Abstract
Background:
Candidemia is an important nosocomial blood stream infection in critically ill patients. Although several studies have addressed candidemia, very few have reviewed the impact of Candida glabrata candidemia in Intensive Care Unit (ICU) patients.
Materials and Methods:
The medical records of ICU patients between 2006 and 2010 were reviewed retrospectively. The epidemiology, clinical features and mortality related risk factors among our adult ICU patients were seen.
Results:
Among 144 episodes of candidemia, C. glabrata (n = 26; 18.05%) was the third most common species isolated. The incidence of C. glabrata candidemia was 0.21/1000 ICU admissions. The most common risk factors were prior exposure to broad spectrum antibiotics (100%), central venous catheter (100%), mechanical ventilation (76.9%), diabetes mellitus (50%), age >65 years (46.15%). Urine (23%) was the most common source of C. glabrata candidemia. Overall in hospital 30 days mortality rate due to C. glabrata fungemia was 53.8%. Patients who were treated with fluconazole showed better outcome than patients treated with amphotericin B. Renal failure requiring hemodialysis was the significantly associated with mortality in our study.
Conclusion:
Candida glabrata was the 3rd most common Candida causing candidemia in our ICUs with a incidence of 0.21/1000 ICU admissions. The outcome of ICU acquired C. glabrata candidemia was poor with 30 days mortality rate of 53.8%. Renal failure requiring hemodialysis was the only risk factor associated with mortality. Further studies are required to identify the other risk factors associated with mortality in C. glabrata candidemia.
Keywords: Candida glabrata candidemia, mortality, risk factors
Introduction
Candidemia is the fourth most common cause of hospital acquired infection. It remains an important cause of morbidity and mortality in critically ill patients in Intensive Care Units (ICU).[1] Despite the availability of effective antifungal therapy, mortality due to candidemia remains high ranging from 30% to 60%.[2,3,4,5] Recent epidemiological data also reveal a mycological shift from Candida albicans to the non-albicans Candida (NAC) species such as Candida glabrata, Candida tropicalis, Candida parapsilosis and Candida krusei.[1,6,7] It is considered that frequent use of fluconazole as antifungal prophylaxis has played a major role in the emergence of NAC species. Some of these species have been correlated with increased virulence and sometimes with increased mortality. C. glabrata candidemia is considered to be associated with higher mortality than other NAC.[8] Although several studies have addressed epidemiological and clinical characteristics of candidemia in ICU, very few have reviewed the mortality related risk factors for C. glabrata candidemia. We therefore reviewed the epidemiology, clinical features and the association of mortality with various risk factors among the ICU patients with C. glabrata candidemia.
Materials and Methods
All adult (>18 years of age) patients, admitted in medical and surgical ICU, with C. glabrata candidemia. During the study period of 2006–2010 were included. Clinical information on each episode was collected on a standardized data form. The medical records of patients were reviewed retrospectively and data including demographic characteristics, clinical features, risk factors (such as presence of central line, prior exposure to fluconazole, antibacterials and anticancer therapy, receipt of total parenteral nutrition (TPN), renal failure requiring hemodialysis, mechanical ventilation, malignancy), source of candidemia, antifungal therapy, and clinical outcome at 30 days were collected for analysis. Episodes of candidemia developing >48 h after ICU admission or 48 h or less following discharge were included in the study. Neonatal or pediatric fungemia cases were not included in the study. A patient was considered to have candidemia if C. glabrata was isolated from at least one blood culture. Source of candidemia was defined as a culture positive site with the same species. Risk factors over 30 days prior to the onset of candidemia were assessed.
Blood samples were processed using the automated system BacT/Alert (Biomerieux). Identification of the Candida was done on Mini API (Biomerieux) till 2009 and on Vitek 2 compact (Biomerieux) thereafter. Statistical analysis was conducted using SPSS for windows version 13.0 (SPSS inc., Chicago, IL). Fisher exact test or Chi-square test was applied for comparison of categorical variables. P < 0.05 were considered as statistically significant.
Results
During our 5-year study period, there were 144 episodes of ICU acquired candidemia in 144 patients from our adult ICUs. C. tropicalis (33.3%) was the most common species followed by C. albicans (29.8%), C. glabrata (18.05%). C. glabrata (n = 26) was the 3rd most common species responsible for candidemia in our adult ICUs. Overall the incidence of candidemia was 1.17/1000 ICU admissions. The incidence of C. glabrata candidemia in ICU ranged from 0.31 to 0.11/1000 admissions (overall incidence – 0.21/1000 ICU admissions) with a peak in the year 2006 and 2010. Figure 1 shows that there is a fluctuating incidence trend of C. glabrata fungemia in our ICUs. The mean age was 60.7 ± 14.3 years and 65.3% were males. The median time from ICU admission to onset of C. glabrata candidemia was 8 ± 13.8 days. Out of total 26 patients, 17 patients were admitted in the medical ICUs and only 9 patients in the surgical ICUs. The most common risk factors were prior exposure to broad spectrum antibiotics (100%), central venous catheter (100%), mechanical ventilation (76.9%), diabetes mellitus (50%), age >65 years (46.15%). 30.7% patients had received prior corticosteroid therapy and 26.9% patients had undergone hemodialysis for renal failure. 26.9% patients had a history of prior fluconazole exposure before blood culture came positive for C. glabrata. One patient each had a history of malignancy and autoimmune disease. Regarding the source of C. glabrata fungemia, urine (23%) was the most common source followed by endotracheal aspirates (19.2%). 50% patients did not have any source of candidemia. Of the 26 C. glabrata candidemia cases 15.3% (4/26), were not treated. This was due to death of the patients (n = 3) and discharge of the patient (n = 1), before blood culture positivity. Treatment regimens of C. glabrata candidemia patients is shown in Table 1. The most common antifungal agent given was the fluconazole (68.1%), followed by liposomal amphotericin B (13.6%). In 9.09% patients, antifungal therapy switched from fluconazole to amphotericin B either due to poor clinical response to fluconazole or identification of infecting species as C. glabrata. In our study, surprisingly 30 day's mortality rate was 42.9% in patients treated with fluconazole while 100% in patients treated with amphotericin B.
Figure 1.
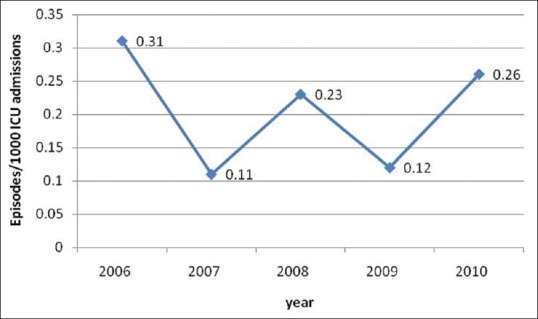
Candida glabrata incidence in Intensive Care Unit
Table 1.
Treatment regimens of Candida glabrata candidemia patients

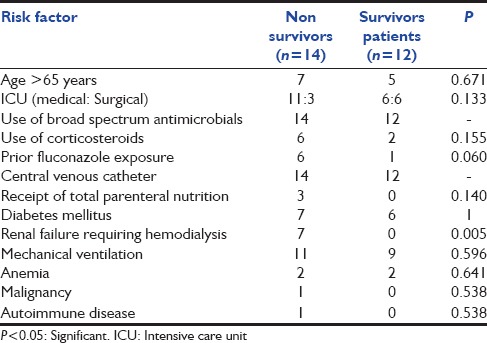
Overall in hospital 30 days mortality rate due to C. glabrata fungaemia was 53.8%, with a median time to death after drawing the first positive blood culture of 6 ± 7.7 days. The most common risk factor associated with mortality is shown in Table 2. Renal failure requiring hemodialysis was the only factor, which was significantly associated with mortality in our study.
Table 2.
Risk factors associated with mortality
Discussion
Candida glabrata currently ranks 2nd as the causative agent of nosocomial systemic Candida infections[8] and is associated with a high mortality rate.[1] According to a report by Pfaller and Diekema C. glabrata was an infrequent cause of blood stream infection in Latin America (7.5%) and Asia Pacific region (10.2%), while there is an increase in C. glabrata frequency in Canada, USA and Europe.[8] In our study, C. glabrata represents about 18.05% of candidemias which is consistent with rates reported in some studies (10–30%).[8,9,10,11,12] In a study done in Taiwan, candidemia incidence of 4.3/1000 ICU admissions was reported, which is quite higher than our incidence of 1.17/1000 ICU admissions.[13] Similar trend is seen in C. glabrata incidence (1.3/1000 ICU admissions in Taiwan when compared to 0.21/1000 ICU admissions in this study) in our study.[13] The incidence of C. glabrata candidemia was highest in the year 2006 (0.31/1000 ICU admissions) with a fluctuating trend in the studied time period in our ICUs. The reason for this could not be ascertained.
In a study done by Ruan et al., 62% of the patients with C. glabrata candidemia was admitted in medical ICUs, which is similar to our data of 65.3%.[13] Many studies have reported an almost equal incidence among males and females; while in our study, 65.3% of C. glabrata candidemia was seen in male patients.[14,15]
Some of the previous studies have suggested that C. glabrata candidemia is more common in elderly patients.[16,17,18,19] This association can be due to antifungal drug pressure or underlying disease. Furthermore, Lockhart et al. demonstrated increased mucosal colonization with C. glabrata among healthy elderly individuals.[20] Our study also supports that C. glabrata candidemia is more common in elderly patients. The mean age in our study was 60.7 ± 14.3 years, which is comparable to other studies.[13,15]
As observed in some of the previous studies, increasing length of ICU stay increases the risk of candidemia.[14,21] Accordingly we report that median duration of ICU stay before C. glabrata candidemia was 8 ± 13.8 days. Choi et al. reports 15 ± 31 ICU days stay before C. glabrata candidemia, which is higher compared to our data.[14] Major risk factors for development of candidemia as reported in various other studies were also observed among our patients.[13,14,15,21] All the patients in our study were on broad spectrum antimicrobials and had central venous catheter. 76.5% patients were on mechanical ventilation, while in a study done by Ruan et al. 93% patients were mechanically ventilated.[13] In our study, only 26.9% patients were on hemodialysis due to renal failure which is quite lower than the rate reported (53%) by Ruan et al.[13] Ruan et al. reports that 53% of C. glabrata candidemia patients received TPN while in our ICU only 11.5% patients received TPN.[13] Although it should be kept in mind that the above factors are common to any patient admitted in ICU, and environment of ICU with various invasive procedures makes it conducive for acquiring fungal infections.
Our data suggests that candiduria is a useful indicator of systemic candidiasis and is more often seen with candidemia.[22] However, other studies report central venous catheter as a more common probable port of entry.[15]
The reported mortality rate for candidemia is very high (38–76%) in adults in developed as well as developing countries.[3,12,21,23,24,25,26] Several studies observed 30 days mortality rate for C. glabrata candidemia as 30%, 48.6%, 50%, 52%, 58%, 60%, which are comparable to our rate of 53.8%.[12,13,14,15,24,27,28] The median time from C. glabrata candidemia to death in our ICU was 6 ± 7.7 days. We also evaluated the modifiable risk factors associated with mortality. On univariate analysis, our data revealed that renal failure requiring hemodialysis was significantly associated with mortality (P = 0.005). Ruan et al., Choi et al., Blot et al. and Klevay et al. also reports renal failure as an independent predictor of mortality in C. glabrata candidemia.[14,15,27,28] They also report prior fluconazole exposure, age, use of steroids as predictors of mortality.
IDSA recommends Amphotericin B or caspofungin as the treatment for C. glabrata candidemia. C. glabrata is relatively resistant to fluconazole when compared with C. albicans. In our study, 68.1% of patients were treated with fluconazole. In a study done in Taiwan, amphotericin B regimen was associated with a lower mortality than regimens without amphotericin B.[13] While in contrast, our data shows that all three patients who were treated with amphotericin B died, while only 50% of patients who received fluconazole died. This can be explained by the fact that in all the patients treated with amphotericin B, the treatment was started late (>72 h).
The present study had several limitations. First, the sample size was limited as the data is from a single institution and data could be influenced by local infection control practices and local antibiotic policies. Furthermore, C. glabrata candidemia is not very common infection. Second, the study was retrospective, which can lead to reviewer bias. Third, small sample size precludes meaningful statistical analysis of mortality related risk factors.
Conclusion
Candida glabrata was the 3rd most common Candida causing candidemia in our ICUs with an incidence of 0.21/1000 ICU admissions. The outcome of ICU acquired C. glabrata candidemia was poor with 30 days mortality rate of 53.8%. Renal failure requiring hemodialysis was the only risk factor associated with mortality. Further studies are required to identify the risk factors associated with mortality in C. glabrata candidemia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 3.Cheng YR, Lin LC, Young TG, Liu CE, Chen CH, Tsay RW. Risk factors for candidemia-related mortality at a medical center in central Taiwan. J Microbiol Immunol Infect. 2006;39:155–61. [PubMed] [Google Scholar]
- 4.Chen TC, Chen YH, Tsai JJ, Peng CF, Lu PL, Chang K, et al. Epidemiologic analysis and antifungal susceptibility of Candida blood isolates in southern Taiwan. J Microbiol Immunol Infect. 2005;38:200–10. [PubMed] [Google Scholar]
- 5.Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc. 1996;95:19–28. [PubMed] [Google Scholar]
- 6.Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122–8. doi: 10.1086/513663. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Rosso R, et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis. 2006;6:21. doi: 10.1186/1471-2334-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ. International Fungal Surveillance Participant Group. Twelve years of fluconazole in clinical practice: Global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10(Suppl 1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 9.Xess I, Jain N, Hasan F, Mandal P, Banerjee U. Epidemiology of candidemia in a tertiary care centre of north India: 5-year study. Infection. 2007;35:256–9. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 10.Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: Pathogenicity and antifungal resistance. J Hosp Infect. 2002;50:243–60. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48:1366–77. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safdar A, Bannister TW, Safdar Z. The predictors of outcome in immunocompetent patients with hematogenous candidiasis. Int J Infect Dis. 2004;8:180–6. doi: 10.1016/j.ijid.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Ruan SY, Lee LN, Jerng JS, Yu CJ, Hsueh PR. Candida glabrata fungaemia in intensive care units. Clin Microbiol Infect. 2008;14:136–40. doi: 10.1111/j.1469-0691.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi HK, Jeong SJ, Lee HS, Chin BS, Choi SH, Han SH, et al. Blood stream infections by Candida glabrata and Candida krusei: A single-center experience. Korean J Intern Med. 2009;24:263–9. doi: 10.3904/kjim.2009.24.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan SY, Huang YT, Chu CC, Yu CJ, Hsueh PR. Candida glabrata fungaemia in a tertiary centre in Taiwan: Antifungal susceptibility and outcomes. Int J Antimicrob Agents. 2009;34:236–9. doi: 10.1016/j.ijantimicag.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, et al. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol. 2002;40:1298–302. doi: 10.1128/JCM.40.4.1298-1302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller MA, Messer SA, Hollis RJ, Jones RN, Diekema DJ. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob Agents Chemother. 2002;46:1723–7. doi: 10.1128/AAC.46.6.1723-1727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller MA, Diekema DJ. Role of sentinel surveillance of candidemia: Trends in species distribution and antifungal susceptibility. J Clin Microbiol. 2002;40:3551–7. doi: 10.1128/JCM.40.10.3551-3557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kauffman CA. Fungal infections in older adults. Clin Infect Dis. 2001;33:550–5. doi: 10.1086/322685. [DOI] [PubMed] [Google Scholar]
- 20.Lockhart SR, Joly S, Vargas K, Swails-Wenger J, Enger L, Soll DR. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res. 1999;78:857–68. doi: 10.1177/00220345990780040601. [DOI] [PubMed] [Google Scholar]
- 21.Akbar DH, Tahawi AT. Candidemia at a University Hospital: Epidemiology, risk factors and predictors of mortality. Ann Saudi Med. 2001;21:178–82. doi: 10.5144/0256-4947.2001.178. [DOI] [PubMed] [Google Scholar]
- 22.Ang BS, Telenti A, King B, Steckelberg JM, Wilson WR. Candidemia from a urinary tract source: Microbiological aspects and clinical significance. Clin Infect Dis. 1993;17:662–6. doi: 10.1093/clinids/17.4.662. [DOI] [PubMed] [Google Scholar]
- 23.Marriott DJ, Playford EG, Chen S, Slavin M, Nguyen Q, Ellis D, et al. Determinants of mortality in non-neutropenic ICU patients with candidaemia. Crit Care. 2009;13:R115. doi: 10.1186/cc7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedini A, Venturelli C, Mussini C, Guaraldi G, Codeluppi M, Borghi V, et al. Epidemiology of candidaemia and antifungal susceptibility patterns in an Italian tertiary-care hospital. Clin Microbiol Infect. 2006;12:75–80. doi: 10.1111/j.1469-0691.2005.01310.x. [DOI] [PubMed] [Google Scholar]
- 25.Aquino VR, Lunardi LW, Goldani LZ, Barth AL. Prevalence, susceptibility profile for fluconazole and risk factors for candidemia in a tertiary care hospital in southern Brazil. Braz J Infect Dis. 2005;9:411–8. doi: 10.1590/s1413-86702005000500009. [DOI] [PubMed] [Google Scholar]
- 26.Levin AS, Basso M, Gobara S, Gomes LB, Medeiros EA, Costa SF Author information Girão E1. Seven-year trend analysis of nosocomial candidemia and antifungal (fluconazole and caspofungin) use in Intensive Care Units at a Brazilian University Hospital. Med Mycol. 2008;46:581–8. doi: 10.1080/13693780802004996. [DOI] [PubMed] [Google Scholar]
- 27.Klevay MJ, Horn DL, Neofytos D, Pfaller MA, Diekema DJ, PATH Alliance. Initial treatment and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. 2009;64:152–7. doi: 10.1016/j.diagmicrobio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Blot S, Vandewoude K, Hoste E, Poelaert J, Colardyn F. Outcome in critically ill patients with candidal fungaemia: Candida albicans vs. Candida glabrata. J Hosp Infect. 2001;47:308–13. doi: 10.1053/jhin.2000.0918. [DOI] [PubMed] [Google Scholar]


