Abstract
Objective
The purpose of present study was to evaluate association between neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) and endometrial hyperplasia (EH).
Methods
One hundred and ten women with abnormal uterine bleeding were included into the study. Blood samples were drawn from all patients to obtain complete blood cell counts, neutrophil-leukocyte ratio and platelet-leukocyte ratio before endometrial curettage procedure initiated. The patients were divided into three groups due to their pathological results: group 1, patients with EH without atypia (n=40); group 2, patients with EH with atypia (n=15); and group 3, patients with neither hyperplasia nor cancer as control group (n=55). Blood cell counts, NLRs and PLRs were compared among these groups.
Results
Based on hemoglobin and platelet counts, there was no significant difference among these groups (P>0.05). Leukocyte and neutrophil counts were higher in group 2 (EH with atypia) than group 1 and group 3 (P<0.01). NLR of group 2 was significantly elevated when compared to group 1 and group 3 (P=0.004). PLR was higher in group 1 and group 2 than control group (P=0.024).
Conclusion
Non-specific inflammatory markers such as NLR and PLR were elevated in women with atypical EH. These markers may be used as a predictor of atypical EH in patients with abnormal uterine bleeding.
Keywords: Atypia, Blood cells, Endometrial hyperplasia, Inflammatory, Markers
Introduction
Endometrial hyperplasia (EH) occurs when endometrial cells exposed to the estrogen for a long time and it is known as one of the most important risk factor of endometrial cancer (EC). There is even 30% risk of EC in patient with EH and atypia [1]. It is well known that risk factors such as hormone treatment, late menopause, anovulation, nulliparity affect EC progress through sex hormone mechanism [2]. At the same time many of these risk factors may also exert pro-inflammatory carcinogenic effects [3]. Inflammatory processes, sex hormones and inflammatory markers play an important role in endometrial growing, shedding and repair of endometrium during menstruation cycles [4,5]. In addition, chronic inflammatory process may play an important role in the development of EC through both intrinsic and extrinsic pathway [6].
Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) which obtained from complete blood count (CBC) in peripheral blood are nonspecific markers of inflammation. NLR may also related in certain pathologies characterized by inflammatory response such as ulcerative colitis and coronary artery disease [7,8]. In addition, high NLR is detected in patients with EC[9]. PLR is currently considered to be a prognostic factor in breast cancer, ovarian and colorectal cancers [10].
Based on these data, we hypothesized that EH as a precursor of EC may also be related with inflammatory process. The aim of this study was to assess association between NLR and PLR with EH in patients with abnormal uterine bleeding.
Materials and methods
This retrospective cross-sectional study consists of 55 patients who had EH and 55 patients who had neither EH nor cancer based on their endometrial biopsy report from pathology department between January 2011 and December 2013 at a university hospital. The study protocol was approved by the Ethics Committee of the Gaziosmanpasa University Hospital. All patients had abnormal uterine bleeding. Menorrhagia, metrorrhagia, menometrorrhagia, and postmenopausal uterine bleeding were considered as abnormal uterine bleeding. The exclusion criteria were use of steroid hormones such as; hormone replacement therapy, oral contraceptives or tamoxifen during the last 6 months, patients with cervical, endometrial, ovarian cancer or other malignancies, endometriosis, hematological disease, inflammatory disease, receiving blood transfusion for any reasons during last 3 months and positive family history of EC.
Physical and gynecological examinations, transvaginal ultrasound scanning and laboratory tests, such as human chorionic gonadotropin, CBC, prothrombin time, activated partial thromboplastin time and international normalized ratio (obtained from 10 mL venous blood samples), were performed for differential diagnosis of abnormal uterine bleeding before endometrial curettage procedure initiated. After evaluation of test results, all patients had undergone endometrial curettage. Blood cells count, NLR (defined as the absolute neutrophil count divided by the absolute lymphocyte count) and PLR (defined as the absolute platelet count divided by the absolute lymphocyte count) were obtained from CBC which obtained before endometrial biopsy procedure.
The histological samples were assessed by the pathologists. The results of EH were classified as four categories: 1) simple EH without atypia, 2) complex EH without atypia, 3) simple atypical EH, and 4) complex atypical EH. The other results of samples, considered as normal, were included proliferative endometrium, secretory endometrium, atrophic endometrium, and endometrial cell fragments. The patients were divided into three groups due to their endometrial pathological results: group 1, EH without atypia (n=40); group 2, EH with atypia (n=15); and group 3, neither hyperplasia nor cancer as control (n=55). Blood cells count, NLR and PLR were compared among the groups.
All data were analyzed using the PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA). Mean±standard deviation and mean (minimum-maximum) were used in variables with and without normal distribution, respectively. Analysis of variance with Scheffe's post hoc test in analysis of variance (ANOVA) was used to analyze the differences among groups for the parametric variables. The Kruskal-Wallis test was used to compare the nonparametric variables. The area under the curve was calculated in a receiver operating characteristic (ROC) analysis to evaluate if the NLR and PLR were determinant to distinguish EH group from control. Pearson correlation test was used to evaluate the association among parametric variables. A value of P<0.05 was considered to indicate statistical significance.
Results
The comparison of demographic parameters among the three groups is given in the Table 1. The mean age of group-1 was significantly lower than group 2 and control (P<0.05). The other features including parity, menopausal status and presence of systemic diseases were similar among all groups (P>0.05).
Table 1. Distribution of demographic features of subjects.
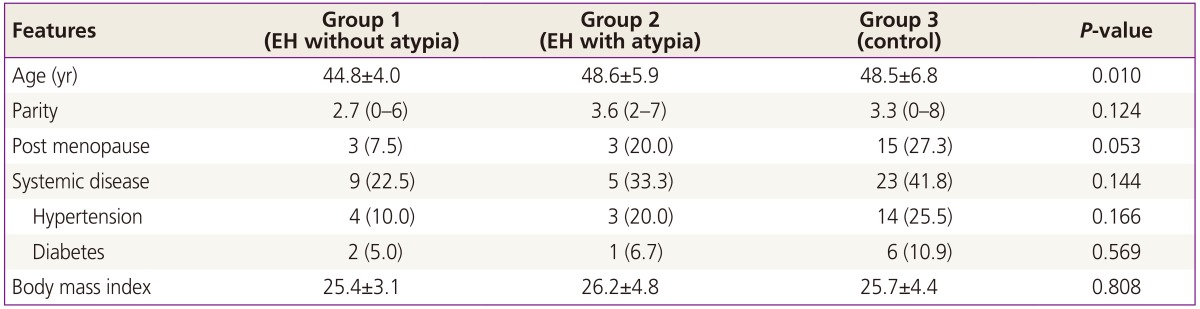
Data are given as mean±standard deviation, mean (min-max), or n (%).
EH, endometrial hyperplasia.
There was no significant difference among groups for hemoglobin levels and platelet counts (Table 2). Leukocyte counts and neutrophil counts as a subgroup of leukocyte were higher in group 2 (EH with atypia) than other groups (P<0.05), whereas there was no significant difference in lymphocyte counts among the groups (P>0.05). There was no significant difference between group 1 (EH without atypia) and control for leukocyte, neutrophil and lymphocyte counts (P>0.05). NLR was found significantly higher in the group 2 when compared with other groups (P=0.004). PLRs of group 1 and group 2 were detected significantly higher than control group (P=0.024). Although there was no predictive value for NLR to distinguish EH from control group, cut-off value of PLR was found as ≥115.3 to predict EH (ROC, 0.636; 95% confidence interval, 0.532 to 0.740; P=0.014). No correlation was found between body mass index (BMI) and these inflammatory markers (NLR and PLR) (P>0.05).
Table 2. Comparison of hematological parameters among the groups.
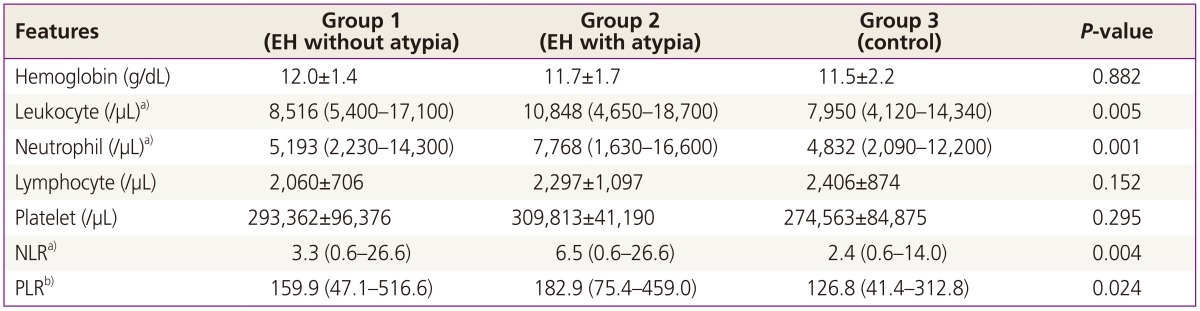
Data are given as mean±standard deviation or mean (min-max).
EH, endometrial hyperplasia; NLR, neutrophil-leukocyte ratio; PLR, platelet-leukocyte ratio.
a)These parameters were higher in group 2 than the others; b)PLR was lower in group 3 than the others.
Discussion
In this study, there were no significant difference in hemoglobin level and platelet counts among patients admitted to the hospital with atypical EH, EH without atypia and normal endometrium because of abnormal uterine bleeding. Even though there are not enough research data in the literature about EH and its relationship with inflammatory markers in peripheral blood, it is known that, preoperative anemia and thrombocytosis have strong relationship with predicting prognosis and staging of the disease in patients diagnosed with EC [11]. Njolstad et al. [12] reported that 5 years of survival rate of patients with anemia is about 61.3%, whereas survival rate of patients without anemia is 87.7%. The same study showed that leukocytosis and thrombocytosis are related to poor prognosis and advance stage of EC. Ayhan et al. [13] reported higher preoperative platelet counts, even in conditions with normal range, may reflect poor prognostic factors such as cervical involvement and high grade among patients with endometrial carcinoma. In addition, a study revealed that even though there were no difference between platelet counts between EC and control groups, the mean platelet volume which is one of morphological parameters of platelet, is higher in EC group than normal control groups [14]. On the other hand, Wang et al. [15] reported that NLR and PLR were found higher in patients with EC with cervical stromal invasion, and suggested the evaluation of these ratios may help select patients who should be particularly watched and tested for cervical stromal involvement.
Acmaz et al. [9] reported that NLR was higher in group with EC than EH and control groups, but there was no difference between EH group and control. PLR is found higher in EH and EC groups than control group, whereas there was no difference between EH and EC groups. At the same study, it found that in cases with EC, leukocyte and neutrophil counts were higher than control groups, whereas there was not difference when compared to EH group.
In our study, EH cases were divided into two groups based on presence or absence of atypia. In the presence of atypia there was absolute elevation of leukocyte and netrophil counts in peripheral blood. Similar to that, in atypical EH group NLR and PLR which are nonspecific inflammatory markers found higher compared to EH without atypia. It is defined that EC is closely related to inflammatory process. Previous studies revealed that inflammatory markers such as C-reactive protein, interleukin (IL)-1, IL-6 elevated in EC cases [16,17]. It is also found that there is higher ratio of gene polymorphism in inflammatory process in such cases [18]. We know that inflammatory process effects development of cervical cancer [19]. In a study which investigated survival of patients with cervical cancer, authors reported that pretreatment NLR may be a costeffective biomarker to stratify risk of recurrence and death in patients with cervical cancer [20]. In contrast, another investigation reported that the pretreatment NLR and PLR are not enough to predict the survival of patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy [21]. But there are not any studies that show relationship between EH, especially with atypia, and inflammatory processes. Since inflammatory processes, have a strong relationship with EC, may also be related to EH. Adipose tissue is source of estrogen production due to aromatization of androgens to estrogens after menopause, and associated with secretion of proinflammatory cytokines [22,23]. Thus, obesity is one of the strongest risk factors for EC [24]. Inflammation may mediate the association between obesity and EC and endometrial carcinogenesis can be promoted by an inflammatory milieu [25,26]. In our study, BMI was not found different among the groups, and there was no correlation between BMI and inflammatory markers (NLR and PLR).
As conclusion, leukocyte count, neutrophile count, NLR, PLR found significantly higher in EH with atypia cases. High level of these inflammatory markers in EH with atypia may be explained via it is a precursor lesion of EC. It might be possible to predict EH with atypia based on routine work up of those parameters from the blood drawn for patients admitted with abnormal uterine bleeding.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Heller DS, Mosquera C, Goldsmith LT, Cracchiolo B. Body mass index of patients with endometrial hyperplasia: comparison to patients with proliferative endometrium and abnormal bleeding. J Reprod Med. 2011;56:110–112. [PubMed] [Google Scholar]
- 2.Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Ann N Y Acad Sci. 2001;943:296–315. doi: 10.1111/j.1749-6632.2001.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 3.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14:2840–2847. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- 4.Salamonsen LA. Tissue injury and repair in the female human reproductive tract. Reproduction. 2003;125:301–311. doi: 10.1530/rep.0.1250301. [DOI] [PubMed] [Google Scholar]
- 5.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kucuk H, Gursoy S, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27:72–76. doi: 10.1002/jcla.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. 2006;17:225–233. doi: 10.1016/j.cytogfr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Acmaz G, Aksoy H, Unal D, Ozyurt S, Cingillioglu B, Aksoy U, et al. Are neutrophil/lymphocyte and platelet/lymphocyte ratios associated with endometrial precancerous and cancerous lesions in patients with abnormal uterine bleeding? Asian Pac J Cancer Prev. 2014;15:1689–1692. doi: 10.7314/apjcp.2014.15.4.1689. [DOI] [PubMed] [Google Scholar]
- 10.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer: a Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Metindir J, Bilir Dilek G. Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin Oncol. 2009;135:125–129. doi: 10.1007/s00432-008-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njolstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013;131:410–415. doi: 10.1016/j.ygyno.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Ayhan A, Bozdag G, Taskiran C, Gultekin M, Yuce K, Kucukali T. The value of preoperative platelet count in the prediction of cervical involvement and poor prognostic variables in patients with endometrial carcinoma. Gynecol Oncol. 2006;103:902–905. doi: 10.1016/j.ygyno.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Oge T, Yalcin OT, Ozalp SS, Isikci T. Platelet volume as a parameter for platelet activation in patients with endometrial cancer. J Obstet Gynaecol. 2013;33:301–304. doi: 10.3109/01443615.2012.758089. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Yang JX, Cao DY, Wan XR, Feng FZ, Huang HF, et al. Preoperative neutrophil-lymphocyte and plateletlymphocyte ratios as independent predictors of cervical stromal involvement in surgically treated endometrioid adenocarcinoma. Onco Targets Ther. 2013;6:211–216. doi: 10.2147/OTT.S41711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedenreich CM, Langley AR, Speidel TP, Lau DC, Courneya KS, Csizmadi I, et al. Case-control study of inflammatory markers and the risk of endometrial cancer. Eur J Cancer Prev. 2013;22:374–379. doi: 10.1097/CEJ.0b013e32835b3813. [DOI] [PubMed] [Google Scholar]
- 17.Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort: a factor analysis. Am J Epidemiol. 2013;177:787–799. doi: 10.1093/aje/kws309. [DOI] [PubMed] [Google Scholar]
- 18.Delahanty RJ, Xiang YB, Spurdle A, Beeghly-Fadiel A, Long J, Thompson D, et al. Polymorphisms in inflammation pathway genes and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:216–223. doi: 10.1158/1055-9965.EPI-12-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deivendran S, Marzook KH, Radhakrishna Pillai M. The role of inflammation in cervical cancer. Adv Exp Med Biol. 2014;816:377–399. doi: 10.1007/978-3-0348-0837-8_15. [DOI] [PubMed] [Google Scholar]
- 20.Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32:1555–1561. [PubMed] [Google Scholar]
- 21.Wang D, Wu M, Feng FZ, Huang HF, Yang JX, Shen K, et al. Pretreatment neutrophil-to-lymphocyte and plateletto-lymphocyte ratios do not predict survival in patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy. Chin Med J (Engl) 2013;126:1464–1468. [PubMed] [Google Scholar]
- 22.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 23.Austin H, Austin JM, Jr, Partridge EE, Hatch KD, Shingleton HM. Endometrial cancer, obesity, and body fat distribution. Cancer Res. 1991;51:568–572. [PubMed] [Google Scholar]
- 24.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 25.Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010;17:1007–1019. doi: 10.1677/ERC-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Rohan TE, Gunter MJ, Xue X, Wactawski-Wende J, Rajpathak SN, et al. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev. 2011;20:971–977. doi: 10.1158/1055-9965.EPI-10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]


