Abstract
Context:
Sepsis is a disease with high incidence and mortality. Among the interventions of the resuscitation bundle, the early goal-directed therapy (EGDT) is recommended.
Aims:
The aim was to evaluate outcomes in patients with severe sepsis and septic shock using EGDT in real life compared with patients who did not undergo it in the Intensive Care Unit (ICU) setting.
Settings and Design:
retrospective and observational cohort study at tertiary hospital.
Subjects and Methods:
All the patients admitted to ICU were screened for severe sepsis or septic shock and included in a registry and followed. The patients were allocated in two groups according to submission or not to EGDT.
Results:
A total of 268 adult patients with severe sepsis or septic shock were included. EGDT was employed in 97/268 patients. The general mortality was higher in no early goal-directed therapy (no-EGDT) then in EGDT groups (49.7% vs. 37.1% [P = 0.04] in hospital and 40.4% vs. 29.9% [P = 0.08] in the ICU, respectively. The general length of stay [LOS] in the no-EGDT and EGDT groups was 45.0 ± 59.8 vs. 29.1 ± 30.1 days [P = 0.002] in hospital and 17.4 ± 19.4 vs. 9.1 ± 9.8 days [P < 0.001] in the ICU, respectively).
Conclusions:
Our study shows reduced mortality and LOS in patients submitted to EGDT in the ICU setting. A simplified EGDT without central venous oxygen saturation is an important tool for sepsis management.
Keywords: Early goal-directed therapy, outcomes, protocol, sepsis
Introduction
Sepsis is a disease with high incidence, high costs and mortality.[1,2,3] Angus et al. estimated 751,000 cases in the U.S. population per year.[3] The average costs per case were US$22,100, with annual total costs of US$16.7 billion nationally.[3] The mortality from severe sepsis and septic shock ranged from 30% to 40%.[3,4,5]
There are worldwide initiatives aiming to reduce mortality associated with sepsis, such as the surviving sepsis campaign (SSC).[6,7,8,9] Among the interventions of the resuscitation bundle by SSC, the early goal-directed therapy (EGDT) is a cardiovascular support protocol. The EGDT was performed using specific criteria for the early identification of high-risk sepsis patients, verified definitions, and a consensus-derived protocol to reverse the hemodynamic perturbations of hypovolemia, vasoregulation, myocardial suppression, and increased metabolic demands.[10]
Since the publication of the original study of EGDT by Rivers et al.[11] and others studies[12,13,14,15,16,17,18,19,20,21,22,23,24] had been developed, generating a lot of discussion regarding the concepts underlying the early pathogenesis of sepsis, the conceptualization of the study, controversies over the treatment algorithm, the salutary effects of EGDT on morbidity and mortality, as well as the generalization and implementation of EGDT.[10] Rivers et al. conducted the EGDT in the emergency department and the time of 6 h of presentation of severe sepsis and septic shock.[11] Currently, EGDT has not yet been implemented fully in actual practice especially in the intensive care setting.[4,5,25]
Therefore, the present study evaluated outcomes in patients with severe sepsis and septic shock who underwent EGDT compared to patients who did not undergo it in the Intensive Care Unit (ICU) setting. We also evaluated the reasons for not employing EGDT and also determined the patients who would be the best candidates for its use according to the severity of their condition. We also conducted an analysis of the reasons for not starting, endpoints and interventions of the EGDT protocol.
Subjects and Methods
Design and setting
A retrospective observational cohort study carried out in the Hospital Mãe de Deus ICU from October 1, 2005 to June 30, 2009. The ICU has 32 beds, with approximately 1700 annual ICU admissions. The Ethics Committee of the Mãe de Deus Hospital approved the study (number 419/10) and waived the need for patients’ written informed consent.
Study population
All the patients admitted to ICU were screened by medical staff of the hospital for severe sepsis and septic shock. After admission in the ICU, the medical staff reviewed cases and confirmed the diagnosis severe sepsis or septic shock. All adults (aged 18 years or older), who met the classical severe sepsis or septic shock criteria[6,7,26] were included in a registry and followed.
Exclusion criteria were patients with do-not-resuscitate orders and patients with nonsepsis diagnosis. The patients were allocated in two groups according to submission or not to EGDT (EGDT and no-EGDT groups, respectively) previous judged by medical staff of the ICU. After initial evaluation of patients with severe sepsis and septic shock, the medical staff followed a preestablished protocol [Appendix 1] based on the SCC,[6,7] according to their individual judgment. All the patients included in the no-EGDT group, the interventions were based on the SSC and hemodynamic resuscitation guided by medical staff without protocol. When analyzing EGDT interventions, we divided patients according to survival at ICU or hospital discharge (survivors [SV] or nonsurvivors [NSV]).
Data collection
For all study patients, the following patient characteristics were recorded: age, sex, site of infection, serum lactate, type of admission (clinical or surgical), time prior to admission in ICU, length of stay (LOS) and mortality in ICU and hospital, and severity of illness using the Acute Physiologic and Chronic Health Evaluation II (APACHE II) score based on the worst values obtained in the first 24 h in the ICU. The reasons for not employing EGDT were observed.
The central venous pressure (CVP), mean arterial pressure (MAP) and central venous oxygen saturation (ScvO2) values were collected during the hemodynamic resuscitation of patients submitted to EGDT. ScvO2 values were recorded hourly by central venous gas analysis performed by laboratory or continuous monitoring (central venous catheter capable of continuous ScvO2 measurement [Edwards Lifesciences]) when available. Implementation and monitoring of EGDT.
After initial evaluation of patients with severe sepsis and septic shock, the medical staff followed a preestablished protocol [Appendix 1] based on the SSC,[6,7] according to their individual judgment. EGDT was applied only if the patient was within the first 6 h of diagnosis of severe sepsis and septic shock. The procedures started as soon as signs of tissue hypoperfusion began demonstrated clinically by arterial hypotension, oliguria, slow capillary bed filling and hyperlactatemia (lactate ≥ 4.0 mmol/L). The resuscitation started with 1000 ml crystalloids in 30 min and faster and larger volumes according to signs of tissue hypoperfusion. The quantities of volume had to be reduced if heart filling pressures increased without improving the hemodynamic status. If blood pressure did not respond to fluids, noradrenaline was initiated.
Hemodynamic resuscitation objectives were: (a) CVP between 8 and 12 mmHg; (b) MAP ≥ 65 mm Hg; (c) diuresis ≥ 0.5 ml/kg/h; (d) ScvO2 ≥ 70%. When this last item did not reach the values recommended, transfusion of red packed blood cells (RPC) had to be considered) if the hematocrit was < 30% l in the first 6 h and also the use of dobutamine infusion until a dose of 20 μg/kg/min.
The following sequence was adopted:
Samples were collected within the 1st h after diagnosis of severe sepsis and septic shock: hematocrit, hemoglobin, ScvO2, and lactate (if not previously collected) were collected by the ICU nursing staff.
Monitoring: the nursing staff of the ICU inserted a urinary catheter. Medical staff placed central venous catheterization, always avoiding delays in infusion. Immediately after installing the catheter, chest X-ray was performed. Installation of CVP was conducted immediately after confirmation of the catheter position. MAP monitoring was ideally carried out on patients using vasopressor.
The steps were recorded hourly during the first 6 h of hemodynamic resuscitation in the group submitted to EGDT: CVP, oximetry, blood glucose measurement, ScvO2, volume infusions (ml), dosis of noradrenaline and dobutamine, RPC units transfused and diuresis.
Statistical analyses
The results were reported as mean ± standard deviation, numbers and percentages. Student's t-test was applied when comparisons were made for parametric data. Nonparametric data were analyzed with the Mann–Whitney U-test. Categorical variables were analyzed using the Chi-square or Fisher's exact tests to find out whether there were differences among groups. All tests were two-tailed, and a P < 0.05 was predetermined for statistical significance. Analyses were done using the SPSS 17.0 software package (SPSS, Chicago, IL).
Results
The study population is summarized in Figure 1. Two patients were excluded with differential diagnosis of sepsis (pulmonary embolism and cardiogenic shock). The demographics of the 268 patients included in the study are summarized in Table 1. EGDT was employed in 97/268 patients (36.2% of the sample). The main causes for not submitting patients to EGDT (no-EGDT group) were ICU admission 6 h after the diagnosis of severe sepsis or septic shock (31%), congestive cardiac failure (6.3%), oliguric acute renal failure (3.0%), previous volemic therapy (1.9%), decision by medical staff (1.9%).
Figure 1.
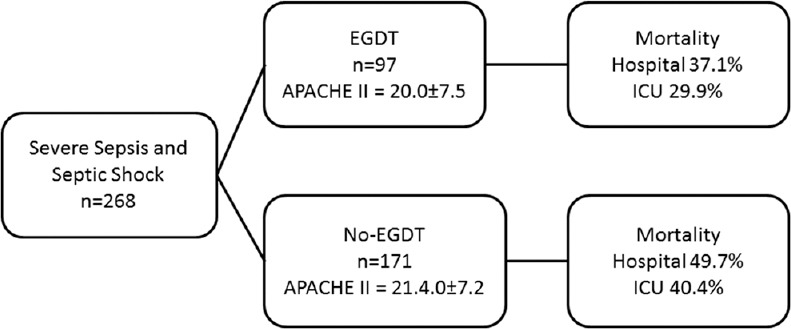
Study population: EGDT: Early goal-directed therapy group; no-EGDT: No Early goal-directed therapy group; APACHE II: Acute Physiologic and Chronic Health Evaluation II; ICU: Intensive Care Unit; This figure shows the patients included in the study with EGDT and no-EGDT groups. Both groups have similar APACHE II scores and the EGDT group has lower mortality
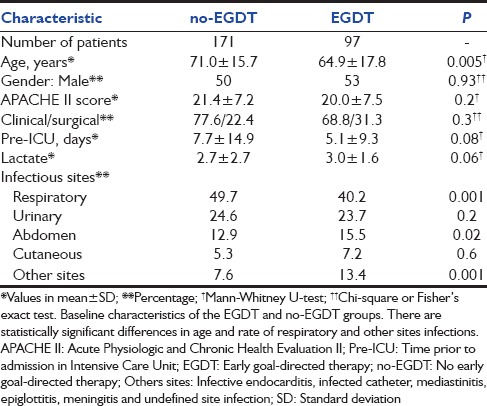
Table 1.
Baseline characteristics
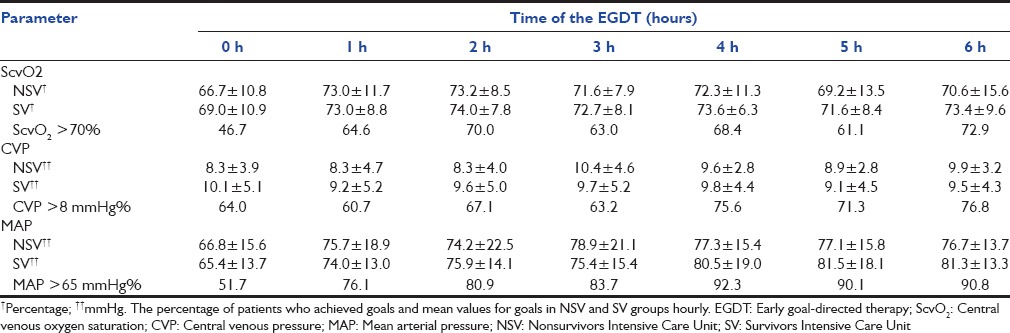
For the group submitted to EGDT, mean CVP, MAP and ScvO2 and rates of goals achieved during the 6 h of EGDT are shown in Table 2. The means and rates were calculated separately for SV and NSV in the ICU. There were no significant differences in achieving EGDT goals in SV and NSV subgroups.
Table 2.
Parameters during the EGDT
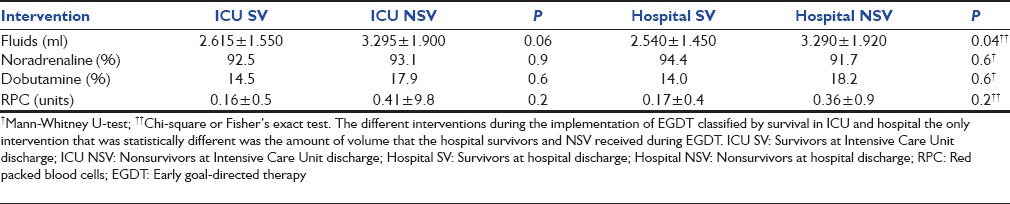
The use of fluids, noradrenaline, dobutamine and the RPC units administered in the group submitted to EGDT is shown in Table 3, according to ICU and hospital survival. The hospital NSV group received more fluid than the SV during EGDT (3.290 ± 1.920 vs. 2.540 ± 1.450 ml [P = 0.04]).
Table 3.
Interventions during EGDT
The general mortality in no-EGDT and EGDT groups was 49.7 vs. 37.1% (P = 0.04) in hospital and 40.4 vs. 29.9% (P = 0.08) in the ICU, respectively. In the subgroup with an APACHE II score lower than 20, mortality in the no-EGDT compared with EGDT was 42.5 vs. 23.5% (P = 0.02) in hospital and 32.5 vs. 17.6% (P = 0.06) in the ICU, respectively [Figure 2].
Figure 2.
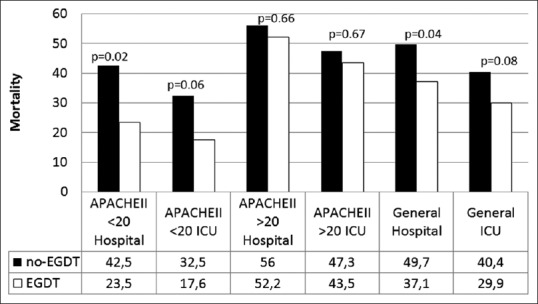
Mortality of the no-EGDT and EGDT groups; Dark = no-EGDT group (no-EGDT); White = EGDT group; EGDT: Early goal-directed therapy; APACHE II: Acute Physiologic and Chronic Health Evaluation II; ICU: Intensive Care Unit; Mortality (%); Chi-square or Fisher's exact test. This figure shows the overall mortality in EGDT and no-EGDT groups. Subgroups were divided according to severity by APACHE II score
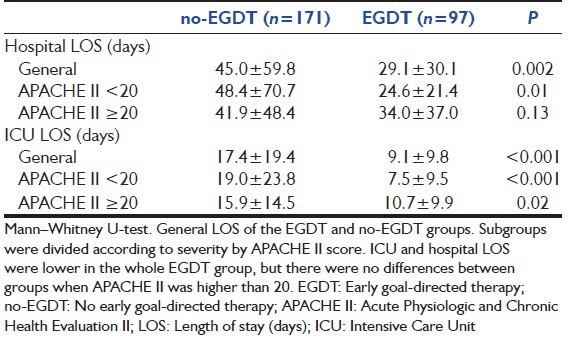
The general LOS in the no-EGDT and EGDT groups was 45.0 ± 59.8 vs. 29.1 ± 30.1 days (P = 0.002) in hospital and 17.4 ± 19.4 vs. 9.1 ± 9.8 days (P < 0.001) in the ICU, respectively. In the subgroup with an APACHE II score lower than 20, the LOS in the no-EGDT and EGDT groups was 48.4 ± 70.7 vs. 24.6 ± 21.4 days (P = 0.01) in hospital and 19.0 ± 23.8 days vs. and 7.5 ± 9.5 days (P < 0.001), respectively [Table 4].
Table 4.
LOS of the EGDT and no-EGDT groups
Discussion
Our study shows the use of EGDT in the ICU setting, without aiming at the implementation or evaluation of the tool. In our study, the use of EGDT reduced mortality and LOS mainly in less severely ill patients as based on the APACHE II score.
Our study was conducted in single open ICU and was a nonrandomized study. The no-EGDT group of patients was older, with more respiratory infections and with a longer time of hospitalization prior to admission in ICU (although not statistically significant). The apparently worse condition of this group may have biased the medical decision to submit or not the patient to EGDT. Otherwise, there were no differences in the APACHE II score and the first value of lactate between both groups, suggesting that they were similarly critical.
We used the APACHE II score to evaluate whether different subgroups would benefit from EGDT before severe organ dysfunction was established. The mean APACHE II score in our group was 20.8. To choose a cutoff value, we reviewed the mean APACHE II scores of other studies using EGDT and who used this score (Rivers et al. was 20.9, Shapiro et al. was 22.6, Gao et al. was 19.5, Kortgen et al. was 33, Trzeciak et al. was 23.8, Micek et al. was 22.5 and Nguyen et al. was 29.8, the mean of all studies was 24.5).[11,12,13,15,16,17,18] and we also evaluated the study that assessed the use of drotrecogin alfa (activated) in patients with severe sepsis at low risk of death, using an APACHE II value of 25 for definition.[27] The objective of this study was to treat patients with established severe organ dysfunction. However, as we wanted to evaluate the use of EGDT before severe organ dysfunction was established the value had to be lower than in this study, and so we arbitrarily defined the value as 20.
Rivers et al. published the first study showing 16% absolute reduction in mortality with the use of EGDT compared with standard care in patients with severe sepsis and septic shock.[11] This study of EGDT was performed in the pre-ICU or ED phase of the disease, within 6 h of the patient's admission. However, the ProCESS and ARISE studies conducted too in the ED showed no differences in outcomes with the use of EGDT protocol-based or protocol-based standard therapy when compared to usual care.[28,29] Our results show the benefits of EGDT in the ICU, even in patients that had not just arrived in hospital with severe sepsis or septic shock, but in patients that may have developed severe sepsis or septic shock in hospital. Others have found results suggesting improvements in outcomes with EGDT compared with historical controls.[12,13,14,15,16,17,18,19,20,21,22,23,24] Shapiro et al. found a 20.3% reduced mortality in the EGDT group compared with 29.4% in historical controls.[13] Sebat et al. showed that the septic subgroup appeared to benefit from the Shock Program. The mortality rate was reduced to 32.6% compared with the septic shock control group whose mortality rate was 46%.[14]
In our study, a reduction of LOS occurred in the EGDT group compared with the traditional group (hospital LOS 29.1 ± 30.1 vs. 45.0 ± 59.8 [P = 0.002] and ICU LOS was 9.1 ± 9.8 vs. 17.4 ± 19.4 [P < 0.001]). These differences persisted in only in the subgroup of patients with APACHE II lower than 20, suggesting that this subgroup benefits more from EGDT. In the Rivers study, the mean LOS in the hospital was similar in both groups. Jones et al. found that the hospital LOS was 1.2 days longer in the EGDT group, whereas the mean ICU LOS was 1.8 days longer in the EGDT group.[19]
Since the publication of the Rivers study, numerous questions have been raised regarding specific components of treatment. In our study, no significant differences were seen between SV and NSV in the end points, suggesting that early treatment may have a greater influence than each goal alone. Trzeciak et al. found that all EGDT end points were successfully achieved in 91% of the EGDT cases.[12] Rivers et al. showed differences in the goals with the use of EGDT,[11] van Beest et al. showed a low incidence of low ScvO2 in septic patients in Dutch ICUs. The mean ScvO2 was 74% compared to 67.8% in our study, 71% in the ProCESS study and 48.9% in the Rivers study.[11,29,30]
Fluids were more used during EGDT in those of our patients who had not survived to hospital discharge (2.540 ± 1.450 vs. 3.290 ± 1.920 L, P = 0.04). In contrast, in the Rivers study those who received more fluids had a better outcome.[11] The Rivers study has been considered by some to be a liberal fluid strategy as the EGDT group received significantly more volume therapy and packed RBCs in the first 6 h of treatment. Other studies show that fluid resuscitation in septic shock with positive fluid balance and elevated CVP may be harmful.[31,32] Our study shows a similar use of dobutamine, noradrenaline and RPC between SV and NSV in the EGDT group.
Our study was conducted in the ICU setting and many patients are not selected to undergo EGDT. The main reason for not performing EGDT was the delay of ICU admission (56%). This may have occurred because the medical staff in the ward and the ED was not trained to recognize patients with severe sepsis and septic shock and to perform the EGDT outside the ICU. Our study was conducted in the ICU setting with staff trained in sepsis diagnosis and management with a specific approach protocol. The presence of an expert team with experience in sepsis management may be considered mainly in the ED and general practice medical-surgical floors. To achieve a consistent level of quality, multiple models of sepsis management with EGDT should be implemented, such as a multidisciplinary sepsis response team and model that rapidly transfers the patient to the ICU from the various locations within the hospital.[33] The early identification of a septic patient with an insidious illness allows the early implementation of EGDT and its benefits. These patients were treated quickly and did not suffer microcirculatory failure and the onset of severe organ dysfunction. However, studies showed that the delayed introduction of EGDT was associated with improved outcomes.[34,35]
Despite the failure of some sepsis bundle interventions during the development of better evidence such as recombinant human activate protein C,[36] the EGDT is still important to guide the management of these patients. Moreover, the external validity of the recent studies is debatable to world reality who developing countries the culture of SSC certainly is not totally incorporate.[28,29] This corroborates that protocols may be useful, however, the individualization performed at the bedside by the professional is best approach the patients with sepsis. Improved recognition and management of sepsis outside the ICU is essential to reduce morbidity and mortality.
Conclusion
Our study shows reduced mortality and LOS in patients submitted to EGDT in an ICU setting. Besides customization, which is necessary to apply EGDT protocol, we think that simplified EGDT without ScvO2 is an important tool for sepsis management.
The crucial point is that to apply this intervention, we need early recognition and management of sepsis. The hospital should have policies that help train the staff in medical and surgical floors in recognition and management of patients with sepsis. It should also enable quicker transfer of patients from the floors to the ICU.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, et al. A prospective, observational registry of patients with severe sepsis: The Canadian Sepsis Treatment and Response Registry. Crit Care Med. 2009;37:81–8. doi: 10.1097/CCM.0b013e31819285f0. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222–31. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: A prospective cohort study. Lancet Infect Dis. 2012;12:919–24. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 8.Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otero RM, Nguyen HB, Huang DT, Gaieski DF, Goyal M, Gunnerson KJ, et al. Early goal-directed therapy in severe sepsis and septic shock revisited: Concepts, controversies, and contemporary findings. Chest. 2006;130:1579–95. doi: 10.1378/chest.130.5.1579. [DOI] [PubMed] [Google Scholar]
- 11.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 12.Trzeciak S, Dellinger RP, Abate NL, Cowan RM, Stauss M, Kilgannon JH, et al. Translating research to clinical practice: A 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129:225–32. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34:1025–32. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 14.Sebat F, Johnson D, Musthafa AA, Watnik M, Moore S, Henry K, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest. 2005;127:1729–43. doi: 10.1378/chest.127.5.1729. [DOI] [PubMed] [Google Scholar]
- 15.Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: A prospective observational study. Crit Care. 2005;9:R764–70. doi: 10.1186/cc3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105–12. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 17.Kortgen A, Niederprüm P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med. 2006;34:943–9. doi: 10.1097/01.CCM.0000206112.32673.D4. [DOI] [PubMed] [Google Scholar]
- 18.Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–13. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 19.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–32. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaieski D, McCoy J, Zeserson E, Chase M, Goyal M. Mortality benefit after implementation of early goal directed therapy protocol for the treatment of severe sepsis and septic shock. Ann Emerg Med. 2005;46:4. [Google Scholar]
- 21.Verceles A, Schwarcz RM, Birnbaum P, Mannam P, Patrick H. S.E.P.S.I.S: Sepsis education plus successful implementation and sustainability in the absence of a rapid response team. Chest. 2005;128:181S–2. [Abstract] [Google Scholar]
- 22.Armstrong R, Salfen SJ. Results of Implementing a Rapid Response Team Approach in Treatment of Shock in a Community Hospital. Presented at: 43rd Annual Meeting of the Infectious Diseases Society of America. 2005 Oct 6-9;:154. [Abstract] [Google Scholar]
- 23.Rogove H, Pyle K. Collaboration for instituting the surviving sepsis campaign in a community hospital. Crit Care Med. 2005;33:110S. [Abstract] [Google Scholar]
- 24.Stenstrom R, Hollohan K, Nebre R, MacRedmond R, Grafstein E, Dodek P, et al. Impact of a sepsis protocol for the management of patients with severe sepsis and septic shock in the emergency department. Can J Emerg Med. 2006;8:S16. [Abstract] [Google Scholar]
- 25.Ferrer R, Artigas A, Levy MM, Blanco J, González-Díaz G, Garnacho-Montero J, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 26.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 27.Laterre PF, Abraham E, Janes JM, Trzaskoma BL, Correll NL, Booth FV. ADDRESS (ADministration of DRotrecogin alfa [activated] in Early stage Severe Sepsis) long-term follow-up: One-year safety and efficacy evaluation. Crit Care Med. 2007;35:1457–63. doi: 10.1097/01.CCM.0000266588.95733.63. [DOI] [PubMed] [Google Scholar]
- 28.ProCESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ARISE Investigators, ANZICS Clinical Trials Group. Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 30.van Beest PA, Hofstra JJ, Schultz MJ, Boerma EC, Spronk PE, Kuiper MA. The incidence of low venous oxygen saturation on admission to the intensive care unit: A multi-center observational study in The Netherlands. Crit Care. 2008;12:R33. doi: 10.1186/cc6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 32.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: A retrospective pilot study. Chest. 2000;117:1749–54. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 33.Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: A multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. 2011;39:252–8. doi: 10.1097/CCM.0b013e3181ffde08. [DOI] [PubMed] [Google Scholar]
- 34.Coba V, Whitmill M, Mooney R, Horst HM, Brandt MM, Digiovine B, et al. Resuscitation bundle compliance in severe sepsis and septic shock: Improves survival, is better late than never. J Intensive Care Med. 2011;26:304–313. doi: 10.1177/0885066610392499. [DOI] [PubMed] [Google Scholar]
- 35.Castellanos-Ortega Á, Suberviola B, García-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodríguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36:542–7. doi: 10.1097/SHK.0b013e3182360f7c. [DOI] [PubMed] [Google Scholar]
- 36.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]


