochem Pharmacol 53:207–214. [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S, Fleischer D, Hurst DP, Lynch DL, Barnett-Norris J, Shi S, Lewis DL, Mukhopadhyay S, Howlett AC, Reggio PH, et al. (2007) Helix 8 Leu in the CB1 cannabinoid receptor contributes to selective signal transduction mechanisms. J Biol Chem 282:25100–25113. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi K, Mercier RW, Pavlopoulos S. (2007) Interaction of a fragment of the cannabinoid CB1 receptor C-terminus with arrestin-2. FEBS Lett 581:5009–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. (1991) Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA 88:7834–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. (2007) Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316:1212–1216. [DOI] [PubMed] [Google Scholar]
- Blume LC, Bass CE, Childers SR, Dalton GD, Roberts DC, Richardson JM, Xiao R, Selley DE, Howlett AC. (2013) Striatal CB1 and D2 receptors regulate expression of each other, CRIP1A and δ opioid systems. J Neurochem 124:808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume L, Eldeeb K, Bass CE, Selley DE, Howlett AC. (2015) Cannabinoid receptor interacting protein (CRIP1a) attenuates CB1R signaling in neuronal cells. Cell Signal 27:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojnik E, Turunç E, Armağan G, Kanıt L, Benyhe S, Yalçın A, Borsodi A. (2012) Changes in the cannabinoid (CB1) receptor expression level and G-protein activation in kainic acid induced seizures. Epilepsy Res 99:64–68. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163. [PubMed] [Google Scholar]
- Daigle TL, Kwok ML, Mackie K. (2008) Regulation of CB1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J Neurochem 106:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. (2000) Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem 75:2434–2444. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. (2007) Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience 146:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. (1976) Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA 73:4225–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Northup JK. (1999) Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol 56:1362–1369. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. (2012) Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Blume LC, Dalton GD. (2010) CB(1) cannabinoid receptors and their associated proteins. Curr Med Chem 17:1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. (1999) Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem 73:493–501. [DOI] [PubMed] [Google Scholar]
- Hu SS, Arnold A, Hutchens JM, Radicke J, Cravatt BF, Wager-Miller J, Mackie K, Straiker A. (2010) Architecture of cannabinoid signaling in mouse retina. J Comp Neurol 518:3848–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain T, Wager-Miller J, Mackie K, Straiker A. (2013) Diacylglycerol lipaseα (DAGLα) and DAGLβ cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons. Mol Pharmacol 84:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Deng L, Chen G. (2004) High Ca(2+)-phosphate transfection efficiency enables single neuron gene analysis. Gene Ther 11:1303–1311. [DOI] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, Chavkin C, Mackie K. (1999) Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci 19:3773–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89:309–380. [DOI] [PubMed] [Google Scholar]
- Lazenka MF, Selley DE, Sim-Selley LJ. (2013) Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci 92:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. (2004) Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem. 279:36013–36021. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Lainé J, Darmon M, Boudin H, Rossier J, Lenkei Z. (2006) Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci. 26:3141–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, McCarthy KD. (1991) Characterization and partial purification of AIM: a plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J Neurochem 57:782–794. [DOI] [PubMed] [Google Scholar]
- Lind GE, Danielsen SA, Ahlquist T, Merok MA, Andresen K, Skotheim RI, Hektoen M, Rognum TO, Meling GI, Hoff G, et al. (2011) Identification of an epigenetic biomarker panel with high sensitivity and specificity for colorectal cancer and adenomas. Mol Cancer 10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludányi A, Eross L, Czirják S, Vajda J, Halász P, Watanabe M, Palkovits M, Maglóczky Z, Freund TF, Katona I. (2008) Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci 28:2976–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. (2007) Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J 21:802–811. [DOI] [PubMed] [Google Scholar]
- Martini L, Thompson D, Kharazia V, Whistler JL. (2010) Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology 35:1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald NA, Henstridge CM, Connolly CN, Irving AJ. (2007) An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol Pharmacol 71:976–984. [DOI] [PubMed] [Google Scholar]
- McIntosh HH, Song C, Howlett AC. (1998) CB1 cannabinoid receptor: cellular regulation and distribution in N18TG2 neuroblastoma cells. Brain Res Mol Brain Res 53:163–173. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Kraichely DM, Wilken GH, Howlett AC. (2000) Inverse agonist properties of N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A) and 1-(2-chlorophenyl)-4-cyano-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxyl ic acid phenylamide (CP-272871) for the CB(1) cannabinoid receptor. In Process Citation Biochem Pharmacol 60:1315–1323. [DOI] [PubMed] [Google Scholar]
- Meyer AH, Katona I, Blatow M, Rozov A, Monyer H. (2002) In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci 22:7055–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ, Davis BJ, Kearn CS, Marcus D, Cook AJ, Wager-Miller J, Straiker A, Myoga MH, Karduck J, Leishman E, et al. (2014) Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J Neurosci 34:5152–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. (2001) CB1 receptor-G protein association. Subtype selectivity is determined by distinct intracellular domains. Eur J Biochem 268:499–505. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. (2005) Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol. 67:2016–2024. [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ. (2012) β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol Psychiatry 71:714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Lewis DL. (2001a) The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience 107:161–167. [DOI] [PubMed] [Google Scholar]
- Nie J, Lewis DL. (2001b) Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J Neurosci 21:8758–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus JL, Liu Y, Wallis KT, Egertová M, Bhartur SG, Mukhopadhyay S, Shi S, He H, Selley DE, Howlett AC, et al. (2007) CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol 72:1557–1566. [DOI] [PubMed] [Google Scholar]
- Oster B, Thorsen K, Lamy P, Wojdacz TK, Hansen LL, Birkenkamp-Demtröder K, Sørensen KD, Laurberg S, Orntoft TF, Andersen CL. (2011) Identification and validation of highly frequent CpG island hypermethylation in colorectal adenomas and carcinomas. Int J Cancer 129:2855–2866. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2008) Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J 22:2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416. [DOI] [PubMed] [Google Scholar]
- Shapira M, Gafni M, Sarne Y. (2003) Long-term interactions between opioid and cannabinoid agonists at the cellular level: cross-desensitization and downregulation. Brain Res 960:190–200. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15:91–119. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Brunk LK, Selley DE. (2001) Inhibitory effects of SR141716A on G-protein activation in rat brain. Eur J Pharmacol 414:135–143. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. (2006) Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol 70:986–996. [DOI] [PubMed] [Google Scholar]
- Singh SN, Bakshi K, Mercier RW, Makriyannis A, Pavlopoulos S. (2011) Binding between a distal C-terminus fragment of cannabinoid receptor 1 and arrestin-2. Biochemistry 50:2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TH, Sim-Selley LJ, Selley DE. (2010) Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br J Pharmacol 160:454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer B, Wallis KT, Wilson SP, Egertová M, Elphick MR, Lewis DL, Hardy LR. (2011) CRIP1a switches cannabinoid receptor agonist/antagonist-mediated protection from glutamate excitotoxicity. Neurosci Lett 503:224–228. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. (2005) Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569:501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. (2009) Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience 163:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. (2009) Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol 76:1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Wager-Miller J, Hutchens J, Mackie K. (2012) Differential signalling in human cannabinoid CB1 receptors and their splice variants in autaptic hippocampal neurones. Br J Pharmacol 165:2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker AJ, Borden CR, Sullivan JM. (2002) G-protein alpha subunit isoforms couple differentially to receptors that mediate presynaptic inhibition at rat hippocampal synapses. J Neurosci 22:2460–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer T, Monastirsky B, Codina J, Birnbaumer L. (1989) Studies on nucleotide and receptor regulation of Gi proteins: effects of pertussis toxin. Mol Endocrinol 3:1115–1124. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. (2003) Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467:60–79. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Valjent E, Firmo C, Mas M, Beslot F, Defer N, Roques BP, Hanoune J, Maldonado R. (2000) Cannabinoid withdrawal is dependent upon PKA activation in the cerebellum. Eur J Neurosci 12:1038–1046. [DOI] [PubMed] [Google Scholar]
- Villares J. (2007) Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience 145:323–334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.