Abstract
Background:
The detection of synchronous metastases at primary diagnosis of breast cancer (BC) affects its initial management. A risk calculator that incorporates many factors to evaluate an individual's risk of harbouring synchronous metastases would be useful to adapt cancer management.
Patients and Methods:
Patients with primary diagnosis of BC were identified from three institutional databases sharing homogeneous work-up recommendations. A risk score for synchronous metastases was estimated and a nomogram was constructed using the first database. Its performance was assessed by receiver characteristic (ROC) analysis. The nomogram was externally validated in the two independent cohorts.
Results:
A preoperative nomogram based on the clinical tumour size (P<0.001), clinical nodal status (P<0.001), oestrogen (P=0.17) and progesterone receptors (P=0.04) was developed. The nomogram accuracy was 87.3% (95% confidence interval (CI), 84.45–90.2%). Overall, the area under the ROC curve (AUC) was 86.1% for the validation set from the Institut Curie-René Huguenin, and 63.8% for the MD Anderson validation set. The negative predictive value (NPV) was high in the three cohorts (97–99%).
Conclusions:
We developed and validated a strong metastasis risk calculator that can evaluate with high accuracy an individual's risk of harbouring synchronous metastases at diagnosis of primary BC.
Condensed abstract:
A nomogram to predict synchronous metastases at diagnosis of breast cancer was developed and externally validated. This tool allows avoiding unnecessary expensive work-up.
Keywords: breast cancer, metastasis, nomogram, risk score
There were about 1 384 155 newly diagnosed breast cancer (BC) cases worldwide in 2008, as reported by the International Agency for Research on Cancer (IARC, 2008). A recent systematic analysis showed a 3.1% annual increase in BC incidence during the last three decades (Forouzanfar et al, 2011). In newly diagnosed cases, systematic body screening will be proposed to achieve accurate staging of the disease (UICC TNM) and to guide the primary treatment strategy (Edge et al, 2010). This work-up aims at identifying the subgroup of patients with distant metastases at diagnosis, who have a distinctly more severe prognosis. Data from the Surveillance Epidemiology and End Results (SEER) programme showed that synchronous distant metastases at diagnosis of primary BC are present in 5% of patients (Howlader et al, 2011).
Accurate staging at diagnosis has clinical and psychological implications, such as the possible avoidance of mastectomy or of certain radiotherapy fields, of prolonged anti-cancer treatment or of anti-osteoclastic drugs. However, many expensive and pointless work-up examinations are performed in women with localised BC. The identification of clinical and tumour factors associated with synchronous metastases may therefore be useful to clinicians. The benefit of systematic body imaging is controversial, but it is very often proposed to all patients in the absence of specific markers of metastases (Carlson et al, 1996; Chen et al, 2000; Gerber et al, 2003). The timing of complete staging is also debated, but clearly doing this evaluation before surgery is relevant because, although surgery is performed most of the time, whole body imaging can influence BC staging and clinical management, particularly in the case of oligometastatic disease. For instance, Yap et al (2001) showed that whole body FDG PET in BC altered the clinical stage in 36% of patients and the clinical management in 60%. The leading professional societies such as the American Society of Clinical Oncology (ASCO) or the European Society of Medical Oncology (ESMO) do not recommend extensive staging in localised BC, except in large tumours, or in the presence of clinically positive axillary lymph nodes.
Identifying high-risk groups would help decreasing the number of unnecessary exams. To date, no statistical model has been developed to predict the likelihood of synchronous metastases at diagnosis. In recent years, nomograms have gained popularity in clinics and have been proposed in oncology to address the individual-based prognosis (Kattan et al, 1998; Hanrahan et al, 2007; Mazouni et al, 2011). Nomograms are statistically based tools that provide the overall probability of a specific outcome for an individual patient. Factors associated with a defined event are incorporated in the nomogram and the calculated probability of the event occurrence is provided in graphical formats.
In this study, we developed a preoperative nomogram based on clinico-pathologic factors to predict the probability of synchronous distant metastases at diagnosis of primary BC. We then validated this nomogram in two independent data sets.
Patients and methods
Training population
The study population consisted of 2059 consecutive women who were diagnosed with primary BC at the One-Stop Unit of the Institut Gustave Roussy (IGR), Villejuif, France, between April 2004 and March 2010; 4461 consecutive patients from the Institut Curie-René Huguenin (CRH), Saint-Cloud, France and 2550 patients treated in the Breast Medical Oncology Department at the MD Anderson Cancer Center. All patients were enrolled over the same period after primary diagnosis of BC and irrespectively of the subsequent treatments (primary surgery and neo-adjuvant chemotherapy). All patients diagnosed with invasive BC at IGR and CRH underwent an initial standardised work-up that consisted in a clinical examination, blood tests including serum markers (CA 15-3 and/or CEA and/or CYFRA 21.1), bone scintigraphy, chest X-ray, abdominal and pelvic ultrasound. At MD Anderson Cancer Center, X-ray, thoraco-abdominal CT and bone scan were performed. Elevated tumour markers alone were not considered as a proof of metastases. The diagnosis of metastases was made based on typical radiological images. Tumour markers were measured and a focused biopsy was carried out in the case of isolated/uncertain lesion(s). The internal Institutional Review Boards of the three centres gave their approval for this study.
Statistical analysis
Variables evaluated at the time of the initial diagnosis included patient age (as a continuous variable), family history of BC, clinical (or radiological if non-palpable) tumour size (cT) (as a continuous variable), oestrogen receptor (ER) and progesterone receptor (PR) status (by immunohistochemical analysis), histological grade and HER2 status. Hormone receptor positivity was defined based on positive staining for ER and/or PR in at least 10% of cancer cell nuclei in France, and ⩾5% at MD Anderson Cancer Center (MDACC). HER2 status was defined according to the ASCO guidelines. The ER, PR and HER2 status were entered as positive or negative.
The factors predictive for the presence of synchronous distant metastases at diagnosis were identified through univariate and multiple logistic regression analyses. Odds ratios were calculated to estimate the strength of the association between individual risk factors and synchronous metastases. Factors found to be significantly associated with synchronous metastases in the univariate analysis (P<0.20) were included in the multivariate analysis.
Using the IGR data set, a logistic regression-based nomogram was developed to predict synchronous metastases at diagnosis. Backward elimination was performed to choose the covariates to be retained in the model. The discriminative power of the model was quantified in terms of discrimination and calibration. Discrimination was quantified by the area under the receiver-operating characteristic (ROC) curve (AUC) and 95% confidence intervals (CIs) were estimated. In all, 200 bootstrap resamples were used for internal validation of the accuracy estimates and to reduce overfit bias. Sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) and their 95% CI were calculated for various cutoff points of the calculated risk score.
External validation was performed by using the nomogram for patients from the two independent validation cohorts. All statistical analyses were two-sided and were performed using the R cran Design package (R Development Core Team, 2010). The characteristics of the three cohorts were compared using the Chi-square or Fisher exact tests for qualitative data and the Kruskal–Wallis test for quantitative data.
Results
A total of 2059 patients diagnosed with primary BC at the One-Stop Unit, IGR, over a 6-year period, were used to develop the nomogram. Among them, 23.7% (488 out of 2059) received neo-adjuvant treatment and 4.4% (91 out of 2059) had synchronous distant metastases at diagnosis.
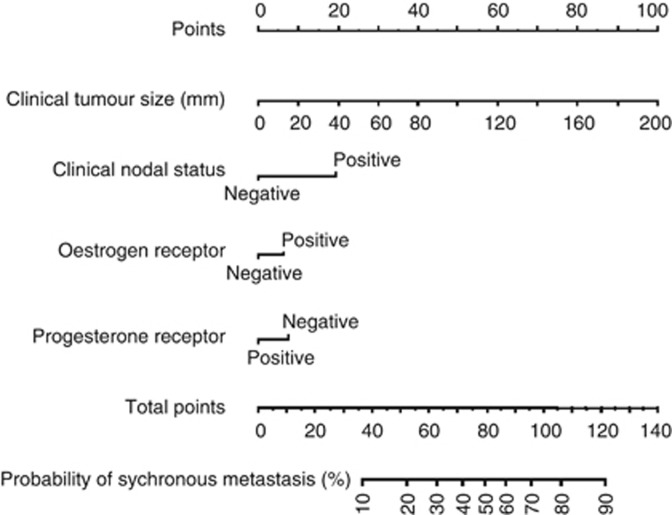
Table 1 shows the univariate and multivariate analyses of potential predictors of synchronous metastases at diagnosis. Clinical tumour size (cT), clinical nodal status (cN), ER, PR and nuclear grade were significantly associated with the risk of synchronous metastases in the univariate analysis and, therefore, they were included in the multivariate analysis (Table 1). The variables used to develop the nomogram were clinical tumour size (cT, P<0.001), clinical nodal status (cN, P<0.001), ER (P=0.17) and PR (P=0.04) (Figure 1). For instance, a woman with a 30-mm tumour (15 points), negative cN status (0 point), ER positive (5 points) and PR negative (8 points) would score 28 points that can be converted in a 4% probability of having synchronous metastases at primary diagnosis.
Table 1. Univariate and multivariate regression analyses.
| Univariate OR (95% CI) | P-value | Multivariate OR (95% CI) | P-value | |
|---|---|---|---|---|
| Age | 1.25 (0.91–1.71) | 0.1702 | – | – |
| Clinical tumour size (mm) | 1.94 (1.72–2.20) | <0.001 | 1.7 (1.5–1.9) | <0.001 |
| Clinical nodal status | 7.8 (5–12) | <0.001 | 4.1 (2.6–6.6) | <0.001 |
| Oestrogen receptor status | 0.66 (0.41–1.07) | 0.09 | 1.7 (0.9–3.4) | 0.13 |
| Progesterone receptor status | 0.53 (0.35–0.81) | 0.0035 | 0.6 (0.3–1) | 0.05 |
| Nuclear grade | 1.3 (0.6–2.8) | 0.49 | ||
| HER2 positive | 1.41 (0.82–2.44) | 0.21 | – | – |
Abbreviations: CI=confidence interval; OR=odds ratio.
Figure 1.
Nomogram based on 2059 patients treated at the Institut Gustave Roussy, for predicting the risk of synchronous metastases at first diagnosis of primary BC. To obtain the predicted probability of synchronous metastases, locate patient values at each axis. Draw a vertical line upward to the ‘Points' axis to determine the points scored by the variable. Sum the points for all variables and locate the sum on the ‘Total points' axis.
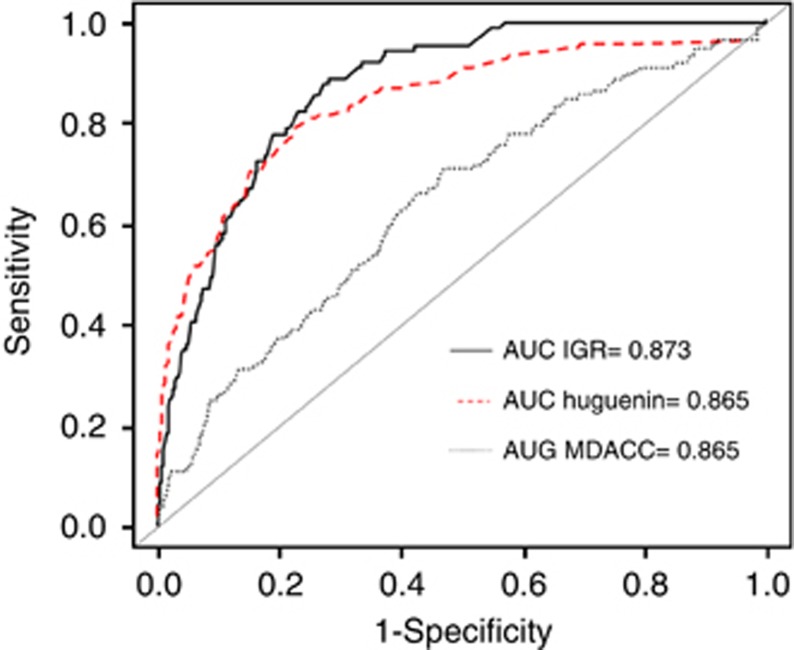
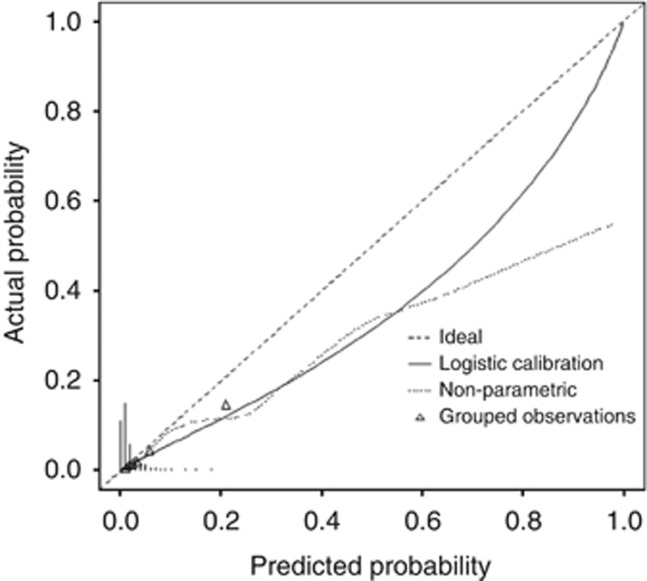
The performance of the risk score to differentiate patients with or without metastases was assessed using the ROC analysis (Figure 2). The AUC was 87.3% (95% CI, 84.4–90.2%). The nomogram predictions were then compared with the actual probability of having synchronous metastases in the 2059 women (Figure 3). The dashed line represents the performance of an ideal nomogram, where the predicted outcome would overlap perfectly with the actual outcome. The performance of the IGR nomogram is shown by the plotted solid line.
Figure 2.
Receiver-operating characteristics curve for the performance of the risk score in identifying patients with synchronous metastases in the three data sets.
Figure 3.
Calibration plot of the internal and external validation cohorts. The x axis shows the prediction calculated using the nomogram, and the y axis shows the observed rates of synchronous metastases for patients in the IGR cohort. The dashed line is the reference line, where an ideal nomogram would lie. The solid line indicates the performance of the IGR nomogram applied to the validation cohort. The solid line is not close to the dashed line of the ideal nomogram and is not always within the 10% margin of error.
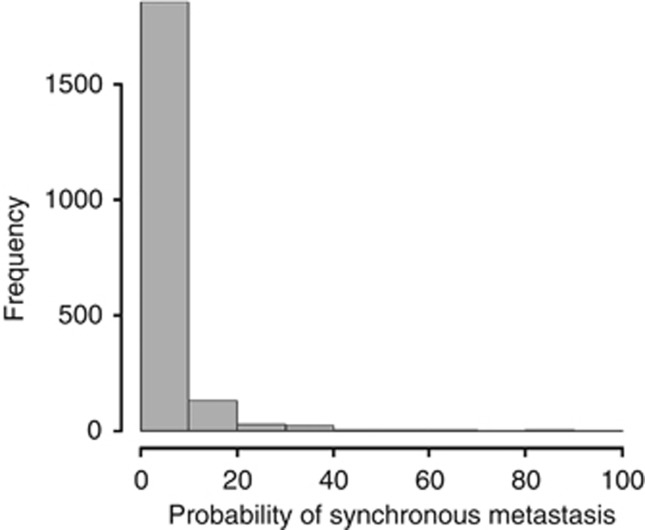
The nomogram was then validated externally using the CRH and MDACC data sets. The CRH cohort characteristics were comparable to those of the training IGR cohort, while those of the MDACC data set were significantly different, particularly cT, ER, PR and HER2 status and histological grade (Table 2). The rates of synchronous metastases were different between the IGR and CRH cohorts (4.4% and 3.2% P=0.02), but not between the IGR and MDACC series (4.4% and 5.1% P=0.35) (Table 2). In the CRH cohort, only 591 (13.2%) patients received neoadjuvant treatment, while 2399 (94%) of the MDACC patients received primary treatment. Figure 4 shows the distribution of the predicted probability of having synchronous metastases for each patient calculated with the nomogram. Most patients (Figure 4) with BC had a predicted metastatic risk lower than 10%. The observed AUC of the constructed nomogram was 86.1% (95% CI, 83.2–89.1%) for the CRH validation cohort and 63.8% (95%CI, 58.8–68.8%) for the MDACC validation set (Figure 2). Therefore, the prediction model based on the IGR data overestimated the actual probability compared with the MDACC data. The respective sensitivity, specificity, NPV and PPV are shown in Table 3. Sensitivity and specificity were good for the IGR and CRH data sets and lower for the MDACC cohort. In all three cohorts, the NPV was higher than 96%.
Table 2. Comparison of the baseline characteristics in the three study cohorts.
| Training set IGR n=2059 | Validation set 1 CRH n=4461 | P* value | Validation set 2 MDACC n=2550 | P** value | |
|---|---|---|---|---|---|
| Age, median (range) | 60 (23–96) | 58 (21–95) | <0.001 | 50 (19–91) | <0.001 |
| Clinical tumour size in mm median (range) | 16 (2–200) | 15 (0–200) | 0.91 | 35 (0–200) | <0.001 |
| Clinical node status (%) | <0.001 | ||||
| Negative | 1693 (82.2) | 3750 (84.1) | 858 (33.6) | ||
| Positive | 366 (17.8) | 711 (15.9) | 1692 (66.4) | ||
| Oestrogen receptor status (%) | 0.65 | <0.001 | |||
| Negative | 400 (19.4) | 890 (20) | 893 (35) | ||
| Positive | 1659 (80.6) | 3571 (80) | 1657 (65) | ||
| Progesterone receptor status (%) | 0.96 | <0.001 | |||
| Negative | 741 (36) | 1601 (35.9) | 1303 (51.1) | ||
| Positive | 1318 (64) | 2860 (64.1) | 1247 (48.9) | ||
| Tumour grade (%) | <0.001 | <0.001 | |||
| 1–2 | 1417 (68.8) | 3357 (75.2) | 921 (36.1) | ||
| 3 | 642 (31.2) | 1104 (24.7) | 1629 (63.9) | ||
| HER2 (%) | <0.001 | <0.001 | |||
| Positive | 260/1807 (14.4) | 492/4349 (11.3) | 382/1909 (20) | ||
| Negative | 1547/1807 (85.6) | 3857/4349 (88.7) | 1527/1909 (80) | ||
| Unknown | 252 (12.2) | 112 (2.5) | 641 (25.1) | ||
| Synchronous metastases | 91 (4.4) | 144 (3.2) | 0.02 | 129 (5.1) | 0.35 |
Abbreviations: CRH= Institut Curie-René Huguenin; IGR= Institut Gustave Roussy; MDACC=MD Anderson Cancer Center.
P* value IGR vs CRH; P** value IGR vs MDACC.
Figure 4.
Distribution of predicted probability of population with synchronous metastases in all data sets. The x axis of Figure 1 shows the prediction calculated using the nomogram, and the y axis shows the observed rates of synchronous metastases for patients in the training cohort.
Table 3. Sensitivity, specificity, PPV, and NPV for the risk calculator.
| Sensitivity (%) | Specificity (%) | NPV | PPV | |
|---|---|---|---|---|
| Training set (IGR) | 89 | 71.8 | 99.3 (87.2) | 12.8 (0.7) |
| Validation set (CRH) | 81.9 (82.6) | 76.1 (75.7) | 99.2 (89.8) | 10.3 (0.8) |
| Validation set (MDACC) | 70.5 | 52.8 | 97.1 | 7.4 |
Abbreviations: CRH=Institut Curie-René Huguenin; IGR=Institut Gustave Roussy; MDACC=MD Anderson Cancer Center; NPV=negative predictive value; PPV=positive predictive value.
Finally, a computer program was developed to help physicians determine the risk of synchronous metastases at primary BC diagnosis. The program called ‘synchronous metastases in BC' is in Java. An internet browser with Java capability is required to run the applets. An example of a screen is shown in Figure 5. The applets will be freely available online through the IGR, CRH and MDACC websites.
Figure 5.
Example of a screen from the computer program ‘synchronous metastases in breast cancer' that was developed from the nomogram described in this study to provide patients and physicians with information to assist them in treatment decision-making with regard to the risk of metastasis.
Development of a nomogram based on the three cohorts
To correct for the lack of reproducibility and verify whether the same criteria were selected, we build a model that incorporated all patients from the three cohorts (n=9070). In univariate analysis, clinical tumour size (cT, P<0.001), clinical nodal status (cN, P<0.001), ER (P<0.001), PR (P<0.001), nuclear grade (P<0.001) and HER2 status (P<0.001) were predictors of the metastatic status. In the final model, cT (P<0.001), cN (P<0.001), PR (P<0.001), nuclear grade (P=0.01) and HER2 (P=0.01) status were retained to build the nomogram. HER2 status could not be assessed in 11% of patients. The AUC for the constructed nomogram was 80.2% (95% CI: 78–82.4%). The sensitivity was 81.8%, the specificity 65.3%, the NPV 91% and the PPV was 1.2%.
Discussion
The estimation of the individual risk of synchronous metastases at diagnosis of primary BC is attractive because it can facilitate the decision regarding the initial staging work-up, thus avoiding pointless examinations. Several nomograms have been developed for patients with BC (Van Zee et al, 2003; Rouzier et al, 2005; Houvenaeghel et al, 2009). Most of these nomograms were developed to predict the nodal status or response to treatment. The present work is the first attempt to propose a diagnostic nomogram to improve BC staging by calculating the risk of initial distant metastases in patients with BC. The nomogram was validated internally and externally using three large data sets from reference cancer centres.
At present, in the presence of synchronous metastases, systemic treatment is proposed in addition to the local treatment of the primary tumour like for local tumours. The level of proof regarding local treatment is low, but the homogeneous results of several retrospective series support this practice (Ali and Le Scodan, 2011). However, mastectomy could be avoided in non-inflammatory cases. Local therapy of metastatic sites might be proposed, as well as bone-targeting treatments in the case of bone involvement.
The place of imaging in BC initial staging remains controversial. Currently, there is no evidence to support routine screening for metastatic disease in asymptomatic women with early operable BC (T1-2, N0-1), and this screening may not be cost effective (Gerber et al, 2003). Imaging for staging is inconsistently recommended by the different National Cancer Institutes worldwide (Ciatto et al, 1988; Harris, 2000; Myers et al, 2001). Most Cancer Societies recommend whole body staging only for large tumours or in the presence of positive nodes (Schnipper et al, 2012; Senkus et al, 2013). Previous studies on cost savings suggest focusing on metastasis screening by chest X-ray and blood tests alone in patients with stage I-II BC with less than five affected axillary lymph nodes (Norum and Andreassen, 2000; Ravaioli and Tassinari, 2000). However, this requires prior surgery. The broader knowledge we have today of tumour biology should be used to identify patients at high risk of metastatic dissemination, and to avoid unnecessary initial and subsequent screenings.
An interesting aspect of our nomogram is the inclusion of classic prognostic factors such as tumour size, nodal clinical, and hormone status. Our model combines independent factors that allow appraising the magnitude of the impact of each factor on the probability of synchronous metastases. The use of classic, well-established clinicopathological factors helps make the nomogram generalisable in the routine clinical practice. The probability of synchronous metastases in a patient is easily calculated by using the nomogram or the web interface. The practical application is the identification of high-risk women by using a combination of factors that together strongly increases the risk of metastases. An acceptable cutoff has yet to be defined with a balance between risk and cost saving. Clearly, most patients with BC have a predicted metastatic risk lower than 10% at diagnosis. We thus propose a definition of low-risk patients based on an observed metastatic risk lower than 4% in agreement with the rates we detected in the two European cohorts. This low threshold has to be prospectively evaluated and patients could be considered at high risk when showing a metastatic risk higher than 10%.
We acknowledge that this study has some limitations. The multicentre cohorts used to validate this nomogram were heterogeneous. Thus, the metastasis rates were variable between centres, with the highest prevalence in the MDACC cohort. This was due to a significantly larger tumour size, higher nuclear grade, and frequent HR negativity in the MDACC population. As a consequence, the prediction model developed in the training set overestimated the actual probability in the MDACC cohort. For high predicted values, especially when higher than 20%, the nomogram tends to overestimate the rate of synchronous metastases. While the two European cohorts were quite similar, the MDACC cohort included mostly patients who received neoadjuvant treatment as they were at higher risk for metastasis. Therefore, we also developed a nomogram based on all patients and in this final model, tumour grading and HER2 status significantly predicted the risk of synchronous metastasis. One limitation of this final model is the high number of patients without HER2 assessment and the absence of external validation. The observed AUC of this nomogram was lower (80.2%) than the one of the nomogram based on the initial training cohort.
In the future, we plan to validate this preoperative nomogram using other data sets and in a prospective manner. This nomogram was based on classic clinico-pathological factors, but emerging biomarkers and genomic patterns could also be tested. It would be interesting to incorporate them into future nomograms to provide greater accuracy of risk estimation for distant metastases in the heterogeneous BC population.
In conclusion, estimation of the risk for distant metastases at primary diagnosis of BC may be helpful to personalise the decision-making strategy in BC patients. In this study, we developed a nomogram for clinical use to estimate this risk and avoid unnecessary expensive work-up in low-risk group which threshold could be <4%. Incorporating significant biological factors in future studies could improve the accuracy of this statistical model.
Acknowledgments
We thank Lorna Saint Ange and Elisabetta Andermarcher for editing.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Ali D, Le Scodan R. Treatment of the primary tumor in breast cancer patients with synchronous metastases. Ann Oncol. 2011;22:9–16. doi: 10.1093/annonc/mdq301. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Goldstein LJ, Gradishar WJ, Lichter AS, McCormick B, Moe RE, Theriault RL. NCCN breast cancer guidelines: the National Comprehensive Cancer Network. Oncology. 1996;10:47–75. [PubMed] [Google Scholar]
- Chen EA, Carlson GA, Coughlin BF, Reed WP, Jr, Garb JL, Frank JL. Routine chest roentgenography is unnecessary in the work-up of stage I and II breast cancer. J Clin Oncol. 2000;18:3503–3506. doi: 10.1200/JCO.2000.18.20.3503. [DOI] [PubMed] [Google Scholar]
- Ciatto S, Pacini P, Azzini V, Neri A, Jannini A, Gosso P, Molino A, Capelli MC, di Costanzo F, Pucciatti MA. Preoperative staging of primary breast cancer: a multicentric study. Cancer. 1988;61:1038–1040. doi: 10.1002/1097-0142(19880301)61:5<1038::aid-cncr2820610530>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A.(eds) (2010AJCC Cancer Staging Manual7th ednSpringer: New York [Google Scholar]
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- Gerber B, Seitz E, Müller H, Krause A, Reimer T, Kundt G, Friese K. Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat. 2003;82:29–37. doi: 10.1023/B:BREA.0000003917.05413.ac. [DOI] [PubMed] [Google Scholar]
- Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- Harris JR.2000Staging of breast cancer Diseases of the BreastHarris JR, Lippman ME, Marrow M, Osborne CK, (eds)2nd edn403–406.Lippincott, Williams and Wilkins: Philadelphia [Google Scholar]
- Houvenaeghel G, Nos C, Giard S, Mignotte H, Esterni B, Jacquemier J, Buttarelli M, Classe JM, Cohen M, Rouanet P, Penault Llorca F, Bonnier P, Marchal F, Garbay JR, Fraisse J, Martel P, Fondrinier E, Tunon de Lara C, Rodier JF. A nomogram predictive of non-sentinel lymph node involvement in breast cancer patients with a sentinel lymph node micrometastasis. Eur J Surg Oncol. 2009;35:690–695. doi: 10.1016/j.ejso.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA.(eds) (2011SEER Cancer Statistics Review, 1975-2008 National Cancer Institute: Bethesda, MD; http://seer.cancer.gov/csr/1975_2008/ Based on November 2010 SEER data submission, posted to the SEER website. [Google Scholar]
- International Agency for Research on Cancer GLOBOCAN (2008. Cancer incidence and mortality worldwide. http://globocan.iarc.fr/globocan2008.htm .
- Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- Mazouni C, Romain S, Bonnier P, Martin PM. A nomogram predicting the probability of primary breast cancer survival at 2- and 5-years using pathological and biological tumor parameters. J Surg Oncol. 2011;103:746–750. doi: 10.1002/jso.21712. [DOI] [PubMed] [Google Scholar]
- Myers RE, Johnston M, Pritchard K, Levine M, Oliver T. Baseline staging tests in primary breast cancer: a practice guideline. CMAJ. 2001;164:1439–1444. [PMC free article] [PubMed] [Google Scholar]
- Norum J, Andreassen T. Screening for metastatic disease in newly diagnosed breast cancer patients. What is cost-effective. Anticancer Res. 2000;20:2193–2196. [PubMed] [Google Scholar]
- R Development Core Team 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/ .
- Ravaioli A, Tassinari D. Staging of breast cancer: recommended standards. Ann Oncol. 2000;11:3–6. doi: 10.1093/annonc/11.suppl_3.3. [DOI] [PubMed] [Google Scholar]
- Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, Buzdar AU, Garbay JR, Spielmann M, Mathieu MC, Symmans WF, Wagner P, Atallah D, Valero V, Berry DA, Hortobagyi GN. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–8339. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- Schnipper LE, Smith TJ, Raghavan D, Blayney DW, Ganz PA, Mulvey TM, Wollins DS. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F. ESMO Guidelines Working Group. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 (Suppl 6:vi7–23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, Borgen PI, Cody HS, 3rd, Kattan MW. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- Yap CS, Seltzer MA, Schiepers C, Gambhir SS, Rao J, Phelps ME, Valk PE, Czernin J. Impact of whole-body 18F-FDG PET on staging and managing patients with breast cancer: the referring physician's perspective. J Nucl Med. 2001;42:1334–1337. [PubMed] [Google Scholar]