Abstract
Objective
This study aimed to assess the association between bone mineral density (BMD) and coronary atherosclerosis in healthy postmenopausal women.
Methods
We performed a retrospective review of 252 postmenopausal women who had visited a health promotion center for a routine checkup. BMD of the lumbar spine (L1-L4) and femoral neck was evaluated using dual-energy X-ray absorptiometry, and coronary atherosclerosis was assessed using 64-row multidetector computed tomography. Participants were divided into normal BMD and osteopenia-osteoporosis groups, according to the T-scores of their lumbar spine or femoral neck.
Results
Participants with osteopenia-osteoporosis had a significantly higher proportion of coronary atherosclerosis than did those with normal BMD at the lumbar spine (P=0.003) and femoral neck (P=0.004). Osteopenia-osteoporosis at the lumbar spine (odds ratio [OR], 2.86; 95% confidence interval [CI], 1.12 to 7.27) or femoral neck (OR, 3.35; 95% CI, 1.07 to 10.57) was associated with coronary atherosclerosis, after controlling for age and cardiovascular risk factors.
Conclusion
Decreased BMD is associated with coronary atherosclerosis in healthy postmenopausal women, independent of age and cardiovascular risk factors. Postmenopausal women with decreased BMD may have a higher risk of developing coronary atherosclerosis.
Keywords: Bone density, Coronary artery disease, Menopause
Introduction
Coronary artery disease (CAD) is a major cause of death among postmenopausal women [1]. As time from the onset of menopause is closely related to the onset of coronary atherosclerosis, the prevalence of CAD has risen in tandem with increased life expectancy. As a result, the number of postmenopausal women who suffer from CAD is expected to increase over time. Therefore, identifying the risk factors associated with CAD in postmenopausal women is critical for reducing related mortality, along with associated healthcare costs.
Previous studies have demonstrated that osteoporosis and atherosclerosis have common etiological factors and mechanisms, suggesting that the presence of osteoporosis is a predictor of atherosclerosis [2,3,4]. According to recent studies, decreased bone mineral density (BMD) is associated with vascular calcification, which has been found to be a predictor of overall CAD incidence and mortality. Furthermore, several prospective studies have reported that low BMD and bone loss are risk factors for CAD-related mortality [5,6,7].
We have previously reported that values of brachial-ankle pulse wave velocity, an indicator of arterial stiffness, were significantly higher in an osteoporosis group (compared to osteopenia and normal BMD groups), and that these values were negatively associated with BMD [8]. These findings indicate that decreased BMD may increase the risk of developing coronary atherosclerosis. However, little is known about the association between decreased BMD and coronary atherosclerosis in postmenopausal woman; therefore, we chose to investigate the association between BMD and coronary atherosclerosis in healthy postmenopausal women.
Materials and methods
1. Participants
The study was approved by the institutional review board of Severance Hospital, Yonsei University College of Medicine. We performed a retrospective review of postmenopausal women who had visited the health promotion center at Gangnam Severance Hospital, Seoul, Korea for a routine checkup from March 2007 to December 2009. Body weight and height were measured in light indoor clothing, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Blood pressure was assessed in the sitting position, after 10 minutes of rest, using an automated device (TM-2665P, A&D Co., Tokyo, Japan). All participants underwent dual-energy X-ray absorptiometry (DEXA) and 64-row multidetector computed tomography (64-MDCT) as part of the routine checkup.
We included postmenopausal women over 45-years-old, with menopause defined as 12 months of amenorrhea, subsequently confirmed by serum follicle-stimulating hormone levels >30 mIU/mL. Exclusion criteria included current smoking status, hypertension, diabetes mellitus, hypercholesterolemia, and cardiovascular disease. Participants who were receiving menopausal hormone therapy (MHT) or medication that might directly affect BMD were also excluded. A total of 252 participants who satisfied these criteria were included in the study analysis.
Physical activity was divided into two categories. Active exercise was defined as exercising three or more times per week for over 30 minutes. Alcohol intake was divided into two categories: those who consumed alcohol and those who did not.
2. Bone mineral density measurement
Areal BMD (g/cm2) was assessed using DEXA (QDR-Delphi; Hologic, Bedford, MA, USA) in the lumbar spine (L1-L4) and femoral neck. Participants were divided into normal BMD and osteopenia-osteoporosis groups, according to the T-scores of their lumbar spine and femur neck. Normal BMD, osteopenia, and osteoporosis were defined according to the World Health Organization's criteria. Osteoporosis was defined as bone density 2.5 or more standard deviations (SD) below the young adult mean (-2.5 SD or lower), and osteopenia was defined as bone density between 1 and 2.5 SD below the young adult mean (-1 to -2.5 SD) [9].
3. Coronary artery assessment
Cardiac computed tomography was performed using a 64-MDCT scanner (Philips Brilliance 64, Philips Medical System, Best, Netherlands). A β-blocker (40-80 mg of propranolol hydrochloride; Pranol, Dae Woong, Seoul, Korea) was administered orally 1 hour before the scan, to decrease the heart rate of women with a heart rate of ≥70 beats/min. Image reconstruction was performed on the scanner's workstation using commercially available software (Extended Brilliance Workstation, Philips Medical System). Coronary atherosclerosis was defined as any size of calcified, or non-calcified, atherosclerotic plaque with luminal narrowing.
4. Statistical analysis
Results were expressed as the mean±SD. The two-sample t-test was used to compare means of continuous variables, and the χ2-test was used to compare proportions of categorical variables. Logistic regression analysis was conducted to examine associations between decreased BMD and coronary atherosclerosis. Analyses were adjusted for age, alcohol intake, exercise, and vascular risk factors, which included BMI, fasting glucose levels, systolic blood pressure, and high-density lipoprotein (HDL) cholesterol levels.
All statistical analyses were conducted using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). A P-value less than 0.05 was considered to be of statistical significance.
Results
The average clinical and laboratory results for all participants are listed in Table 1. Age was significantly higher, and BMI was significantly lower, among women with osteopenia-osteoporosis compared to women with normal BMD. BMD values for the lumbar spine in the normal and osteopenia-osteoporosis groups were 1.01±0.09 and 0.85±0.10 g/cm2 respectively. BMD values for the femoral neck in the normal and osteopenia-osteoporosis groups were 0.77±0.06 and 0.62±0.07 g/cm2 respectively (Table 1).
Table 1. Clinical and laboratory characteristics of participants.
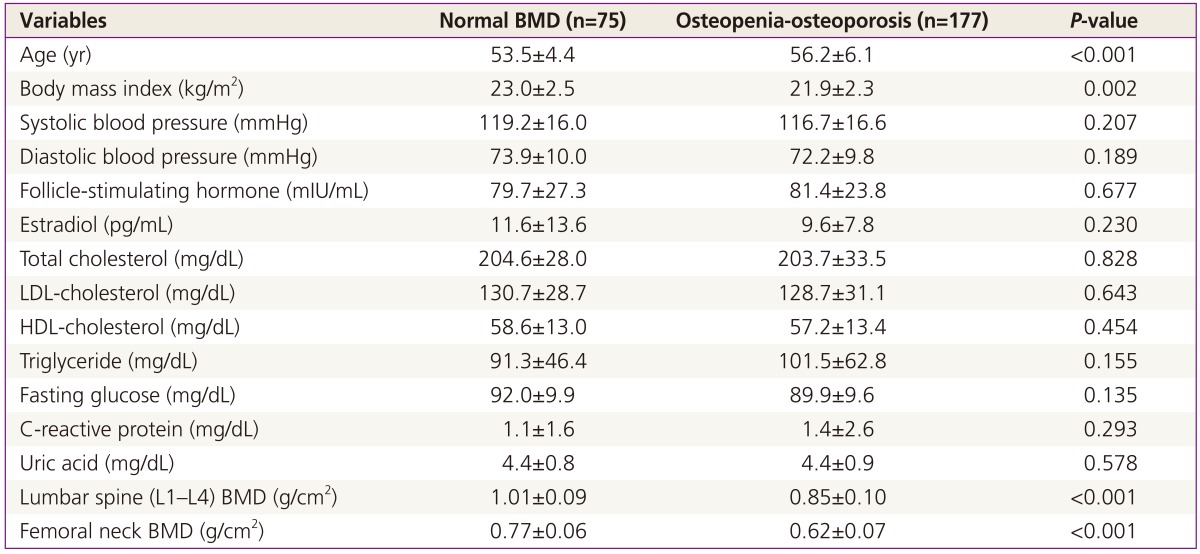
BMD, bone mineral density; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
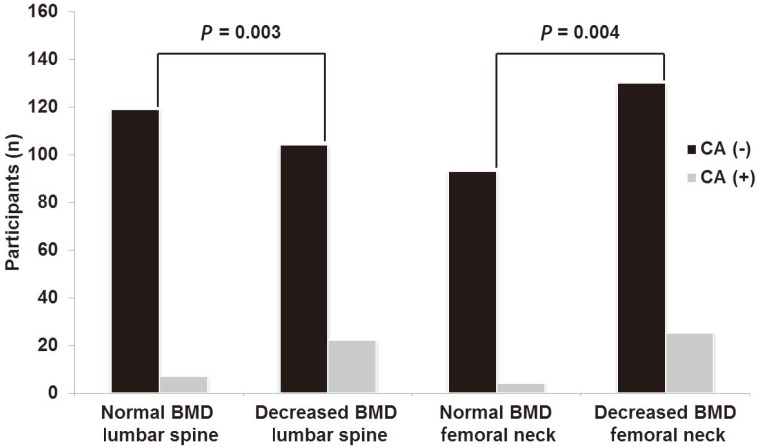
Fig. 1 shows the proportion of coronary atherosclerosis in the normal BMD and osteopenia-osteoporosis groups. Participants with osteopenia-osteoporosis had a significantly higher proportion of coronary atherosclerosis at the lumbar spine (P=0.003) and femoral neck (P=0.004), when compared to those with normal BMD.
Fig. 1. Distribution of CA (-) and CA (+) patients in normal and osteopenia-osteoporosis groups. CA, coronary atherosclerosis; BMD, bone mineral density.
Multiple regression analysis was performed to investigate the association between decreased BMD and coronary atherosclerosis. Osteopenia-osteoporosis at the lumbar spine (odds ratio [OR], 2.855; 95% confidence interval [CI], 1.121 to 7.23) and femoral neck (OR, 3.354; 95% CI, 1.065 to 10.569) was associated with coronary atherosclerosis, after controlling for age and vascular risk factors (Table 2).
Table 2. Logistic regression analysis of association between osteopenia-osteoporosis and atherosclerosis.
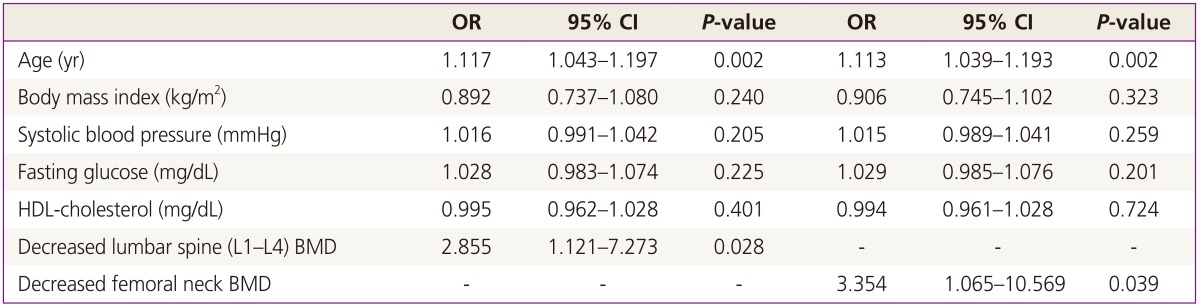
Adjusted for age, alcohol intake, exercise, and vascular risk factors, including body mass index, systolic blood pressure, fasting glucose, and HDL-cholesterol.
OR, odds ratio; CI, confidence interval; HDL, high-density lipoprotein; BMD, bone mineral density.
Discussion
The present study revealed that decreased BMD was significantly associated with coronary atherosclerosis, independent of other risk factors. These findings suggest that postmenopausal women with decreased BMD may have a higher risk of developing coronary atherosclerosis. Our results confirmed a significant relationship between osteoporosis and coronary atherosclerosis, as previous studies have suggested, as well as a relationship between osteopenia and coronary atherosclerosis.
Menopause is a natural aging process, characterized by a decrease in estrogen and an increase in ferritin levels, during which a woman passes from her reproductive to non-reproductive years [10]. Estrogen deficiency impairs the normal bone remodeling cycle by increasing osteoclast resorption activity, without a corresponding increase in osteoblast activity, resulting in a net loss of bone [11]. Free iron deposits in tissues also create a pathological condition (iron overload), which impairs bone remodeling by inhibiting osteoblast proliferation and differentiation [12]. Meanwhile, it is well known that estrogen prevents the development of atherosclerosis, and that risk of CAD increases for postmenopausal women. In addition, excess iron in the tissues may catalyze the formation of highly reactive oxygen free radicals, which can trigger the development of atherosclerosis. Our previous study has shown that ferritin levels were associated with an increased risk of subclinical coronary atherosclerosis in postmenopausal women [13]. These facts suggest that decreased BMD may be associated with coronary atherosclerosis, especially in postmenopausal woman.
In postmenopausal women, osteoporosis and CAD are major causes of morbidity and mortality. Although MHT has been used to prevent these two diseases, MHT is no longer recommended as a first-line therapy for osteoporosis and CAD. Therefore, identification of individual with a high risk of the diseases is important for the prevention. As estrogen deficiency and iron overload in the postmenopausal period are common risk factors for both osteoporosis and CAD, measuring BMD may be a useful screening tool for identifying postmenopausal women with high-risk of developing osteoporotic fractures or CAD.
The key pathological step in atherosclerosis is thought to be the formation of calcified plaque on the endothelial surface. However, the results from previous studies are in disagreement regarding whether osteoporosis truly influences vascular calcification. In contrast to our results, some authors have indicated that there is no relationship between osteoporosis and atherosclerosis [14,15]. In another study, osteoporosis and coronary atherosclerosis were found to be independent processes in postmenopausal women, after adjusting for age, smoking status, diabetes mellitus, hypertension, and hypercholesterolemia [16]. However, other researchers have found that cardiovascular mortality is higher in women with low BMD [6]. Recent studies have also found that atherosclerotic calcification is associated with bone loss in postmenopausal women [17,18], and that a decrease in BMD may be related to subclinical atherosclerosis [7,19,20,21]. The mechanisms explaining the relationship between osteoporosis and CAD have not yet been elucidated, though it is believed that many other factors may be responsible; these factors have yet to be investigated.
We attempted to investigate the association between BMD and coronary atherosclerosis using 64-MDCT, which can accurately detect coronary atherosclerosis, even in asymptomatic individuals. Reported sensitivity and specificity of 64-MDCT range from 85% to 99% and 86% to 96%, respectively [22,23,24,25]. 64-MDCT has also improved the detection of non-calcified plaques, which, compared to calcified plaques, are considered more likely to be unstable and vulnerable to sudden rupture, subsequently increasing the risk of fatal ischemic cardiac events [26,27]. In another study, a considerable proportion of non-calcified plaques were found in asymptomatic low-risk patients with no, or mild, coronary calcium [28]. Moreover, we included only healthy postmenopausal women by excluding participants with a medical history of hypertension, diabetes mellitus, hypercholesterolemia, or cardiovascular disease. Therefore, correlation between BMD and CAD in previous studies and our study BMD is a considerable screening method for coronary atherosclerosis in healthy postmenopausal women, thereby facilitating early treatment and better outcomes.
Although our results are encouraging, the retrospective nature of the study precludes the possibility of investigating the causal relationship between bone loss and coronary atherosclerosis. In addition, our hospital-based design, and the limited number of participants, restricted our ability to reach a precise conclusion regarding the association between bone loss and coronary atherosclerosis. Larger longitudinal studies would likely be required to fully investigate the effects of bone loss on coronary atherosclerosis in postmenopausal women and clarify BMD is reliable screening method for CAD.
In conclusion, our results demonstrate that decreased BMD is associated with coronary atherosclerosis in healthy postmenopausal women, independent of age and cardiovascular risk factors. Therefore, postmenopausal women with low BMD may have a higher risk of developing coronary atherosclerosis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 2.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 3.Frye MA, Melton LJ, 3rd, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, et al. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–194. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 4.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 6.Browner WS, Seeley DG, Vogt TM, Cummings SR Study of Osteoporotic Fractures Research Group. Nontrauma mortality in elderly women with low bone mineral density. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 7.Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 8.Seo SK, Cho S, Kim HY, Choi YS, Park KH, Cho DJ, et al. Bone mineral density, arterial stiffness, and coronary atherosclerosis in healthy postmenopausal women. Menopause. 2009;16:937–943. doi: 10.1097/gme.0b013e3181a15552. [DOI] [PubMed] [Google Scholar]
- 9.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10:259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 10.Jian J, Pelle E, Huang X. Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal. 2009;11:2939–2943. doi: 10.1089/ars.2009.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li GF, Pan YZ, Sirois P, Li K, Xu YJ. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int. 2012;23:2403–2408. doi: 10.1007/s00198-012-1982-1. [DOI] [PubMed] [Google Scholar]
- 13.Seo SK, Yun BH, Chon SJ, Lee YJ, Han EJ, Park JH, et al. Association of serum ferritin levels with metabolic syndrome and subclinical coronary atherosclerosis in postmenopausal Korean women. Clin Chim Acta. 2015;438:62–66. doi: 10.1016/j.cca.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Hyder JA, Allison MA, Criqui MH, Wright CM. Association between systemic calcified atherosclerosis and bone density. Calcif Tissue Int. 2007;80:301–306. doi: 10.1007/s00223-007-9004-6. [DOI] [PubMed] [Google Scholar]
- 15.Aoyagi K, Ross PD, Orloff J, Davis JW, Katagiri H, Wasnich RD. Low bone density is not associated with aortic calcification. Calcif Tissue Int. 2001;69:20–24. doi: 10.1007/s002230020003. [DOI] [PubMed] [Google Scholar]
- 16.Sinnott B, Syed I, Sevrukov A, Barengolts E. Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int. 2006;78:195–202. doi: 10.1007/s00223-005-0244-z. [DOI] [PubMed] [Google Scholar]
- 17.Burnett JR, Vasikaran SD. Cardiovascular disease and osteoporosis: is there a link between lipids and bone? Ann Clin Biochem. 2002;39(Pt 3):203–210. doi: 10.1258/0004563021902134. [DOI] [PubMed] [Google Scholar]
- 18.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 19.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 20.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 21.Jung YS, Hwang HJ, Yun BH, Chon SJ, Cho S, Choi YS, et al. Renal function is associated with bone mineral density and arterial stiffness in healthy postmenopausal women. Gynecol Obstet Invest. 2014;78:124–129. doi: 10.1159/000363746. [DOI] [PubMed] [Google Scholar]
- 22.Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244:419–428. doi: 10.1148/radiol.2442061218. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, Lin C, Davidson R, Dong C, Liao Y. Diagnostic value of 64-slice CT angiography in coronary artery disease: a systematic review. Eur J Radiol. 2008;67:78–84. doi: 10.1016/j.ejrad.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28:3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 25.Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X, et al. 64-slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–1393. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 26.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 27.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki K, Matsumoto T, Aono H, Furukawa H, Samukawa M. Prevalence of subclinical atherosclerosis in asymptomatic patients with low-to-intermediate risk by 64-slice computed tomography. Coron Artery Dis. 2011;22:18–25. doi: 10.1097/MCA.0b013e32833b20a5. [DOI] [PubMed] [Google Scholar]