Abstract
The processes that regulate T cell memory generation are important for therapeutic design and the immune response to disease. However, what allows a subset of effector T cells to survive the contraction period to become memory cells is incompletely understood. The Bcl-2 family is critical for T cell survival, and Bcl-2 has been proposed to be important for the survival of memory cells. However, previous studies have relied on double-knockout models, potentially skewing the role of Bcl-2, and the use of Bcl-2 as a marker in adoptive transfer experiments, a method required to confirm the memory potential of cell subsets, has not been possible due to the intracellular localization of the protein. In this study, we present a novel Bcl-2-reporter mouse model and show for the first time that a distinct subset of effector T cells, and also a subset within the CD127hiKLRG1lo memory precursor effector cell (MPEC) population, retains high Bcl-2 expression at the peak of the CD8+ T cell response to Listeria monocytogenes. Furthermore, we show that Bcl-2 correlates with memory potential in adoptive transfer experiments using both total responding CD8+ T cells and MPECs. These results show that even within the MPEC population, Bcl-2 confers a survival advantage in a subset of effector CD8+ T cells that allows differentiation into memory cells and cement Bcl-2 as a critical factor for T cell memory.
Keywords: T cell, memory, apoptosis
Introduction
Memory T cell induction is critical for efficient responses to disease and for therapeutic efforts such as vaccination. During an acute response, antigen-specific T cells undergo expansion and contraction phases that ultimately lead to the formation of a stable memory population. However, what allows the survival of the memory cells while the other ~90% of effector T cells die is still unclear. A number of factors, including signal strength, inflammation, and cytokines, have been proposed to contribute to the formation of T cell memory [Reviewed in (1)]. Similarly, a number of surface molecules have been proposed to have functional and/or identifying roles in the cells fated to become memory.
Expression of the IL-7Rα subunit (CD127) has been shown to mark effector cells fated to become memory cells, which is consistent with the evidence that IL-7 is important for memory cell survival (2, 3). However, some data have cast doubt on the true importance of CD127 and IL-7 signaling in memory formation because cells in an IL-7−/− environment exhibit relatively normal CD127 modulation and establishment of memory (4). Other studies showed that CD127 expression alone is not sufficient for the establishment of T cell memory, calling CD127 expression a “permissive” rather than an “instructive” feature (5, 6). CD127 has been used with another surface marker, killer cell lectin-like receptor G1 (KLRG1), to distinguish memory precursor effector cells (MPECs) from short-lived effector cells (SLECs) at the peak of the immune response (7). The factors that promote MPEC survival are still unclear, but MPECs have been shown to express higher levels of Bcl-2 (7, 8).
Because of the important roles of the anti-apoptotic proteins of the Bcl-2 family in T cell survival, these proteins are strong candidates for regulating T cell memory. Both Bcl-2 and Mcl-1 are expressed in memory T lymphocytes, whereas Bcl-xL is upregulated in effector cells (9–13). Using a conditional knockout model, Bcl-xL was shown to be dispensable for T cell memory (14). The roles of Mcl-1 and Bcl-2 in memory formation have been more difficult to assess using knockout models because of the importance of these proteins in thymocyte and naïve T cell survival (11, 12, 15–18). An examination of Bim+/−Bcl-2−/− cells (which partially escape Bcl-2−/−-induced death in the thymus and periphery due to the loss of one allele of Bim) showed that Bcl-2 was not required for memory to lymphocytic choriomeningitis virus (LCMV) infection (19), but the decreased Bim and/or the lymphopenic environment may have affected the requirement for Bcl-2 in this system. A follow-up study concluded that the Bim/Bcl-2 balance is indeed important for the survival of effector cells to become memory (8). While making great strides toward confirming a role for Bcl-2 in T cell memory, it is important to note that these studies largely relied on a system in which Bim expression was altered either through genetic manipulation or by pharmacologic agents, and previous studies have suggested that alterations in Bim, including loss of a single allele, can shift the balance between Bcl-2 and Mcl-1 in terms of their importance in T cell survival (20). Because of this interplay between many of the Bcl-2 family members, alternate approaches are needed to assess the in vivo role of Bcl-2 under endogenous gene expression conditions.
Although previous studies have implicated a role for Bcl-2 in T cell memory, it has been difficult to directly assess its importance due to the lack of appropriate conditional knockout models. Furthermore, because Bcl-2 is an intracellular protein, it cannot be used to define populations for the live-cell sorting and subsequent functional studies that are essential for determining the memory potential of effector T cells. In this study, we introduce a Bcl-2 reporter mouse that allows not only the analysis of Bcl-2 expression in effector T cells in the context of infection, but also the sorting of these cells to determine the memory potential of Bcl-2-expressing cells. Using this model, we identify effector CD8+ T cells that express relatively high levels of Bcl-2 both within the total responding population and, notably, within the MPEC subset. Furthermore, we show that Bcl-2 expression correlates with the establishment of memory to the bacterial pathogen Listeria monocytogenes in both the total effector CD8+ T cell pool and within the MPEC population. These studies confirm the important role of the prosurvival protein Bcl-2 in the formation of T cell memory and provide a useful model for future studies on Bcl-2 and T cell memory, including studies on vaccination and the response to disease.
Materials & Methods
Generation of Bcl-2YFP and OT1YFP mice
The modifications of the BAC were performed as described by Sparwasser et al. with minor modifications (21). The original shuttle vector, pLD53.RecA, was a kind gift from Dr. G. Eberl (Institut Pasteur, Paris, France), and the original insert was removed by Not I and Asc I digestion and replaced with the recombination cassettes. The cassettes were generated by overlap PCR using Pfu Ultra High-Fidelity Polymerase (Stratagene, La Jolla, CA). The annealing temperatures were usually 60°C.
The Bcl-2 YFP recombination cassette was designed to insert the cDNA sequence of a membrane-targeted version of YFP at the translational start of Bcl-2, deleting the first 72 nucleotides of exon 1 of Bcl-2. The two ~1-kb-long flanking fragments (box A and box B) were amplified from BAC RP23-405G16 with the primers 5′-TTG GCG CGC CGC CCT TCG GAG TTT AAT CAG-3′/5′-CAT CCT TCC CCG AAA AGA AGC TGC-3′ and 5′-GCT ACG AGT GGG ATG CTG GAG ATG-3′/5′-ATG CGG CCG CCA AGC TTA GCT ATG AAT TCC AGG-3′. The membrane-targeted version of YFP was amplified from the pLD53.RecA vector using the primers 5′-GCA GCT TCT TTT CGG GGA AGG ATG CTG TGC TGT ATG AGA AGA ACC-3′ and 5′-CAT CTC CAG CAT CCC ACT CGT AGC TTA CTT GTA CAG CTC-3′. The three PCR products were gel-purified and used as templates in an overlap PCR reaction to generate the Bcl-2 YFP recombination cassette, which was ligated into pLD53.RecA as described above to obtain the shuttle vector pBcl-2 YFP. The vector content was confirmed by sequencing.
BAC DNA was linearized with Nru I, and the band of the correct size was excised from the gel and electroeluted. The DNA was diluted to 2 ng/μl with microinjection buffer and was injected into the pronucleus of FVB/N fertilized eggs. The progeny was screened by PCR for the successful integration of the BAC by PCR. To detect YFP, the primers for screening were: 5′-CGT TTC GGA AAG CGC GTT GG-3′ and 5′-CGG TGG TGC AGA TGA ACT TC-3′. The primers for detection of the BAC ends were as follows: 5′ BAC end – 5′-GCT CTG GAG TGA ATA CCA CGA CGA-3′/5′-GGC ATG ATG AAC CTG AAT CGC CAG-3′ and 3′ BAC end – 5′-GGC CTA CCC ACT AGT CAA TTC GGG-3′/5′-GAA GGC TTC AGT CGC TCC TCC T-3′. The Bcl-2YFP mice were crossed onto the C57BL/6 background or the C57BL/6 variant expressing the congenic marker CD45.1 for at least 5 generations before analysis experiments and for at least 15 generations for transfer experiments. To generate OT1YFP mice, Bcl-2YFP mice were bred to OT1 mice, and the progeny were screened for both YFP and the OT1 TCR Vα2 by flow cytometry. C57BL/6, CD45.1, and OT1 mice were all obtained from Jackson Laboratories (Bar Harbor, ME, USA).
Flow cytometry
Organs (thymus and spleen) were removed and reduced to a single-cell suspension in FACS buffer (2% FBS in PBS). Fc receptors were blocked by incubating with 25–50% 24G2 hybridoma supernatant in FACS buffer for 10 min on ice. Surface molecules were stained by adding 0.25–1 μl fluorescently labeled antibody per 106 cells in a 100–200 μl volume of FACS buffer. Cells were stained on ice for at least 15 minutes then were washed and resuspended in FACS buffer with or without propidium iodide (PI; Sigma) at 2 μg/ml. Flow cytometry was performed on a FACScan or a FACSCanto cytometer (BD Biosciences, San Jose, CA), and the results were analyzed using FlowJo software (Tree Star, Ashland, OR). All antibodies to surface molecules (CD4, CD8, B220, Mac-1, Gr-1, CD25, CD44, CD127, KLRG1, Vα2) were from eBioscience or Biolegend (both San Diego, CA). Although YFP could be detected in both the FITC and the PE channel, unstained YFP+ cells were used to adjust the compensation between these channels such that the signal could be detected in only one channel.
For intracellular staining of Bcl-2 and Ki67, the single cell suspensions that had been stained for surface markers were washed with FACS buffer and resuspended in 100 μl FACS buffer. The cells were fixed with 100 μl 4% paraformaldehyde (EMD Biosciences, San Diego, CA) in PBS for 20 min at 4°C, permeabilized with 250 μl 0.1% saponin (Sigma) in FACS buffer for 20 min at 4°C, and incubated for 1 h in ice with an anti-Bcl-2-PE antibody (BD Biosciences) at a 1:5 dilution or an anti-Ki67-eFluor 450 antibody (eBioscience) at a 1:40 dilution in 100 μl 0.1% saponin in FACS buffer. The cells were washed with 0.1% saponin in FACS buffer and were resuspended in 250 μl 0.1% saponin in FACS buffer for data acquisition.
Infection of Bcl-2YFP mice with LM-OVA
For the initial analysis of Bcl-2 expression, Bcl-2YFP mice were infected directly. For the sorting experiments, 50,000–100,000 OT1YFP cells were transferred by intraperitoneal injection into congenically marked recipients one day before infection. Briefly, spleens from OT1YFP mice were removed and made into a single cell suspension. RBCs were lysed by incubation in ACK buffer for 1–2 min at room temperature. Cells were resuspended in 2% FBS in PBS for CD8+ T cell enrichment prior to sorting. CD8+ T cells were enriched using the EasySep CD8+ T Cell Enrichment Kit (Stem Cell Technologies, Vancouver, BC, Canada) following the manufacturer’s instructions. Cells were resuspended in PBS for injection (200 l/mouse).
Recombinant Listeria monocytogenes expressing chicken ovalbumin (LM-OVA) was a kind gift from Dr. M. Bevan (University of Washington, Seattle, WA). Frozen stocks of the LM-OVA were grown in brain-heart infusion broth (Difco) supplemented with 5 μg/ml erythromycin (Sigma). Bacterial culture samples were grown to mid-log phase as measured by OD600, aliquoted, and frozen at −80°C. Doses were confirmed by spreading bacterial samples on brain-heart infusion agar (Difco) plates. Immediately prior to infection, the bacteria were thawed, rinsed, and diluted in PBS for intravenous injection (100–200 μl/mouse). For the primary infections and the recall infections of recipient mice from transfer experiments, a dose of 104 CFU/mouse was used. For the recall infections in Bcl-2YFP mice, a dose of 106 CFU/mouse was used.
Analysis of effector CD8+ T cells
Seven or eight days after infection, spleens were removed and prepared to a single-cell suspension in 2% FBS in PBS as above. Cells were incubated with 24G2 hybridoma supernatant for 10 min on ice to block Fc receptors then with free mouse IgG1 (BD Biosciences) for 10 min. Then, a pre-prepared mixture of OVA peptide (American Peptide Company, Sunnyvale, CA) bound to DimerX (an H-2Kb-IgG1 fusion protein; BD Biosciences; bound to OVA overnight in PBS at 37°C) with secondary antibody (PE-anti-mouse IgG1; BD Biosciences) was added to the cells along with antibodies to surface molecules. Staining was allowed to proceed for 30–90 minutes, and cells were analyzed on a FACSCanto cytometer (BD Biosciences).
Sorting and adoptive transfer of OT1YFP CD8+ T cell populations
Day 7 or 8 splenocytes were prepared and enriched for CD8+ T cells as described above. Typically, 3–4 mice were pooled for each experiment. Enriched CD8+ cells were stained with antibodies to CD8, CD44, the congenic marker CD45.1/CD45.2, the OT1 TCR subunit Vα2, CD127, and KLRG1 (all from Biolegend) for at least 20 minutes. Cells were sorted on a FACSDiva sorter (BD Biosciences). After sorting, cells were washed once with PBS and resuspended in PBS for intravenous injection into the tail vein of naïve, congenically marked recipient mice. Approximately 10,000 cells from each population were transferred (see figure legends for the cell number transferred for each experiment). In some experiments, 5 × 106 unsorted, enriched CD8+ cells were transferred as a positive control.
Memory analysis of recipient mice
After transfer of OT1YFP cells, at least five weeks were allowed to pass before secondary challenge of the recipient mice. A secondary LM-OVA infection and T cell analysis were performed as described above with the exception that the analysis was performed on day 4 or 5. Splenic cellularity was determined by counting cells using a Countess cell counter (Invitrogen). For the flow cytometric analysis, 50,000 total events were collected to assess the percentage of CD8+ cells in the spleen (only live cells were included based on PI exclusion). Then, a high number of live CD8+ cells was collected to determine the frequency of the relatively rare donor cells. The total number of OT1YFP cells per spleen was calculated based on the observed frequencies and the total cell number. The percent recovery or fold expansion of the donor cells was calculated by dividing the calculated number of OT1YFP cells in the spleen by the number of cells transferred. For mice that received unsorted CD8+ cells, the number of OT1YFP cells transferred was determined based on the percentages observed during sorting and was used as the denominator to calculate recovery/expansion.
Short-term analysis of recipient mice
Splenocytes from 8 to 12 donor mice were pooled on day 7 of infection, and enriched CD8+ cells were sorted into populations V-VIII as described. A total of 25,000–50,000 cells (four- and seven-day experiments) or 100,000 cells (five-week experiments) were transferred by intravenous injection (200 μl in PBS). Because of the rarity of the sorted populations and the unavoidable loss of some volume of the cell preparation, only 1–4 mice could be included per group per experiment. The data shown for four- and seven-day experiments are the combination of three separate experiments. The number of donor cells recovered and the percent recovery were calculated as described above, and YFP fluorescence was detected by flow cytometry.
Statistical Analyses
The data were analyzed using Prism software v. 5 and 6 (GraphPad Software, La Jolla, CA). The significance of the data was assessed using an unpaired Student’s t test or, for comparisons across multiple groups, a one-way ANOVA with Tukey’s multiple comparison test. P values less than 0.05 were considered to indicate statistical significance. In expression level assays in which the different groups from within an individual mouse were compared, a paired analysis was used.
Results
Bcl-2YFP reporter mouse
To more closely examine the regulation and importance of Bcl-2 during an immune response, we generated a reporter mouse in which the gene encoding yellow fluorescent protein (YFP) was inserted into the translation initiation site of the Bcl-2 locus on a bacterial artificial chromosome (BAC) transgene (Bcl-2YFP mice; Fig. 1A). The BAC including the Bcl-2 gene spans approximately 250 kb due to the presence of a large intron between exons 2 and 3. To confirm that the complete Bcl-2YFP BAC was inserted (and thus any potential gene control regions located on the BAC), we used PCR to detect both the Bcl-2YFP fusion gene and both ends of the BAC in founder mice (Fig. 1B and data not shown). Using thymocytes, we confirmed that YFP expression directly parallels that of endogenous Bcl-2 (Fig. 1C). Furthermore, YFP expression mirrored known patterns of Bcl-2 expression in thymocyte populations (Fig. 1D) and in T and B cells and granulocytes in the spleen (Fig. 1E). For example, Bcl-2 is expressed in most of the CD4+ T cells, CD8+ T cells, and B cells, but not in the Mac1+Gr-1+ granulocytes.
Figure 1. Bcl-2YFP reporter mouse.

A. Schematic of BACs containing Bcl-2 and the YFP insert. B. PCR of BAC or founder mice tail DNA to detect the Bcl-2YFP allele and the 3′ end of the BAC. The founder with the highest level of Bcl-2 expression established the Bcl-2YFP line. C. Bcl-2 vs. YFP fluorescence in the thymus. D. YFP expression in thymocytes stained with CD4/CD8 to distinguish DN, DP, and SP populations and CD44/CD25 to separate DN subsets. YFP fluorescence in each gated population is shown. E. YFP expression in CD4+, CD8+, B220+ (B cells), and Mac-1+Gr-1+ (granulocytes) cells in the spleen. For D/E, open histograms represent splenocytes from mice that do not contain the YFP transgene (negative control), and shaded histograms represent Bcl-2YFP cells.
Analysis of Bcl-2 (YFP) expression in effector and memory CD8+ T cells responding to LM-OVA
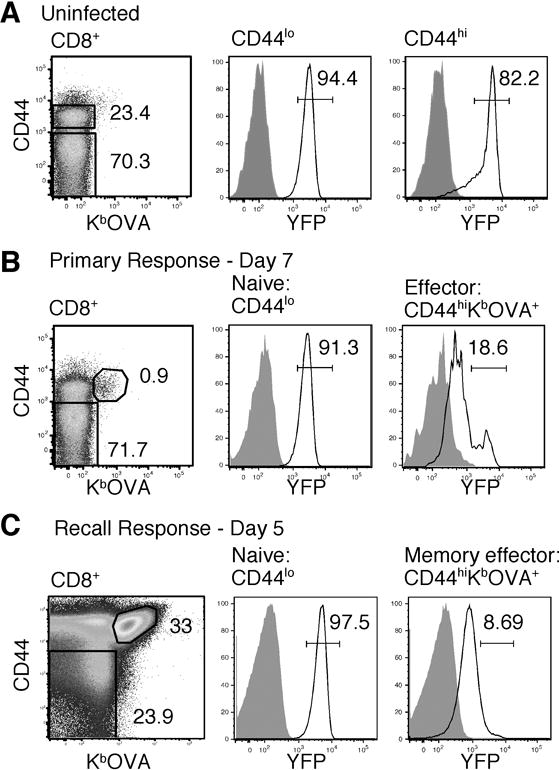
Using YFP as a marker, we confirmed that both naïve and memory CD8+ T cells expressed high levels of Bcl-2 (Fig. 2A). To examine the regulation of Bcl-2 during an immune response to infection, we utilized an infection model in which Bcl-2YFP mice were infected with a sublethal dose of Listeria monocytogenes expressing OVA (LM-OVA), which induces a dominant CD8+ T cell response to the peptide OVA257–264 presented by MHC class I H-2Kb. Consistent with previously reported patterns of Bcl-2 expression (9, 10), most activated (CD44hi) OVA-specific CD8+ T cells downregulated YFP compared to naïve cells seven days after infection (Fig. 2B). However, a small population (typically ~15–20%) of effector cells expressed high levels of Bcl-2 at this timepoint (Fig. 2B). A similar trend was observed in memory CD8+ T cells responding to a recall challenge (Fig. 2C). While previous studies examined gene expression on a population level, the YFP reporter system identified this previously unrecognized subpopulation of high Bcl-2-expressing effector cells, which we hypothesized were the cells that ultimately survive to establish CD8+ T cell memory.
Figure 2. Bcl-2 expression in naïve, effector, and memory CD8+ T cells in response to LM-OVA.

A. Expression of YFP in naïve (CD44lo) and memory (CD44hi) CD8+ T cells from uninfected mice. B. YFP expression in naïve (CD44lo) and effector (CD44hiKbOVA+) cells seven days after LM-OVA infection. C. YFP expression in naïve and memory effector cells five days after recall challenge. For all panels, the number represents the percentage within the parent population. For histograms, the gate marks “high” Bcl-2 expression. White histograms are YFP+ cells, and shaded histograms are YFP− cells.
Effector CD8+ T cells with higher Bcl-2 have greater memory potential
To determine whether there is a difference in the potential of Bcl-2-expressing populations to become memory cells, we sorted effector T cells based on Bcl-2 expression and transferred these cells to naïve animals. We avoided using Kb-OVA binding to identify OVA-specific cells because Kb-OVA binding to the TCR could have biological effects in responding T cells. Therefore, we crossed Bcl-2YFP mice onto a congenically marked (CD45.1/CD45.2) OT1 TCR-transgenic background (OT1YFP). To avoid complications from directly infecting the OT1YFP mice (due to the overwhelming number of antigen-specific cells and the lack of CD4+ cells), 50,000–100,000 OT1YFP CD8+ T cells were transferred to wild-type recipient mice one day before infection. This number of cells, while supraphysiologic, has been used in similar studies, and in our Bcl-2YFP system, the phenotype of the responding CD8+ T cells was similar to that observed in the endogenous responders when Bcl-2YFP mice were infected directly (data not shown).
Cells were first gated on CD8+CD44hiOT1YFP (OT1 identified as CD45.1+Vα2+) cells. Then, we gated four populations based on Bcl-2 expression (populations I–IV), each consisting of approximately 15–20% of the total and together representing the full range of Bcl-2 expression (Fig. 3A). Consistent with previous observations that the MPEC population expresses higher Bcl-2 than SLECs (7, 8), the Bcl-2hi population (I) was most enriched for MPECs, and the percentage of MPEC cells decreased with Bcl-2 levels with the exception of the Bcl-2lo cells (IV) (Fig. 3B). A post-sort analysis (not shown) indicated that population IV contained a higher percentage of contaminating host cells, which would appear in the MPEC gate due to the similarities of the MPEC markers with resting T cell markers, than the other three populations.
Figure 3. Memory potential of effector T cell populations sorted based on YFP (Bcl-2) expression.

A. Sorting of effector T cells. CD8-enriched splenocytes gated on effector (CD44hi) donor (CD45.1+Vα2+) cells were sorted into populations I-IV based on YFP expression. Numbers represent the percentage of the parent population within the gate. B. Percentage of MPECs in the sorted populations (numbers indicate percent of parent). C. Number of donor cells detected in secondary recipients after recall challenge. The number of donor (CD8+CD44hiCD45.1+Vα2+) cells was calculated by multiplying the frequency by the total number of splenocytes (only live cells included). The mean + standard deviation (SD) for each group is shown. Data are representative of two independent experiments (four mice/group each). Significance was assessed using an ANOVA (*p = 0.0143) with Tukey’s multiple comparisons test (*p<0.05).
After at least five weeks, mice transferred with populations I-IV were challenged with LM-OVA to assess the establishment of memory. A flow cytometry analysis revealed that as the Bcl-2 level of the donor cells decreased, so did the memory potential (Fig. 3C). Bcl-2hi cells (population I) consistently yielded the highest number of memory cells, while Bcl-2lo cells (population IV) did not yield a notable response (Fig. 3C). The effect of Bcl-2 on memory outcome was statistically significant as assessed by ANOVA (Fig. 3C, p = 0.0143). In one experiment (shown in Fig. 3C), there was a trend toward a graded decrease in memory potential with decreasing Bcl-2, and in a second experiment, we only detected donor cells in the mice that received population I (Bcl-2hi) (not shown). These results indicate that the highest Bcl-2 expressers have the capacity to efficiently become memory cells.
Memory potential correlates with Bcl-2 expression in MPECs
While the data above indicated that higher levels of Bcl-2 correspond to increased memory potential, it remained possible that the differences between populations I-IV in establishing memory were due to the differences in MPEC percentages between these populations, not Bcl-2 expression per se. Therefore, in a set of preliminary experiments, we separated effector OT1YFP cells into SLECs, which were uniformly Bcl-2lo, and Bcl-2hi and Bcl-2lo MPECs using different gating strategies (Fig. S1A). Five to ten million unsorted CD8+ T cells were transferred into separate mice as a positive control, and we could detect an expansion of the memory population (as measured by the calculated number of OT1YFP cells recovered/the number transferred) in the mice that received unsorted CD8+ T cells (Fig. S1B). Consistent with the results of studies in CD8+ T cells using LCMV as a pathogen (7), we did not detect an appreciable expansion of donor cells upon secondary challenge in mice transferred with the SLEC population (Fig. S1B). Within the MPEC population, different gating strategies yielded different results. When we assigned the Bcl-2hi and Bcl-2lo gates based on what appeared to be a distinct break in YFP expression (top 15% vs. bottom 69%), the Bcl-2lo population was better at conferring memory (Fig. S1B, left). However, when we defined these populations to include the highest tertile and the lowest tertile, the high population appeared to be better (Fig. S1B, right). An intermediate gating strategy yielded intermediate results (Fig. S1B, center). These results suggest that Bcl-2int-hi MPECs, specifically those representing the top 20–50% of MPECs, may have the best memory potential.
To further define the relation between Bcl-2 expression levels and memory potential within the MPEC population, we divided MPECs into equal-sized populations based on Bcl-2 expression and compared the memory potential between high and low expressors (Fig. 4A). A post-sort analysis confirmed that all of the sorted MPEC populations were >96% pure for OT1YFP cells and demonstrated the difference in the YFP mean fluorescence intensity (MFI) between the populations (Fig. S2). Interestingly, the CD127 MFI increased along with YFP expression, but the dynamic range of CD127 was much smaller than that of YFP expression (Fig. S2). Whether this is because of a difference in the actual range of expression levels or rather in the limitations of the detection of the signal is not clear.
Figure 4. Memory potential of Bcl-2 “high” and “low” MPEC populations.
A. Sorting of OT1YFP cells on day 7 of LM-OVA infection. Following OT1YFP pretransfer and LM-OVA infection, CD8+-enriched splenocytes were gated as shown (CD8+CD45.1+CD44hiVα2+). MPECs (CD127hiKLRG1−) were split into populations V-VIII based on Bcl-2-YFP expression. The numbers indicate the percentage of the parent population within the gate. B. Ki67 expression in YFP-sorted MPECs. OT1YFP cells were transferred, and mice were infected with LM-OVA as for sorting. On day 7 following infection, cells were stained for surface markers and Ki67. The percent Ki67+ cells (mean + SD) is shown for each group (as gated in A) within individual mice. Significance was calculated using a paired ANOVA (****p<0.0001) with Tukey’s multiple comparisons (NS p≥0.05; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). C. Analysis of mice that received Bcl-2 high and low populations (VI and VIII, respectively) five days after recall challenge. The number of OT1YFP cells per spleen was calculated, and the mean + SD of the mice within each group (four mice per group) is shown (**p<0.01 using Student’s t test). Data are representative of three independent experiments.
To better characterize our sorted populations, we examined Bcl-2 and Ki67 expression in the corresponding OT1YFP effector cells. As expected, the MFI of Bcl-2 protein staining correlated with the assigned group as defined by YFP expression, confirming that the downregulation of the reporter, YFP, accurately matched that of the protein Bcl-2 in effector cell subpopulations and did not represent an artifact of the reporter system (data not shown). To determine the relative proliferative capacity of the different populations, we stained the cells for Ki67, a marker of dividing cells, to obtain a “snapshot” of the level of proliferation ongoing in each population. Interestingly, the percent of cells that was Ki67+ was inversely correlated to YFP expression, indicating that the cells with the highest levels of Bcl-2 had the lowest level of proliferation at this timepoint (Fig. 4B). These data are consistent with numerous reports that Bcl-2 has a negative effect on cell cycle progression (22–25) and indicate that any advantage we observe for Bcl-2high cells in the memory response is likely due to survival, not enhanced proliferation.
Upon recall challenge and analysis of the mice that were transferred with these populations, we observed that MPECs with higher Bcl-2 had significantly greater memory potential than Bcl-2lo MPECs (Fig. 4C). Because our earlier results suggested that the highest Bcl-2 expressors of the MPEC population may be less fit to become memory, we selected Bcl-2int-hi (population VI) cells to represent Bcl-2 “high” cells for a comparison with Bcl-2lo cells (population VIII). Interestingly, the trend that the Bcl-2highest MPECs are somewhat less fit to become memory was observed in two out of three experiments in which we compared all four populations V-VIII (Fig. S3). Together, these results indicate that Bcl-2 can be used as a functional marker of memory potential, even within the MPEC population.
Engraftment and YFP expression of sorted cells immediately following transfer and before recall
To confirm that all sorted populations responded equally to the ex vivo procedures and that the generation of memory was not due to any differences in short-term survival following sorting/transfer, we transferred greater numbers of cells from populations V-VIII (as shown in Fig. 4), and assessed engraftment four and seven days as well as five weeks after transfer. Although the cell numbers were quite small, we could detect a small population of Vα2+ (OT1 TCR) CD45.2+ (or CD45.1+) cells in the spleen at all timepoints (Fig. 4A). The number of cells recovered and the percent recovery (number of cells normalized to number transferred) were indistinguishable between populations at days 4 and 7 (Fig. 5B, 5C). The number of cells remaining five weeks after transfer matched the trend observed in the memory response, although the differences were not statistically significant, likely due to the small number of cells detected (Fig. 5B, 5C, right). These data indicate that differences in long-term, but not short-term, survival of the different populations are responsible for the differences in memory responses. Strikingly, relative YFP fluorescence between the groups did not change over the week following injection (Fig. 5D), indicating that the populations as defined by Bcl-2 expression are stable over this time period. However, at the later timepoint, the YFP expression within each group was more variable and some groups were indistinguishable, indicating that those cells that did survive likely altered Bcl-2 expression to return some baseline over time.
Figure 5. Short-term analysis of transferred OT1YFP populations.

OT1YFP cells from 8–12 donor mice infected with LM-OVA were pooled on day 7 and sorted into populations V-VIII as shown in Fig. 4. The splenocytes of recipient mice were examined four and seven days as well as five weeks after transfer. A. Representative flow cytometry plots for the identification of transferred cells. B. Total number of recovered donor cells per spleen. The percentage of donor cells (CD8+CD44hiCD45.1+Vα2+) was multiplied by the total number of splenocytes, and the mean + SD is shown. For transfers of four and seven days, three experiments of n=1–2 mice/group were combined; for five weeks, a single experiment (n=4) is shown. The only significant differences observed between groups were with the mice that did not receive cells (“no cells”, ANOVA). B. Percent recovery of donor cells. Because the number of cells transferred slightly varied between experiments, the number of cells recovered was normalized to the number transferred, and the mean + SD is shown. C. YFP fluorescence. The YFP MFI of donor cells was determined for each recipient mouse. The mean + SD is shown.
Discussion
We have shown that the dynamic regulation of Bcl-2 during the effector phase of the immune response is important for establishing T cell memory. Using the OT1YFP LM-OVA infection system, we found that a subset of cells retains high Bcl-2 expression at the peak of the immune response, an observation that was not possible in previous studies using gene expression techniques, and that this population contains a high percentage of the CD127hiKLRG1− MPEC population. Using adoptive transfer, we confirmed the observations of other groups that the CD127hiKLRG1− population are indeed enriched for memory precursors. Our results indicate that high Bcl-2 expression can be used as an alternative marker to CD127/KLRG1 to mark effector CD8+ T cells with high memory potential. Although MPECs have been shown to express higher levels of Bcl-2 than SLECs, using the reporter mouse, we identified a gradient in Bcl-2 expression even within the MPEC population and showed that Bcl-2 can be used as a marker of memory potential within the MPEC population. The identification of the subset of MPECs with the highest memory potential is important because it will allow a more refined comparison of the phenotypic and functional characteristics of memory precursor cells in the future to identify subtle changes that promote memory development.
Intriguingly, based on the different results obtained with different gating strategies (Fig. S1, S2), it appears that the MPECs that express intermediate-high, but not the highest, levels of Bcl-2 may be the best at establishing memory. In two out of three experiments comparing populations V–VIII, it was not the MPEC-Bcl-2hi population (V), but the MPEC-Bcl-2med-hi population (VI), that yielded the highest recovery of memory cells, while MPEC-Bcl-2low cells were consistently poor memory cells (Fig. S2). This was consistent with the results of the preliminary experiment in which we tested different MPEC gates (Fig. S1) and with the pre-recall numbers of transferred cells (Fig. 5). While initially these results seem to contrast the results of the experiments in which effector T cells (not gated on MPECs) were transferred (Fig. 3), we must consider the fact that the higher Bcl-2 population (population I) in these experiments was more enriched for MPECs. The population I gate likely encompasses a relatively large percentage of the MPEC curve (corresponding to population V and population VI), and therefore, the gating strategy used for total effector cells does not distinguish between MPEC-Bcl-2hi and MPEC-Bcl-2med-hi cells.
The trend that MPEC-Bcl-2highest cells may be less fit is an interesting observation that should be addressed in future studies. It is likely that factors other than Bcl-2 are required for memory formation, and the highest Bcl-2-expressing cells may be less fit when other factors are considered. One potential explanation for this phenomenon is the differences in proliferation between the different populations. Consistent with a negative role for Bcl-2 in cell cycle progression, we showed that proliferation in MPECs was inversely correlated with Bcl-2 expression. Intuitively, it is rational that those cells programmed for long-term survival are less proliferative at the effector phase of the response. However, it is possible that the best cells strike a balance between survival and proliferation, expressing high Bcl-2, while allowing some proliferation. Our system can be used to identify other factors that may contribute to memory by comparing MPEC-Bcl-2hi and MPEC-Bcl-2med-hi cells.
Together, our data show that relatively high levels of Bcl-2 lead to memory in CD8+ T cells. The system presented here will provide a useful model to examine factors that influence T cell memory. As discussed above, comparing the populations defined here could identify cell-intrinsic factors important for memory. Furthermore, using this system, we can identify cell-extrinsic factors that are important for memory by altering the pre-transfer or recipient environment. This model not only allows an assessment of memory formation but also offers a readout of the effects of environmental changes specifically on Bcl-2, which we have proven to be an important modulator of T cell memory.
Supplementary Material
Acknowledgments
Support for flow cytometry and sorting was provided by the Duke Comprehensive Cancer Center’s shared flow cytometry resource. The authors express special thanks to Drs. Wei Jia and Jian Guo for their technical and logistical assistance.
This work was supported by NIH grant No. AI074754.
Abbreviations
- BAC
bacterial artificial chromosome
- LM-OVA
Listeria monocytogenes-ovalbumin
- KLRG1
killer cell lectin-like receptor G1
- MFI
mean fluorescence intensity
- MPEC
memory precursor effector cell
- SLEC
short-lived effector cell
- YFP
yellow fluorescent protein
References
- 1.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunologic research. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 3.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 4.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting Edge: IL-7-Independent Regulation of IL-7 Receptor {alpha} Expression and Memory CD8 T Cell Development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 7.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol. 2011;186:5729–5737. doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 10.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 11.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 12.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, He YW. The antiapoptotic protein Bcl-xL is dispensable for the development of effector and memory T lymphocytes. J Immunol. 2005;174:6967–6973. doi: 10.4049/jimmunol.174.11.6967. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci U S A. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC, Fields LE, Lucas PJ, Stewart V, Alt FW, et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 17.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki Y, Nakayama K, Nakayama K, Tomita T, Isoda M, Loh DY, Nakauchi H. Role of bcl-2 in the development of lymphoid cells from the hematopoietic stem cell. Blood. 1997;89:853–862. [PubMed] [Google Scholar]
- 19.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007 doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunkle A, Dzhagalov I, He YW. Cytokine-dependent and cytokine-independent roles for Mcl-1: genetic evidence for multiple mechanisms by which Mcl-1 promotes survival in primary T lymphocytes. Cell death & disease. 2011;2:e214. doi: 10.1038/cddis.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparwasser T, Gong S, Li JY, Eberl G. General method for the modification of different BAC types and the rapid generation of BAC transgenic mice. Genesis. 2004;38:39–50. doi: 10.1002/gene.10249. [DOI] [PubMed] [Google Scholar]
- 22.Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proceedings of the National Academy of Sciences. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazel S, Burtrum D, Petrie HT. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. The EMBO journal. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 25.Vairo G, Innes KM, Adams JM. Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


