Abstract
Interleukin-25 (IL-25) promotes Type-2 immunity by inducing the expression of Th2-associated cytokines. While it is known that the IL-25R (IL-17RB) recruits the adaptor protein ACT1, the IL-25R signaling mechanism remains poorly understood. While screening for IL-25R components, we found that IL-25 responses were impaired in Traf4 –/– cells. Administering IL-25 to Traf4 –/– mice resulted in blunted airway eosinophilia and Th2 cytokine production. Notably, IL-25R recruitment of TRAF4 was required for the ACT1/IL-25R interaction. Mechanistically, TRAF4 recruited the E3-ligase SMURF2, to degrade the IL-25R-inhibitory molecule DAZAP2. Silencing Dazap2 increased ACT1/IL-25R interaction and IL-25 responsiveness. Moreover a tyrosine within the IL-25R elicited DAZAP2 interference. This study indicates that TRAF4-SMURF2-mediated DAZAP2 degradation is a crucial initiating event for the IL-25 response.
Introduction
Excessive inflammation in response to otherwise innocuous allergens contributes to the pathology associated with asthma. An important question is how Th2 cell type immune responses (type-2 responses) are initiated in response to allergen exposure and linked to allergic inflammation. Recent exciting studies have begun to reveal the mechanisms by which the epithelium modulates type 2 responses through the production of a group of epithelial-derived Th2 cell-driving cytokines, including IL-25, IL-33, and TSLP. These epithelial-derived Th2 cell-driving cytokines maintain the balance of host immune homeostasis and defense against various pathogens, whereas dysregulation of these cytokines contributes to excessive type-2 responses and inflammation associated with asthma. In particular, IL-25 induced in airway epithelial cells in response to allergens, has been demonstrated to promote allergic inflammation by directly stimulating Th2-associated cytokine and chemokine production from the airway epithelium as well as from T-cells to exacerbate the pathophysiology of asthma (1-6).
IL-25 is the most structurally divergent member in the IL-17 cytokine family, exerting distinct physiologic responses (7). While IL-17 cytokines, such as IL-17A, are known to induce neutrophil mobilizing cytokines and chemokines resulting in neutrophil recruitment, IL-25 is the only member demonstrated to initiate type-2-driven inflammation (8-10). Administration of IL-25 in mice leads to production of the Th2-associated cytokines IL-4, IL-5, IL-9 and IL-13, with eosinophil recruitment and IgE production (1, 4, 8, 11-13). Elevated levels of IL-25 and its receptor were detected in asthmatic lung tissues, linking their roles in allergic pulmonary inflammation (6). In allergic asthma models, mice deficient in IL-25 exhibited reduced cell-infiltrate into the lungs and diminished type-2 cytokine production (3, 4, 14, 15). IL-25 signaling in multiple cell-types, including epithelial cells, type-2 innate lymphoid cells (ILC2s) and T-cells, contribute to IL-25-mediated pathology. Thus, emerging studies have been devoted to target the IL-25 signaling pathway for the development of new strategies for the treatment of asthma and other allergic inflammatory diseases.
The receptors for IL-17A (IL-17RA and IL-17RC for IL-17R) and IL-25 (IL-17RA and IL-17RB for IL-25R) belong to a common superfamily, defined by a highly conserved SEFIR domain (Similar Expression to FGF genes and IL-17 receptors) in the cytoplasmic region (13, 16, 17). The SEFIR domain facilitates homotypic interactions with other SEFIR domain containing molecules. Work from our group and others have shown that the adaptor molecule known as ACT1 (Activator of NFkB –1, or CIKS), harbors a SEFIR domain, and is a key component in IL-17A and IL-25 signaling (4, 12, 18, 19). We have reported that while mice deficient in Act1 have impaired IL-17-induced pulmonary neutrophil recruitment, Act1 deficiency also abolishes IL-25-induced Th2 cytokines and eosinophil recruitment (4, 12). As a result, Act1–/– mice have reduced allergen-induced pulmonary eosinophilia and inflammatory cytokine production (3, 4). Upon IL-17 or IL-25 stimulation, ACT1 is recruited to the IL-17R and IL-25R respectively through its SEFIR domain. Additionally, ACT1 has an E3-ligase U-box domain and TNF-receptor associated factor (TRAF)-binding sites (20). These domains allow ACT1 to recognize and ubiquitinate TRAF molecules for subsequent downstream signaling. Specifically, ACT1 mediates K63-linked polyubiquitination of TRAF6 and TRAF5, which are critical for IL-17-induced NFkB activation and mRNA stabilization of cytokines/chemokines, respectively (20-22). Although there has been much progress in defining the signaling pathways activated by IL-17A, the molecular mechanism of IL-25R-ACT1 induced signal transduction remains elusive.
In this study we screened for TRAF involvement in the IL-25R signaling cascade in primary epithelial cells derived from TRAF −3, −4 and −6 deficient mice. Using this strategy, we found a striking defect in IL-25 responsiveness in the primary TRAF4-deficient T cells and epithelial cells, and as well as abolished type-2 responses in Traf4–/– mice. Mechanistically, TRAF4 mediates the recruitment of the E3-ligase, smadubiquitin regulatory factor 2 (SMURF2), to the IL-25R. Moreover, SMURF2 is required for IL-25-induced degradation of the inhibitory molecule deleted in azoospermia (DAZ)-associated protein 2 (DAZAP2). Thus, TRAF4-mediated SMURF2-dependent degradation of DAZAP2 is an essential step in order for IL-25-signaling to commence.
Materials and Methods
Mice
TRAF4-deficient (Traf4–/–) C57/BL/6 (B6) mice were generated as described previously (23). SMURF2-deficient (Smurf2–/–) mice were provided by Dr. Ying Zhang (National Cancer Institute, Bethesda, Maryland) and generated as described in (24). IL-17RB-deficient (IL-17rb–/–) mice were obtained from Dr. Wenjun Ouyang (Genentech, San Francisco, California). All experiments used gender and age –matched littermates aged 6-8 weeks. The Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation approved all animal experiments.
Cell culture, transfection and reagents
Primary kidney epithelial cells were isolated from kidneys taken from the indicated mice aged 10-20 days as described previously (25). HEK293 cells were maintained in DMEM plus 10% FBS. Human bronchial epithelial cell-line (Bet1a) were maintained in LHC9 media (Lonza, Gibco, Life Technologies). Cells were grown on pre-treated dishes and plates with coating media; PureCol (Advanced Biomatix), 1 μg/ml BSA (Gibco), 5 μg/ml Fibronectin (Cal Biochem) diluted in LHC Basal Media (Gibco) and passed through a 0.22μM filter. Bet1a cells were passed with trypsin-versene solution (Lonza) and trypsin neutralizing solution (Gibco). SiRNA against TRAF4 was purchased from Qiagen Flexitube siRNA described previously (Zepp et al., 2012). SiRNA was transfected into Bet1a cells using Lipofectamine 2000 (Invitrogen). Proximity-based ligation assays were performed in Hela cells according to the manufacturers instructions (Duolink™Assay, Sigma Aldrich). Experiments involving T-cells and polarization were conducted as described previously (3).
For plasmid transfection 1-3 μg of plasmid was transfected using Lipofectamine 2000 reagent (Invitrogen) following the manufacturers’ specifications. MEF cells stably expressing the IL-25R were described previously (26). Non-targeting shRNA and shRNA targeting murine Dazap2 were purchased from Sigma Aldrich's mission On-Target shRNA in lentiviral vectors or subcloned into an Adenoviral vector. Lentivirus was generated by co-transfection of shRNA plasmids with pCl-VSVG and ps-PAX2 (Addgene) using Lipofectamine 2000 into packaging HEK293T cells cultured for 48 hours. MEFs were then transduced with lentiviral particles in the presence of polybrene (5 μg/ml) for 24 hours after which the cells were maintained in 0.5 μg/ml puromycin containing media. Primary T-cells were transduced with lentivirus as described previously in (27). Antibodies for immunoblots used are as follows; rabbit anti-ACT1 was described previously in (19), goat anti-TRAF4 (N16), mouse anti-Omni tag, rat anti-IL-17RB (TJ5), rabbit anti-Smurf2 (H50), mouse anti-P-ERK1/2 and goat anti-ACTIN were from Santa Cruz Biotech. Rabbit anti-P-STAT6, rabbit anti-P-P38, rabbit anti-HA tag, mouse anti-Myc tag, rabbit anti-P-JNK1/2 were from Cell Signaling Technologies. Rabbit anti-DAZAP2 was from Abcam. Mouse anti-HA tag was from Sigma Aldrich, Rabbit anti-HA was from Cell Signaling Technologies. Mouse anti-V5 Tag was from Invitrogen. Rabbit antibodies against K63- and K48-linked ubiquitination were from Millipore. RNA isolations were performed using TriZol reagent (Invitrogen) according to the manufacturers’ instructions. cDNA was generated from 0.5-1μg of total RNA using Superscript II reverse transcription kit (Invitrogen). All primers used in this study were previously reported (4). Primers for murine Dazap2 are F; CCAGTTGGTCCCATCTATCC, R; AGGAGGTGGAGGAGGAATGT.
Co-immunoprecipitation and western blotting
For Co-immunoprecipitations, cells were pelleted and lysed in Co-IP Buffer (0.5% Triton X-100, 20 mM HEPES [pH 7.6], 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 1mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride [PMSF] and protease inhibitor cocktail tablets [Roche]) The lysates were then incubated overnight with Protein-G sepharose beads conjugated to the indicated antibodies. The beads were then pelleted and wash 4 times with Co-IP buffer. For experiments involving P-Tyr immunoblotting following Co-IP against V5-IL-25R procedure for ubiquitination assay (below) was used except with the addition of Na3VO4 throughout procedure.
Ubiquitination assays
Cells were harvested by washing in cold PBS and then lysed in 1% SDS solution. The lysates were sonicated on high setting for 15s in 4°C water sonicator (Diagenode). Then the lysates were boiled for 10 minutes at 100C after which the boiled samples were diluted with Co-IP buffer to 0.1% SDS and the centrifuged at 13,200 rpm for 10 min, the pellet was discarded. The supernatant was applied to Protein-G sepharose beads and antibodies against HA-tag (Sigma Aldrich) and were rotated overnight at 4°C. The beads were then pelleted and washed three times with Co-IP buffer, the precipitates were resolved by SDS-PAGE, transferred to nitrocellulose membranes and subjected to western blotting with the indicated antibodies.
Plasmids
Plasmids for Myc- and V5- tagged IL-17RB (including TRAF domain mutations), Omni-TRAF4, Myc-Smurf2 WT and CG mutant were described previously (24-26). Plasmid with full-length Dazap2 was purchased from Genecoepia and subcloned into pCDHCMV (GFP) lentiviral vector (System BioSciences). For adenoviral expression of Il-17rb, the mIL-17rb gene was first subcloned into pENTR/D-TOPO vector. Site-directed mutagenesis was carried out using PFU turbo Taq polymerase (Affymetrix); cDNA sequences were all verified by capillary sequencing. The verified cDNA was then moved to pAd/CMV/V5 DEST adenoviral vector (Invitrogen) according to the manufacturers’ protocol.
Adenovirus production and infection of primary cells
Adenovirus production was conducted following the manufacturers’ protocol (Invitrogen). Briefly, PacI linearized pAd/CMV/V5-IL-17rb Destination vector was transfected into HEK293A producer cells (Invitrogen). 8-10 days later viral particles were released from pelleted cells by repeated freeze-thaw cycles. The viral stock was then applied at limiting dilutions on confluent cultures of HEK293 cells, plaques were observed within 5 days and enumerated to calculate MOI. Viruses were further titrated in primary kidney epithelial cells, after which western blot for V5-tag was performed. Primary kidney epithelial cells or MEFs were transduced with adenovirus at an MOI of 3 for 24 hours. Fresh media was added and cells were incubated for an additional 24 hours. Adenovirus expressing Cre-GFP or null-GFP were purchased from Vector BioLabs and used at a concentration of 1 × 106 PFU/ml.
Intratracheal instillation of IL-25 and IL-17
Instillation of recombinant cytokine was carried out as described previously (4). Mice were first anesthesized with ketamine/xylazine cocktail, then 50 μl of recombinant cytokine mix was instilled via sterile syringe and tubing passed through the trachea via the oral cavity (non-surgical). For IL-25 induced airway inflammation, mice were sacrificed and tissue harvested 4 days after the instillation procedure.
Induction of allergic airway inflammation
Mice were sham immunized or OVA-immunized via intra-peritoneal (i.p.) injection with 100 μg OVA (Grade V, Sigma Aldrich, St. Louis, MO) emulsified with 2 mg Alum in a total volume of 200 μl in PBS on Day 0 and Day 10. Mice receiving OVA-immunization were subsequently challenged via intra-tracheal administration of 200 μg OVA in 50 μl PBS on day 21, 23 and 25. Mice were sacrificed and BAL fluids and tissue harvested 24 hours after the last challenge.
Brochoalveolar lavage and tissue collection
Mice were sacrificed at the times indicated. BAL fluid and cytospins were prepared as described previously (4) CytoSpin III (Shandon/Thermo Fisher Scientific). Cytospin preparations stained using Quik-Diff Giemsa Stain (Thermo Fisher Scientific). Lung tissue was either snap-frozen in liquid nitrogen for further processing for RNA with Trizol or placed in 10% Formalin for paraffin-embedding and H&E and PAS/AB staining.
Statistical analysis and graphing
Data were then analyzed using non-parametric Mann-Whitney rank-sum test. P-values less than or equal to 0.05 were considered statistically significant. All statistics and graphical representations were conducted with Prism 5.0 software for Mac (Graphpad).
Results
Differential requirements for TRAF proteins in the IL-25 response
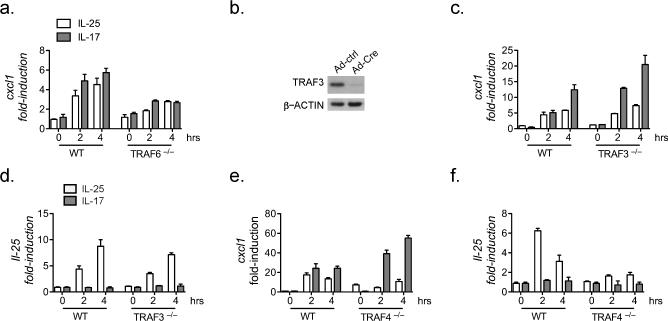
Previous studies have shown that ACT1 is the key adaptor molecule recruited to the IL-17 and IL-25 receptors following ligand stimulation (4, 12). ACT1 functions as an E3-ubiquitin ligase and interacts with specific TRAF proteins to direct downstream signaling pathways. Given the differential responses elicited by IL-17 and IL-25 in vivo, we sought to test how specific TRAF proteins may participate in the IL-25 response. As reported previously, we observed a marked reduction in IL-17- and IL-25-induced Cxcl1 gene expression and loss of ligand-induced IκBα phosphorylation in the TRAF6-deficient cells, confirming the critical role of TRAF6 for IL-17- and IL-25-mediated NFkB-dependent gene induction (Fig. 1a and data not shown) (28, 29). Since TRAF3 and TRAF4 have been reported as negative regulators for IL-17-induced NFkB activation, we next aimed to determine the roles of these two TRAFs in IL-25 signaling. We isolated Traf3flox/flox kidney cells and infected with an adenovirus expressing Cre recombinase to mediate Traf3 deletion (data not shown). There was no significant difference in IL-25-induced expression of Cxcl1 or IL-25 in the TRAF3-deficient cells compared to that in wild-type cells (Fig. 1b-c), whereas Traf3 –/– cells were indeed hyper-responsive to IL-17 (Fig. 1b) (30). These results indicate that TRAF3 is dispensable for the IL-25 response. Importantly, whereas the primary kidney epithelial cells derived from the Traf4 –/– mice were also hyper-responsive to IL-17-induced Cxcl1 expression, IL-25-induced expression of Cxcl1 or IL-25 was abolished in TRAF4-deficient cells (Fig. 1d-e). These data suggest that TRAF4 is differentially required for IL-17- and IL-25-mediated signaling (25). In summary, the screening of TRAF proteins revealed an unexpected essential role for TRAF4 in mediating an IL-25 response.
Figure 1. Screening for TRAF involvement in IL-25R-dependent response.
Primary cells were transduced with adenovirus encoding IL-25R for 48 hours (Suppl. Fig. 1). Cells were treated with IL-25 or IL-17A for the indicated times, RNA was isolated and real-time qPCR performed. (a) Cxcl1 gene expression in MEFs from Traf6 –/– or WT mice. (b) Kidney epithelial cells (KEC) were isolated from Traf3 f/f mice. Cells were transduced with –AdCre for 48 hrs and lysate prepared and subjected to SDS-PAGE and immunoblotting as indicated. (c-d) KECs from (b) were then stimulated as indicated and Cxcl1 (c) or Il-25 (d) was measured. (e and f) KEC isolated from WT or Traf4 –/– mice were infected and treated same as in (a-c). All data are representative results from at least 2 or 4-independent experiments; gene expression data are normalized to β-actin, error bars represent mean ± SEM.
TRAF4 is required for IL-25-dependent airway inflammation
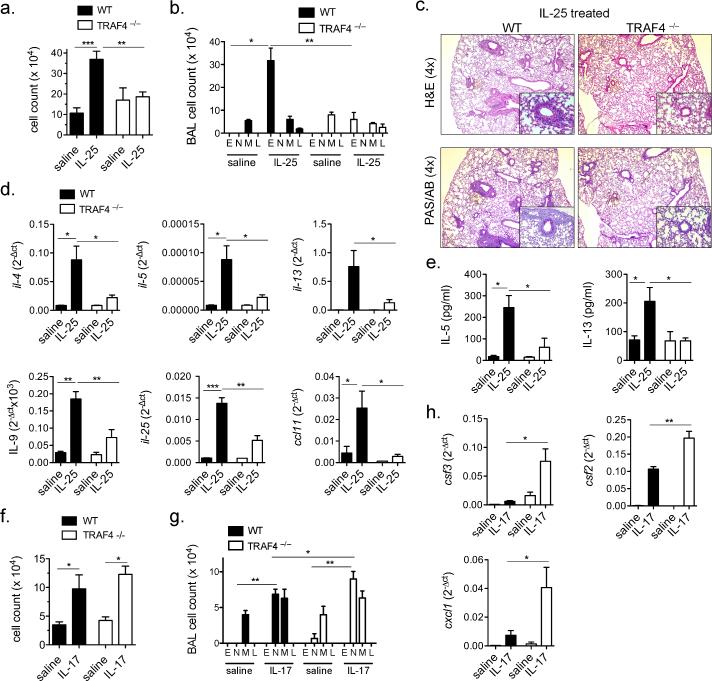
IL-25 was first reported as a cytokine capable of initiating Type-2 associated pathology and cytokine production (1). IL-25 administration into the airways of mice induces the production of IL-4, IL-5, IL-13 and subsequent eosinophilia (4, 12, 13). Thus, in order to corroborate our in vitro findings we tested whether TRAF4 is critical for IL-25 responses in vivo. We administered recombinant IL-25 via intra-tracheal injection to Traf4 –/– and WT littermates. Although we observed significant cell accumulation in the bronchoalveolar lavage (31) fluid of control mice treated with IL-25, this effect was abolished in the Traf4 –/– mice (Fig. 2a). The BAL differential cell counts revealed that IL-25-induced eosinophilia was greatly reduced in the Traf4 –/– mice compared to that in the littermate control mice (Fig. 2b). Further examination of the lung histology revealed substantial IL-25-induced accumulation of immune cells and mucus production in the small airways of control mice, which was absent in the Traf4 –/– mice (Fig. 2c). We assessed IL-25-induced expression of type-2 cytokines, including Il-4, Il-5, Il-13, Il-9, Il-25 and Ccl11 (Eotaxin), all of which were greatly reduced in the Traf4 –/– lung tissue compared to that of the wild-type mice (Fig. 2d). Moreover, IL-5 and IL-13 recovered from the BAL fluid were also significantly reduced in the Traf4 –/– mice (Fig. 2e). Taken together, these observations further support a critical role for TRAF4 in the IL-25-induced Type-2 response in vivo. Notably, we also tested the impact of TRAF4 deficiency on IL-17 response in vivo and found that Traf4 –/– mice exhibited increased IL-17-induced neutrophil accumulation and target gene expression in the lungs (Fig. 2fh). These data further corroborate the specificity of TRAF4 for the IL-25 signaling pathway.
Figure 2. TRAF4 regulates IL-25 responses in vivo.
WT and Traf4 –/– mice were injected with IL-25 (5μg per mouse) via intra-tracheal administration. After 4 days (a) Total cellularity recovered from bronchial-aveolar lavage fluid was enumerated. (b) Cell differential from BAL determined by Wright-Geimsa stain. M, macrophage, N, neutrophil, E, eosinophil, L, lymphocyte. (c) IL-25-treated lungs were fixed in formalin and stained with H&E and PAS/AB, shown are representative images. (d) Lung tissue RNA profile for the indicated genes from treated and untreated mice as determined by RT-qPCR normalized to β-actin. (e) ELISA for IL-5 and IL-13 from the BAL fluid of untreated and treated mice. (f) WT and Traf4–/– mice were administered IL-17A (5 μg/mouse) via intratracheal route, 24 hours later, BAL fluid was collected and total cellularity determined. (g) Cell differential from BAL fluid from (f). (h) Lung tissue RNA was extracted and RT-qPCR was performed for the indicated gene normalized to β-actin. Results shown are representative data with n=4-5 mice per experimental group from 2 independently performed experiments, error bars represent ± SEM, * indicates p<0.05, ** p<0.01, *** p<0.001.
The OVA-sensitization and challenge model has been used extensively to recapitulate allergen-induced airway inflammation in mice. Moreover, several studies have implicated IL-25 as a critical initiator of many downstream consequences following allergen exposure (1, 3, 4, 14, 15). Since we found that TRAF4 is an essential signaling component of the IL-25-induced Type-2 response, we then examined the impact of TRAF4 deficiency on OVA-induced airway inflammation. We observed that OVA-induced BAL cellularity was substantially reduced in Traf4 –/– mice compared to that in wild-type control mice (Fig. 3a). Specifically, eosinophil and lymphocyte recruitment ware markedly decreased in the absence of TRAF4 (Fig. 3a). Importantly, the marked reduction in inflammation in Traf4 –/– mice was similar to defects observed in Il-17rb –/– mice (Suppl. Fig. 2). Moreover, the Traf4 –/– mice exhibited a reduced gene expression profile for type 2 cytokines (Il-4, Il-9, Il-13, Il-25) as well as reduced Eotaxin-1 and Muc5ac (Fig. 3c). Together, these data implicate TRAF4 as a crucial molecule in allergen-induced IL-25-dependent airway inflammation.
Figure 3. TRAF4 mediates allergic airway inflammation.
WT and Traf4–/– mice were immunized with OVA emulsified in Alum on Day 0 and Day 10 followed by 3 subsequent intratracheal challenges with OVA on Day 21-23. (a) BAL wash was performed and total cellularity and cell differential was determined. (b) Lung tissue from OVA-treated mice were fixed and H&E stained, representative sections are shown. (c) Lung tissue RNA was extracted and RT-qPCR was performed for the indicated genes normalized to β-actin. Data is representative from two independent experiments with n=4-5 mice per group, error bars represent mean ± SEM, * indicates p<0.05, *** p<0.001.
TRAF4 is required for IL-25 response in T-cells and airway epithelial cells
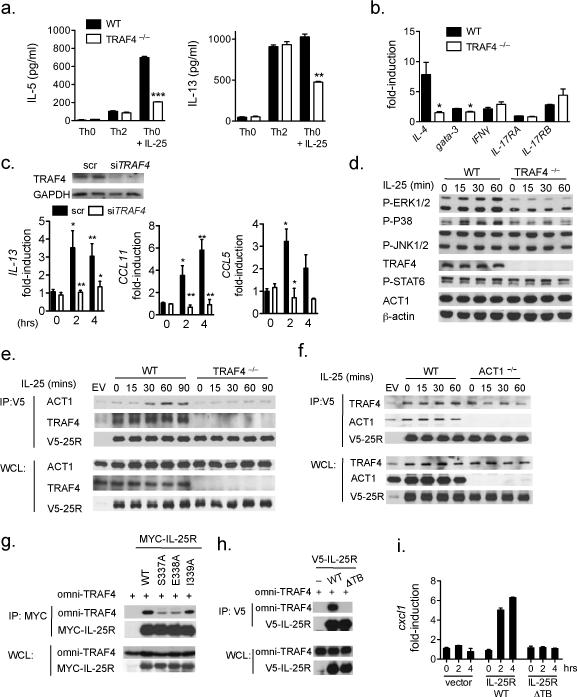
Using cell-type specific ACT1-deficient mice, we have previously reported that T-cell- and epithelial-derived Act1 expression is critical for the IL-25 response in vivo (3, 4). Thus we tested the role of TRAF4 in IL-25-mediated responses in these cell-types. Whereas Traf4 –/– Th2 cells (polarized by IL-4) exhibited no difference in cytokine production compared to that in littermate controls, Traf4 –/– T-cells conditioned with IL-25 showed substantially reduced IL-5 and IL-13 production and expression of Il-4 and Gata-3 compared to that in wild-type Th25 cells (Fig. 4a-b). TRAF4 deficiency in T cells had no impact on Il-25r (Il-17rb), Il-17ra and Ifnγ expression or cell proliferation (Fig. 4b and data not shown). To assess the role of TRAF4 in mediating the IL-25 response in airway epithelial cells, we knocked down TRAF4 in a human airway epithelial cell-line, Bet1A (Fig. 4c). IL-25-induced expression of Il-13, CCL11 (eotaxin) and CCL5 (rantes) were greatly reduced in the TRAF4-silenced (siTRAF4) cells compared to the cells transfected with non-targeting scrambled siRNA (Fig. 4c). As a control, we found that IL-1β-induced CXCL1 expression was unaffected by TRAF4-silencing compared to the control cells (data not shown). These data indicate that TRAF4 is specifically required for IL-25 responses in T-cells as well as in human airway epithelial cells.
Figure 4. Cell-intrinsic IL-25 responses are TRAF4-dependent.
Naïve CD4-positive T-cells were isolated from WT and Traf4 –/– mice and subsequently activated with plate-bound CD3/CD28 and in the indicated polarizing conditions. (a) ELISA for IL-5 and IL-13 performed from supernatants of activated T-cells. (b) RNA was isolated from activated T-cells in Th0+IL-25 conditions and RT-qPCR for the indicated genes normalized to β-actin. (c) Top, Immunoblot from human airway epithelial cell-line (Bet1a) transfected with siRNA against human Traf4 or scrambled (scr) (100 nM each). Bet1a cells transfected with siRNA were treated with hIL-125 (100 ng/ml) for the indicated times, RNA was isolated and RT-qPCR was performed for the indicated genes normalized to GAPDH. (d) Activated T-cells in Th2 polarizing conditions from WT and Traf4 –/– mice were treated with IL-25 (100 ng/ml) for the indicated times, lysates were subjected to SDS-PAGE followed by immunoblotting for the indicated proteins. (e and f) WT and Traf4 –/– or (f) Act1 –/– , KECs were isolated and infected with Ad-V5-Il-17rb (V5-25R). Cells were then stimulated with IL-25 (100 ng/ml) for the indicated timepoints and lysates prepared and subjected to co-immunoprecipitation with antibody against V5. (g and h) HEK293 cells were transfected with the indicated vectors, followed by coimmunoprecipitation for 24 hours using antibodies against tagged IL-25R. Coimmunoprecipitate and whole cell lysate (WCL) were separated by SDS-PAGE and immunoblotted with the indicated antibodies. (i) Il-17rb –/– KECs were transduced with the indicated vectors followed by IL-25 stimulation, RT-qPCR was performed and normalized to β-actin. All data are representative of at least 3 independently performed experiments. Error bars represent mean ± SEM, * indicates p<0.05, ** p<0.01, *** p<0.001.
Consistent with the decreased IL-25-dependent T-cell cytokine response, IL-25-induced phosphorylation of ERK1/2 and P38 were abolished in the Traf4 –/– T cells as compared to that in wild-type T cells (Fig. 4d). One important question is how TRAF4 participates in the IL-25 signaling cascade. We performed co-immunoprecipitation with the IL-25R to assess whether TRAF4 is recruited to the receptor complex. Although there was constitutive interaction of TRAF4 with the IL-25R, IL-25 stimulation enhanced the recruitment of TRAF4 to IL-25R (Fig. 4e). Unexpectedly, we observed a substantial defect in the recruitment of ACT1 to the IL-25R in Traf4 –/– cells (Fig. 4e). On the other hand, TRAF4 was still able to interact with IL-25R in Act1 –/– cells (Fig. 4f). These data suggest that the interaction of TRAF4 to the IL-25R is required for yet independent of the recruitment of ACT1. Mutations within the putative TRAF binding site (334-341: VYPSEICF, to S337A, E338A and I339A) in IL-25R reduced but did not abolish the interaction with TRAF4 (Fig. 4g). However, when we deleted the TRAF binding domain (ΔTBD) it completely ablated the interaction with TRAF4 as well the response to IL-25 in the restored Il-17rb –/– cells (Fig. 4h-i). Collectively, these results suggest that IL-25-induced IL-25R/TRAF4 interaction may be a pre-requisite for subsequent ACT1 recruitment and downstream signaling processes.
TRAF4 and SMURF2 cooperate to mediate the IL-25 response
Since it has been well defined that ACT1 is recruited to IL-25R through SEFIR-SEFIR domain interaction, it is critical to identify a possible mechanism that could explain the TRAF4-dependent recruitment of ACT1 to the IL-25R (4, 26, 32). One possibility is that TRAF4 is recruited to IL-25R to remove a pre-bound inhibitory molecule of the receptor to permit the recruitment of ACT1. Previous yeast hybrid screening with the intracellular portion of IL-25R as bait identified a novel binding partner known as deleted in azospermia (DAZ)-associated protein 2 (DAZAP2) (33). In that study, SMURF2 (SMAD-ubiquitin regulatory factor) was identified as the E3-ligase capable of promoting DAZAP2 degradation. However, the functional importance of DAZAP2 or SMURF2 in IL-25 signaling has not been established. Notably, several reports have shown that TRAF4 interacts with SMURF2 in several contexts, including during migration in metastatic breast cancer and in the TGFβ signaling pathway (34-36). Our results here show that TRAF4 is required for IL-25-induced recruitment of ACT1 to the IL-25R. Thus we hypothesize that if DAZAP2 inhibits the recruitment of ACT1 to the IL-25R then TRAF4 may mediate the recruitment SMURF2 to the IL-25R complex in order to degrade DAZAP2 and remove its inhibitory activity.
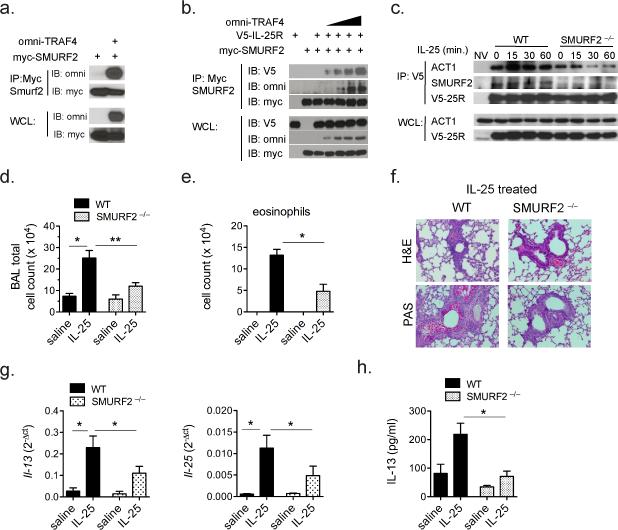
To test this hypothesis, we first assessed the impact of SMURF2 in IL-25 signaling. Consistent with previous reports, we confirmed the TRAF4-SMURF2 interaction (Fig. 5a) (34, 35). More importantly, SMURF2 interacts with the IL-25R and the binding of SMURF2 to the IL-25R was substantially enhanced with increasing amounts of TRAF4 (Fig. 5b), suggesting that TRAF4 has the capacity to further promote the recruitment of SMURF2 to IL-25R. Next we assessed the recruitment of ACT1 to the IL-25R in Smurf2 –/– cells. Similar to what we observed in the Traf4 –/– cells, the ACT1/IL-25R interaction was substantially diminished in Smurf2–/– cells (Fig. 5c). These data suggest that TRAF4 can facilitate SMURF2 recruitment to the IL-25R complex, which is required for subsequent recruitment of ACT1. To further substantiate the involvement of SMURF2 in IL-25 signaling, we tested the impact of SMURF2 deficiency on IL-25 responses in vivo. We found that IL-25-induced BAL cellularity was reduced in Smurf2–/– mice compared to WT mice (Fig. 5d). Consistent with this, IL-25-induced eosinophilia was significantly reduced in the Smurf2 –/– mice (Fig. 5e). Similarly, inflammation and mucus production were also diminished in the lung tissue of Smurf2–/– mice (Fig. 5f). Moreover, the known IL-25 target genes, Il-25 and Il-13, were also both significantly reduced in the lung tissue and in the BAL fluid of Smurf2–/– mice (Fig. 5g-h). Together, these results support the hypothesis that TRAF4 and SMURF2 are both required for the IL-25 response.
Figure 5. SMURF2 is a positive mediator of the IL-25-response.
(a and b) HEK293 cells were transfected with the indicated vectors. After 48 hours the cell lysate was prepared followed by co-immunoprecipitation for 24 hours using antibodies against MYC-tag SMURF2 (a) or V5-tag IL-25R (b). WT and Smurf2 –/– KECs were isolated and infected with Ad-V5-Il-17rb (V5-25R). Cells were then stimulated with IL-25 (100 ng/ml) for the indicated timepoints and lysates prepared and subjected to co-immunoprecipitation with antibody against V5. The co-immunoprecipitate and whole cell lysate (WCL) were separated by SDS-PAGE and immunoblotted with the indicated antibodies. (a-c) Data are representative results of at least 2 independently performed experiments. (d) WT and Smurf2 –/– mice were injected with IL-25 by intra-tracheal route. After 4 days BAL wash was performed and total cellularity (d) and eosinophil in the BAL (e) were enumerated. (f) Lungs were sectioned and stained as indicated. Representative images from treated mice are shown. (g) RNA was prepared from total lung tissue and RT-qPCR performed for Il-25 and Il-13 normalized to β-actin. (h) IL-13 ELISA from BAL fluid of treated mice. Data presented are from a representative experiment with n=4 mice per experimental group, these experiments were repeated 2 times with similar results. Error bars represent mean ± SEM, * indicates p<0.05, ** p<0.01.
TRAF4 and SMURF2 are required for IL-25-induced degradation of the inhibitory molecule DAZAP2
Considering the previous report about the connection between SMURF2 and DAZAP2, we next examined the impact of DAZAP2 on the IL-25 response. Primary mouse T-cells were transduced with control shRNA or lentiviral shRNA targeting endogenous Dazap2 (Fig. 6a). We then polarized the shDazap2 or scrambled shRNA control T-cells with IL-25 and then measured cytokine expression. We observed substantially more IL-25-induced Il-5 and Il-13 gene expression as well as IL-5 protein production in the T-cells with shDazap2 compared to the control (Fig. 6b-d). To further determine the impact of DAZAP2 on IL-25-induced signaling, we also generated a Dazap2-knockdown MEF cell line (shDazap2) (Fig. 6e). Compared to the control cell-line (scrambled shRNA-expressing, scr), the shDazap2 cells exhibited enhanced IL-25-induced activation of ACT1, JNK1/2, ERK1/2 and P38 (Fig. 6f). Importantly, IL-17 induced comparable activation of ACT1, JNK1/2, ERK1/2 and P38 in the scr and shDazap2 cells (Fig. 6g), indicating that DAZAP2 specifically inhibits IL-25R-mediated signaling. Moreover, we observed a significant increase in the interaction of ACT1 with the IL-25R in the shDazap2 cells compared to the control cells (Fig. 6h). These observations suggest that DAZAP2 hinders the recruitment of ACT1 to the IL-25R, resulting in the inhibition of IL-25 signaling.
Figure 6. DAZAP2 negatively impacts IL-25 responses.
(a) Primary murine T-cells were transduced with lentivirus expressing shRNA targeting Dazap2 or non-targeting scrambled. RNA was isolated and RT-qPCR was performed 5 days after initial transduction and normalized to actin. (b) T-cells from (a) were activated by plate bound CD3/CD28 in the indicated conditions. RNA was isolated and RT-qPCR for the indicated genes was performed and expression normalized to β-actin. (d) IL-5 ELISA from T-cell supernatant in (b-c) (e) IL-25R expressing MEF cell line were transduced with lentiviral expressed shRNA targeting Dazap2, RNA was prepared and RT-qPCR performed and normalized to actin. (f and g) MEFs from (e) were treated with IL-25 (f) or IL-17A (g) for the indicated times, lysate was prepared and immunoblotted for the indicated proteins. (h) MEF cells were treated with IL-25, lysate was prepared and coimmunoprecipitated with antibodies against ACT1, then immunoblotted for the indicated proteins. (i) Adenovirus encoding shRNA was injected i.t. to wild-type mice, lung tissue protein was isolated and lysate subjected to SDS-PAGE and immunoblotting. (j) BAL eosinophilia after IL-25 i.t. injection was enumerated. (k) Levels of IL-5 and IL-13 detected by ELISA from lung tissue lysates from IL-25 treated mice. (l) Representative histology from the indicated mice. Data are representative results from 2-3 independently performed experiments. For i-l, n=5 mice per group, this experiment was repeated two times with similar results. Error bars represent mean ± SEM, * indicates p<0.05, ** p<0.01.
Next, we sought to test the involvement of DAZAP2 in regulating IL-25 responses in vivo. For this we administered an adenovirus encoding shRNA directed against Dazap2 via intratracheal (i.t.) injection into wild-type mice, three days later IL-25 was delivered via i.t. route. In line with our in vitro data, the shDazap2 group exhibited enhanced IL-25-induced eosinophilia and elevated levels of IL-25-target genes, IL-5 and IL-13 in the lung tissue (Fig. 6i-l).
The results thus far indicate that TRAF4 and SMURF2 are required for ACT1 recruitment to the IL-25R. In contrast to this, DAZAP2 hinders the ACT1/receptor interaction and inhibits downstream IL-25 signaling. An important point to address is how IL-25-stimulation can overcome DAZAP2 inhibition, and whether TRAF4 and SMURF2 participate in this process. We examined the impact of IL-25 stimulation on endogenous DAZAP2 in polarized Th2 from WT, Traf4 –/– or Smurf2 –/– mice. DAZAP2 levels were substantially reduced within 15 minutes of IL-25 treatment and this reduction was ablated in Traf4 –/– or Smurf2 –/– cells (Fig. 7a). The expression levels of Il17rb in the KO cells by RT-qPCR were similar to that in WT cells (data not shown). It was previously reported that SMURF2 is the E3-ligase capable of promoting proteasome-dependent DAZAP2 degradation (33). Interestingly, we found IL-25 stimulation induced K48-, but not K63-linked polyubiquitination of DAZAP2 within 5 minutes of IL-25 stimulation in MEFs (Fig. 7b). Moreover, MG132 blocked IL-25-induced DAZAP2 degradation, resulting in accumulation of K48-linked polyubiquitination of DAZAP2 (Fig. 7b). These results suggest that IL-25 stimulation can lead to DAZAP2 ubiquitination and degradation, thereby relieving DAZAP2-mediated inhibition. Consistent with the results in MEFs and Th2 cells, IL-25 also induced DAZAP2 degradation in primary kidney epithelial cells within 2-5 minutes of stimulation, which was abolished in the Traf4 –/– cells (Fig. 7c).
Figure 7. IL-25 stimulation promotes DAZAP2 degradation.
(a) Primary CD4+ TH2 cells derived from the indicated mice were treated with IL-25, cell lysate was prepared and subjected to SDS-PAGE and immunoblotting for DAZAP2. Representative blots from at least 2 or 3 independently performed experiments are shown, ratio is relative to GAPDH loading control. (b) IL-25R expressing MEF cells with stable expression of HA-tagged DAZAP2 were treated with IL-25 for the indicated timepoints, cell lysates were prepared and co-immunoprecipitated with antibodies against HA-tag (top), then immunoblotted for the indicated proteins and endogenous Ub. (b, bottom) quantification of whole cell lysate DAZAP2/GAPDH ratio. MG132 was incubated for 2 hrs prior to lysate preparation where indicated. (c) Primary KECs derived from WT or Traf4 –/– were transduced with viruses encoding HA-Dazap2 and V5-IL-25R after which, cells were stimulated with IL-25 for the indicated times, cell lysate was prepared and separated by SDS-PAGE followed by immunoblotting for HA-tag. (d and e) HEK293 cells were transfected with the indicated vectors, lysate was prepared and then separated by SDS-PAGE. MG132 was incubated for 2 hrs prior to lysate preparation where indicated. All blots shown are from one representative experiment performed 2 or 3 times.
SMURF2 was previously implicated as the E3 ligase mediating DAZAP2 degradation. We indeed found that wild-type SMURF2, but not an E3-ligase defective mutant SMURF2-CG, was able to promote DAZAP2 degradation, which was also blocked by MG132 (Fig. 7d). Furthermore, co-expression of WT SMURF2 but not the E3-defective mutant could promote poly-ubiquitination of DAZAP2 (Fig. 7e). This ubiquitination was K48-linked, as a K48R mutant Ub failed to assemble on DAZAP2 (Fig. 7f). These data clearly suggest that TRAF4 and SMURF2 mediate IL-25 signaling by promoting IL-25-induced K48-linked polyubiquitination of DAZAP2 and consequent degradation, thereby alleviating the inhibition of ACT1 recruitment imposed upon by DAZAP2.
IL-25R recruits DAZAP2 through p-Tyr 355 residing within the SEFIR domain
We next further investigated the molecular basis for DAZAP2-mediated inhibition on ACT1 recruitment to the IL-25R. DAZAP2 contains three putative SH2 domains (Fig. 8a) (37). It is well known that SH2 domains facilitate protein-protein interaction through the recognition of phosphorylated tyrosine residues. Interestingly, we indeed noted multiple tyrosine residues (six) in the intracellular portion of the IL-25 receptor (Fig. 8a). It is important to note that five of these tyrosine residues reside within the SEFIR domain, which is the requisite domain for ACT1-SEFIR interaction. Thus we hypothesized that the IL-25R may harbor phosphorylated tyrosines (in the SEFIR domain) that are essential for the recognition and binding of the SH2 domains in DAZAP2, hindering the recruitment of ACT1 to the SEFIR domain of the receptor.
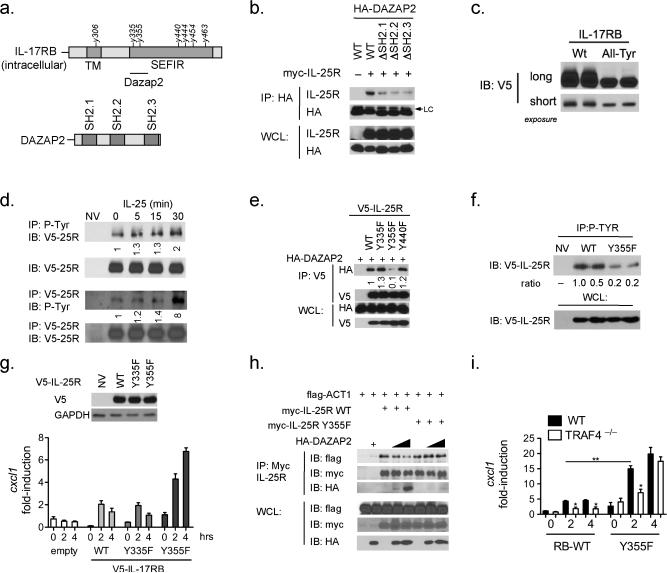
Figure 8. IL-25R contains tyrosine residues that modulate its function.
(a) Schematic diagram showing tyrosine residues on the intracellular domain of IL-17RB and previously mapped DAZAP2 binding region, (right) schematic of SH2 domains on DAZAP2. (b) HEK293 cells were transfected with the indicated vectors, cells were pelleted and lysates were prepared and co-immunoprecipitated with antibodies against HA-tag, following this lysates were subjected to SDS-PAGE and immunoblotting. (c) WT KEC were transduced with Ad-IL-17rb (WT) or Ad-IL-17rb with all intracellular tyrosines mutated to phenylalanine (All-Tyr) for 48 hours followed by SDS-PAGE and immunoblotting. (d) WT KEC were transduced with Ad-IL-17RB and treated with IL-25 for the indicated times, cells were pelleted and lysates were prepared and coimmunoprecipitated with antibody against P-Tyr (upper) or V5 (lower), after which lysates were subjected to SDS-PAGE and immunoblotting. (e) HEK293 cells were transfected with the indicated vectors followed by co-immunoprecipitation against V5-tag and immunoblotting. (f) Il17rb –/– KECs were transduced with adenovirus encoding for IL-17rb WT or Y355F mutant. Cells were pelleted and lysates were prepared and coimmunoprecipitated with antibody against P-Tyr and resolved by SDS-PAGE. (g) Il17rb –/– KECs were transduced with adenovirus encoding for IL-17rb WT or the indicated single Y to F mutants, (top) is immunoblot for the transduced receptors (bottom) KEC were treated for the indicated time with IL-25, RNA was isolated and RT-qPCR performed and normalized to β-actin. (h) HEK293 cells were transfected with the indicated plasmids and Co-immunoprecipitated and resolved on SDS-PAGE followed by immunoblotting as indicated. (i) Traf4 –/– KECs were transduced with adenovirus encoding for IL-17rb WT or the indicated single Y to F mutants, cells were treated for the indicated time with IL-25, RNA was isolated and RT-qPCR performed and normalized to β-actin. Representative data of 2 or 3 independently performed experiments are shown, error bars represent. Error bars represent mean ± SEM.
To determine the importance of SH2 domains in DAZAP, we generated SH2-domain deletion mutants of DAZAP2. Deletion of any one of the SH2 domains impaired DAZAP2's interaction with the IL-25R (Fig. 8b), which suggests the possible recognition of phosphorylated tyrosine residues on the IL-25R. We then generated a compound tyrosine mutant in which all six of the tyrosine residues in the IL-25R were mutated to phenylalanine (All-Tyr mutant). We observed a dramatic shift in the mobility of the All-Tyr mutant compared to the wild-type IL-25R when they were re-expressed in IL-25R-deficient kidney epithelial cells (Fig. 8c). These results suggest that the IL-25R is highly modified and that the tyrosine residues on the intracellular portion contribute to this modification. Using anti-p-Tyr antibody, we indeed detected tyrosine phosphorylation in IL-25R, which was further induced by IL-25 stimulation (Fig. 8d).
To test whether these tyrosines are required for DAZAP2 recruitment to the receptor, we generated site-specific mutants and co-expressed with DAZAP2. While WT IL-25R, Y335F and Y440F retained the interaction with DAZAP2, Y355F lost the interaction with DAZAP2 (Fig. 8e). In order to study the regulation and function of the Y355F mutant, we re-introduced this variant and WT IL-25R into IL-17rb –/– kidney epithelial cells. Interestingly, Y355F showed much reduced basal tyrosine phosphorylation compared to wild-type IL-25R, suggesting that phosphorylation at this site probably takes place in the absence of IL-25 stimulation (Fig. 8f). Furthermore, while wild-type IL-25R could restore IL-25 response in IL-17rb –/– cells, we actually saw a markedly enhanced response to IL-25 in the Y355F restored kidney epithelial cells (Fig. 8g). These results indicate that the mutant IL-25R lacking the tyrosine necessary for DAZAP2 recruitment is capable of transducing a stronger IL-25 response, confirming the inhibitory role of DAZAP2 on IL-25 signaling. Since this tyrosine residue resides in the SEFIR domain, it is logical to propose that the recruitment of DAZAP2 to this site probably would directly interfere with the ACT1/IL-25R interface (mediated by the SEFIR domain). We indeed observed that DAZAP2 could no longer inhibit ACT1's interaction with the mutant IL-25R Y355F compared to the wild-type IL-25R (Fig. 8h).
IL-25R Y355F mutant mediates IL-25 response in the absence of TRAF4
Since TRAF4 is required for IL-25-induced DAZAP2 degradation, one would predict that the mutant IL-25R Y355F (lacking the tyrosine necessary for DAZAP2 recruitment) would bypass the need for TRAF4 to mediate the IL-25 response. To test this, we transduced wild-type and IL-25R Y355A mutant into wild-type and Traf4 –/– kidney epithelial cells, followed by treatment with IL-25. Importantly, we found that while the wild-type IL-25R still requires TRAF4 to respond to IL-25, IL-25R Y355F mutant was able propagate an IL-25 response in the absence of TRAF4 (Fig. 8i). Overall these results indicate that post-translational modification of the IL-25R determines DAZAP2 recognition and thus TRAF4 involvement. Collectively, the TRAF4-SMURF2 regulatory axis is essential for DAZAP2 degradation, and most likely prepares the receptor to recruit the key adaptor molecule ACT1, an essential permissive event to initiate IL-25 signaling (Suppl. Fig. 3).
Discussion
In this study we identified a novel role for TRAF4 in mediating the IL-25 response. TRAF4 deficiency impaired the IL-25-induced Th2 response in vivo, in cultured T-cells and epithelial cells. While TRAF4 interacted with the IL-25R in an ACT1-independent manner, ACT1 interaction with the IL-25R was impaired in Traf4 –/– cells. We demonstrated that the positive activity of TRAF4 was to recruit SMURF2, an E3-ubiquitin ligase, to promote the degradation of DAZAP2. Silencing of Dazap2 led to increased ACT1/IL-25R interaction and an enhanced IL-25 response. Moreover, a tyrosine residue within the IL-25R-SEFIR domain was critical for DAZAP2 interference. Taken together, this study demonstrates that TRAF4-SMURF2-mediated DAZAP2 degradation is a critical step for the recruitment of ACT1 to IL-25R to initiate IL-25 signaling and airway inflammation.
The identification of TRAF4 as a critical component of the IL-25 response was unexpected. We reported that in the IL-17A signaling cascade, TRAF4 is a negative regulator of NFkB activation and IL-17-associated disease pathology (25). Under the IL-17A pathway, TRAF4 binds to ACT1 and occupies the TRAF-Binding-Domains (TBDs) on ACT1 thereby blocking TRAF6. However, while TRAF4-IL-25R interaction is dependent on a putative TRAF binding domain (334-341: VYPSEICF) in the IL-25R, TRAF4 is required for ACT1 recruitment to the IL-25R. This suggests that TRAF4 interacts directly with the IL-25R and precedes ACT1. Indeed, TRAF4 deficiency blunted IL-25-responses in primary cells and in IL-25-driven models of airway inflammation. The mechanism for how TRAF4 facilitates the recruitment of ACT1 to the IL-25R was indirect. Rather, an inhibitory molecule, DAZAP2, hinders the recruitment of ACT1 to the IL-25R. IL-25 stimulation elicits TRAF4 to the receptor complex to remove this inhibitory effect. It is important to note that DAZAP2 has been shown to reside in the cytoplasm and nucleus. Previously DAZAP2 was shown to reside in the cytoplasm following IL-25 stimulation. We further confirmed that the IL-25R/DAZAP2 interaction exists only in the cytoplasm, based on a proximity-based ligation imaging analysis in transfected cells (unpublished observations).
DAZAP2 is highly conserved among mammals and its secondary structure features several SH2 and SH3 domains (37, 38). We identified tyrosine residue 355 of the IL-25R serves as the docking site for the SH2 domain of DAZAP2. It is important to note that both the TRAF binding domain and tyrosine-355 reside in the SEFIR domain of IL-25R, which is known to interact with the SEFIR domain of ACT1 (26, 32). Since silencing Dazap2 increased ACT1 recruitment to the IL-25R and enhanced IL-25 responses in vivo, it is likely that DAZAP2's binding to the p-Tyr in the SEFIR domain creates a steric interference for the SEFIR-SEFIR interaction between ACT1 and IL-25R. Our data suggests that binding of TRAF4 to the N-terminal end of the SEFIR (SEFIR: 329-477 versus TRAF binding site: 334-341) is cooperative for SEFIR-SEFIR interaction. Furthermore, since ACT1 also contains the TRAF binding site, TRAF4 may be released from the IL-25R and form an ACT1-TRAF4 complex after the engagement of SEFIRSEFIR domains. The IL-25 response in Traf4 –/– cells was restored with IL-25R-Y355F, suggesting that TRAF4 may be dispensable beyond its effects on DAZAP2. However, genome wide gene array studies will be helpful to assess whether TRAF4 is cooperative with ACT1 following the removal of DAZAP2.
Our observations reveal that TRAF4 participates in IL-25 signaling by partnering with another E3 ligase, SMURF2, to mediate K48-linked polyubiquitination and consequent degradation of DAZAP2, an event that is required for ACT1 recruitment to the IL-25R. Since both TRAF4 and SMURF2 are required for IL-25-induced ACT1 recruitment and IL-25 signaling, their roles in this IL-25-dependent process must be non-redundant. Our results demonstrated that TRAF4 is directly recruited to IL-25R in response to ligand stimulation. The TRAF4 interaction with the IL-25R can facilitate SMURF2 recruitment, which in turn, interacts with and degrades DAZAP2. SMURF2 was previously identified as the E3-ligase capable of inducing proteasome-dependent DAZAP2 degradation (33). In our study we further determined that SMURF2 is the E3-ligase capable of promoting K48-linked ubiquitination of DAZAP2. It is important to note that neither IL-25-induced modifications nor degradation of TRAF4 or SMURF2 were ever observed in our study (unpublished observations). Therefore, for the IL-25R pathway we conclude that the main activities of TRAF4 and SMURF2 are conducted in a cooperative manner to promote DAZAP2 degradation.
Notably, this is the first example of the IL-25R, or any IL-17R, being modified at the post-translational level. Moreover, we detected tyrosine phosphorylation of IL-25R in the absence of IL-25 stimulation. Thus, the IL-25R might contain phosphorylated tyrosines even in the basal state. In line with this hypothesis, the Y355F IL-25R mutant showed reduced basal tyrosine phosphorylation when transduced into Il-17rb –/– cells. This suggests that constitutive phosphorylation at Tyr-355 may represent a critical impediment for IL-25R activity. The identity of the tyrosine kinase is being investigated. In silico analyses suggest that janus kinases (JAK2 and JAK3) as well as growth factor receptor kinases such as vascular endothelial growth factor (VEGFR) and platelet derived growth factor (PDGFR) have predicted sites on the IL-25R. Our preliminary results show constitutive interaction of JAK2 with IL-25R (unpublished observations). Thus, it is possible that JAK2 phosphorylates Tyr-355 in untreated cells, which attracts DAZAP2 to the IL-25R. What is noteworthy is that P-Tyr of IL-25R increased with ligand stimulation. In a separate study, we found that STAT5 is recruited to IL-25R through the recognition of another tyrosine in a TRAF4-dependent but ACT1-independent manner (40). Thus, it is conceivable that the removal of DAZAP2 by the TRAF4-SMURF2 axis also facilitates the phosphorylation of other tyrosines by JAK2, thereby recruiting the SH2-domain containing STAT5. Future studies are required to define the role of each potential tyrosine on the IL-25R, as each may serve a function in cellular signaling or impinge on receptor conformation.
The complexity of the regulation imposed on the IL-25R underscores the impact of IL-25 in mediating pathological inflammation. Unlike other type-2 cytokines such as IL-13, IL-25 has a profound capacity to initiate the type-2 response. This activity is due in part to the abundance of IL-25R across multiple cell-types and tissues. Further, in settings of allergic inflammation, IL-25 activity is compounded by the fact that it signals to naïve and activated T-cells to promote Th2 cytokine production (1, 3, 5, 14). More recently, IL-25 has been shown to be critical for mounting allergic Type-2 responses from the resident ILC2 cell populations. Thus it would be important to evaluate this TRAF4-dependent signaling pathway in ILC2's in the future. Thus, given the importance of IL-25, it is not surprising that its receptor is strictly regulated. It would be interesting to determine how DAZAP2 is transcriptionally regulated. Indeed, promoter methylation resulting in DAZAP2 down-regulation has been described in multiple myeloma (37, 39); perhaps an aberrant down-regulation of DAZAP2 is present in atopic asthmatics. Furthermore, identifying potential tyrosine kinases or the status of the phosphorylated IL-25R may serve as important therapeutic targets or potential biomarkers, respectively. In summary, this study defined an IL-25R-specific regulatory axis that controls the cellular response to IL-25, which has broad implications in airway inflammation and allergic type-2 responses.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01-NS071996 (NINDS) and P01-HL103453 (NHLBI) to X.L.
References
- 1.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. The Journal of experimental medicine. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang Z, Swaidani S, Yin W, Wang C, Barlow JL, Gulen MF, Bulek K, Do JS, Aronica M, McKenzie AN, Min B, Li X. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(−)c-Kit(+) innate cell population. Immunity. 2012;36:821–833. doi: 10.1016/j.immuni.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaidani S, Bulek K, Kang Z, Gulen MF, Liu C, Yin W, Abbadi A, Aronica M, Li X. T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation. Journal of immunology. 2011;187:3155–3164. doi: 10.4049/jimmunol.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. Journal of immunology. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. The Journal of allergy and clinical immunology. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 6.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. The Journal of experimental medicine. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine & growth factor reviews. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 8.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 9.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 10.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. The Journal of clinical investigation. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, Van GY, Clarkin K, Nguyen HQ, Yu YB, Jing S, Senaldi G, Elliott G, Medlock ES. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 12.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, Siebenlist U. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. Journal of immunology. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. Journal of immunology. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 14.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nature immunology. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzukawa M, Morita H, Nambu A, Arae K, Shimura E, Shibui A, Yamaguchi S, Suzukawa K, Nakanishi W, Oboki K, Kajiwara N, Ohno T, Ishii A, Korner H, Cua DJ, Suto H, Yoshimoto T, Iwakura Y, Yamasoba T, Ohta K, Sudo K, Saito H, Okumura K, Broide DH, Matsumoto K, Nakae S. Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, is crucial for murine asthma. Journal of immunology. 2012;189:3641–3652. doi: 10.4049/jimmunol.1200461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends in biochemical sciences. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 17.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. Journal of immunology. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 18.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. The Journal of biological chemistry. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 19.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Science signaling. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, Lauer M, Hascall V, Misra S, Chance MR, Aronica M, Hamilton T, Li X. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nature immunology. 2011;12:844–852. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nature immunology. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiels H, Li X, Schumacker PT, Maltepe E, Padrid PA, Sperling A, Thompson CB, Lindsten T. TRAF4 deficiency leads to tracheal malformation with resulting alterations in air flow to the lungs. The American journal of pathology. 2000;157:679–688. doi: 10.1016/S0002-9440(10)64578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang LY, Yamashita M, Coussens NP, Tang Y, Wang X, Li C, Deng CX, Cheng SY, Zhang YE. Ablation of Smurf2 reveals an inhibition in TGF-beta signalling through multiple mono-ubiquitination of Smad3. The EMBO journal. 2011;30:4777–4789. doi: 10.1038/emboj.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zepp JA, Liu C, Qian W, Wu L, Gulen MF, Kang Z, Li X. Cutting edge: TNF receptor-associated factor 4 restricts IL-17-mediated pathology and signaling processes. Journal of immunology. 2012;189:33–37. doi: 10.4049/jimmunol.1200470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Liu C, Qian W, Han Y, Li X, Deng J. Crystal structure of IL-17 receptor B SEFIR domain. Journal of immunology. 2013;190:2320–2326. doi: 10.4049/jimmunol.1202922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, Liu C, Herjan T, Misra S, Carman JA, Gao J, Dongre A, Han S, Bunting KD, Ko JS, Xiao H, Kuchroo VK, Ouyang W, Li X. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nature immunology. 2013;14:72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. The Journal of experimental medicine. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartupee J, Liu C, Novotny M, Sun D, Li X, Hamilton TA. IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. Journal of immunology. 2009;182:1660–1666. doi: 10.4049/jimmunol.182.3.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, Liu Y, Zhao L, Li X, Shi Y, Qian Y. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. The Journal of experimental medicine. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. The Journal of allergy and clinical immunology. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Swaidani S, Qian W, Kang Z, Sun P, Han Y, Wang C, Gulen MF, Yin W, Zhang C, Fox PL, Aronica M, Hamilton TA, Misra S, Deng J, Li X. A CC' loop decoy peptide blocks the interaction between Act1 and IL-17RA to attenuate IL-17- and IL-25-induced inflammation. Science signaling. 2011;4:ra72. doi: 10.1126/scisignal.2001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popova A, Kzhyshkowska J, Nurgazieva D, Goerdt S, Gratchev A. Smurf2 regulates IL17RB by proteasomal degradation of its novel binding partner DAZAP2. Immunobiology. 2012;217:321–328. doi: 10.1016/j.imbio.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Lu K, Wang J, An L, Yang G, Chen H, Cui Y, Yin X, Xie P, Xing G, He F, Zhang L. Ubiquitin ligase Smurf1 targets TRAF family proteins for ubiquitination and degradation. Molecular and cellular biochemistry. 2010;338:11–17. doi: 10.1007/s11010-009-0315-y. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Jin C, Tang Y, Tang LY, Zhang YE. Ubiquitination of tumor necrosis factor receptor-associated factor 4 (TRAF4) by Smad ubiquitination regulatory factor 1 (Smurf1) regulates motility of breast epithelial and cancer cells. The Journal of biological chemistry. 2013;288:21784–21792. doi: 10.1074/jbc.M113.472704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Zhou F, Garcia de Vinuesa A, de Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li Y, Bauer A, Rousseau A, Sheppard KA, Mickanin C, Kuppen PJ, Lu CX, Ten Dijke P. TRAF4 promotes TGF-beta receptor signaling and drives breast cancer metastasis. Molecular cell. 2013;51:559–572. doi: 10.1016/j.molcel.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Shi YW, Shen R, Ren W, Tang LJ, Tan DR, Hu WX. Molecular features and expression of DAZAP2 in human multiple myeloma. Chinese medical journal. 2007;120:1659–1665. [PubMed] [Google Scholar]
- 38.Shi Y, Luo S, Peng J, Huang C, Tan D, Hu W. The structure, expression and function prediction of DAZAP2, a down-regulated gene in multiple myeloma. Genomics, proteomics & bioinformatics. 2004;2:47–54. doi: 10.1016/S1672-0229(04)02007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo SQ, Hu JP, Qu Q, Li J, Ren W, Zhang JM, Zhong Y, Hu WX. The effects of promoter methylation on downregulation of DAZAP2 in multiple myeloma cell lines. PloS one. 2012;7:e40475. doi: 10.1371/journal.pone.0040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Zepp JA, Qian W, Yin W, Bunting KD, Aronica M, Erzurum S, Li X. A novel IL-25-signaling pathway through STAT5. 2014. Submitted manuscript. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.