Abstract
Importance
Advances in retinal imaging have led to the development of optical coherence tomography (OCT) instruments that incorporate spectral domain (SD) technology. Understanding measurement variability and relationships between retinal thickness measurements obtained on different machines is critical for proper use in clinical trials and clinical settings.
Objectives
Evaluate reproducibility of retinal thickness measurements from OCT images obtained by time domain (TD) (Zeiss Stratus) and SD (Zeiss Cirrus and Heidelberg Spectralis) instruments and formulate equations to convert retinal thickness measurements from SD-OCT to equivalent values on TD-OCT.
Design
Cross-sectional observational study. Each study eye underwent two replicate Stratus scans followed by two replicate Cirrus or Spectralis (real time image registration utilized) scans centered on the fovea.
Setting
Private and institutional practices
Participants
Diabetic persons with at least one eye with central-involved diabetic macular edema (DME), defined as Stratus central subfield thickness (CST)≥250μm. An additional normative cohort, individuals with diabetes but without DME, was enrolled.
Main Outcome Measure(s)
OCT CST and macular volume
Results
The Bland-Altman coefficient of repeatability for relative change in CST (the degree of change that could be expected from measurement variability) was lower on Spectralis compared with Stratus and Cirrus scans (7%, 12–15%, and 14%, respectively). For each cohort, the initial Stratus CST was within 10% of the replicate Stratus measurement 92% of the time; the conversion equations predicted a Stratus CST within 10% of the observed thickness 86% and 89% of the time for Stratus/Cirrus and Stratus/Spectralis groups, respectively. The Bland-Altman limits of agreement for relative change in CST between machines (the degree of change that could be expected from measurement variability, combined within and between instrument variability) were 21% for Cirrus and 19% for Spectralis, comparing predicted versus actual Stratus measurement.
Conclusions and Relevance
Reproducibility appears better on Spectralis than Cirrus and Stratus. Conversion equations to transform Cirrus or Spectralis measurements to Stratus-equivalent values, within 10% of the observed Stratus thickness values, appear feasible. CST changes beyond 10% when using the same machine or 20% when switching machines, after conversion to Stratus equivalents, are likely due to a change in retinal thickness and not measurement error.
Introduction
Advances in retinal imaging have led to the development of multiple optical coherence tomography (OCT) instruments that incorporate spectral domain (SD) technology, which addresses limitations that were imposed by Time Domain (TD) technology. High-speed A scan acquisition available with SD-OCT yields higher B scan resolution images, reduces motion artifact and provides time-efficient scan sampling density. SD-OCT instruments also provide registration of images obtained in the same eye from different encounters. This point-to-point direct comparison between scans, performed in real time or by post image acquisition warping, provides a more efficient means to reproducibly evaluate retinal change over time.
The management of diabetic macular edema (DME) frequently involves measurements from OCT devices. Stratus OCT was used widely in clinical practice and was the principal instrument employed in numerous studies conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) until 2011.1, 2 Advantages of using SD-OCT instruments have led to their rapid adoption and the Cirrus (Carl Zeiss Meditec, California) and the Spectralis (Heidelberg, Germany) devices have commonly replaced Stratus instruments in DRCR.net clinical centers.
The DRCR.net uses macular retinal thickness measurements to guide eligibility, to apply re-treatment criteria, and as an outcome measure of DME in clinical trials. DRCR.net clinics presently have a variety of OCT instruments, each determining retinal thickness based on different locations for the outer retinal boundary. Measured retinal thickness will vary between machines and is typically greater when images are obtained with SD-OCT as compared with TD-OCT.
An objective of this study is to compare macular thickness measurements between Stratus and Cirrus and between Stratus and Spectralis with the intent to develop and assess conversion equations that translate thickness measurements from these SD-OCT machines into a standardized and comparable TD-OCT Stratus value. Valid conversion equations would enable trials to pool data from these three instruments and compare results within and between protocols across groups that are using these instruments. An additional objective of this study is to compare the reproducibility of thickness measurements from Stratus, Cirrus, and Spectralis using their respective software segmentation analysis algorithms.
Methods
This DRCR.net observational study was conducted at 31 clinical sites. It was funded by the National Eye Institute (National Institutes of Health, U.S. Department of Health and Human Services). The protocol and Health Insurance Portability and Accountability Act compliant informed consent forms (or the ability to obtain verbal consent) were approved by institutional review boards. Each subject gave informed consent for study participation. The protocol is available at www.drcr.net.
Study Population
Eligible individuals were at least 18 years old with type 1 or type 2 diabetes, and media clarity adequate to obtain OCT images. Cohort 1 (‘primary cohort‘) enrolled both eyes of participants with DME in at least one eye (Stratus central subfield thickness [CST] 250 μm or greater). Cohort 2 (‘normative cohort’) enrolled participants with at least one eye meeting the normative criteria detailed in a separate publication (in which the objective was to collate a “normal” macular thickness database specific to the Spectralis instrument).3 Both eyes were enrolled if each eye met the criteria, otherwise a single eye was enrolled. The primary cohort enrollment goal was 75 eyes in each of four Stratus CST strata (<250 μm, 250–300 μm, 301–450 μm, and >450 μm), within each ‘SD/TD’ OCT group (the ‘Cirrus/Stratus’ group and the ‘Spectralis/Stratus’ group).
Study Procedures
For each study eye (following pupil dilation), a single certified operator was to obtain two replicate TD-OCT Stratus scans followed by two replicate Cirrus or Spectralis SD-OCT scans. Stratus scans consisted of the fast macular thickness scan (6 6.0 mm radial 128 A scans/B scan) analyzed with Stratus software version 4.0 or higher. Cirrus Macular Cube 512×128 protocol (6 mm × 6 mm) scans were analyzed with software version 3 or higher. Spectralis volume scans were acquired with 49 high speed B-scans (512 A scans/B scan) covering 20° × 20° roughly 6 mm × 6 mm) with Automatic Real Time mean of 16 and analyzed with software version 5.1 or higher. For images obtained in version 5.1, which limits analysis to the region within which the data points were acquired, the observed retinal volume value was converted to volume as determined by version 5.3a, which uses the entire 6mm ETDRS grid, using the formula footnoted in Table 1. The first Spectralis scan was set as a reference such that the second scan underwent real-time image registration.
Table 1.
Conversion Equation Data
| CST | Macular Volume | |||
|---|---|---|---|---|
|
| ||||
| Cirrus/Stratus (N=479) | Spectralis/Stratus (N=689) | Cirrus/Stratus (N= 420) | Spectralis/Stratus (N=632) | |
| Distribution of Differences | (Cirrus minus Stratus, in μm) | (Spectralis minus Stratus, in μm) | (Cirrus† minus Stratus, in mm3) | (Spectralis minus Stratus, in mm3) |
| Min | −61 | −56 | 0.1 | 0.8 |
| 5th percentile | 5 | 29 | 0.5 | 1.1 |
| 10th percentile | 14 | 41 | 0.7 | 1.2 |
| 25th percentile | 28 | 56 | 0.9 | 1.4 |
| Median | 43 | 67 | 1.1 | 1.6 |
| 75th percentile | 56 | 75 | 1.3 | 1.7 |
| 90th percentile | 65 | 82 | 1.4 | 1.8 |
| 95th percentile | 70 | 86 | 1.5 | 1.9 |
| Max | 117 | 162 | 1.8 | 2.6 |
|
| ||||
| Conversion Equation* |
(N= 240) Stratus = −43.12 + 1.01*Cirrus |
(N= 342) Stratus= −72.76 + 1.03* Spectralis |
(N=212) Stratus=−1.21 + 1.02*Cirrus† |
(N= 322) Stratus =−2.05 + 1.06* Spectralis |
All conversion equations were determined from a random half sample of participants, and using only measurement 1 values; from repeated measures models with generalized estimating equations to account for correlation between two study eyes.
Using transformed cirrus volume, calculated from the 9 subfields as [(((CST*(4/9) + InnerSup*(8/9) + InnerTemp*(8/9) + InnerInf*(8/9) + InnerNas*(8/9) + OuterSup*3 + OuterTemp*3 + OuterInf*3 + OuterNas*3)*3*3*3.14)/16)/1000].
CST = Central Subfield Thickness
Statistical Methods
Central subfield thickness and macular volume were the primary parameters used in both the conversion and reproducibility analyses. eTable1 summarizes which scans were evaluated by the reading center and which values were used for each analysis.
Reproducibility for each parameter was evaluated separately for each OCT machine, within each of the SD/TD groups, and separately for the normative cohort. The relationships of differences between the test-retest scans (scan1-scan2) of each machine were explored using Bland-Altman methods. Computations of the Bland-Altman coefficient of repeatability (CR) and the 95% confidence interval (CI)used the standard method 1.96*√2*√MSE, where MSE is the mean squared error from repeated measures regression models, with the dependent variable being the parameter measurement and the independent variables being the participant and eye nested within participant CR were computed on both the original outcome measurement scale as well as the relative difference scale.4 The reproducibility of each type of thickness measurement was compared between machines using linear mixed models that evaluated the relative absolute differences as the dependent variable and the machine as the independent variable, and accounted for the correlation within participants (between study eyes and machines).
Conversion equation analyses for CST and macular volume were evaluated within each of the SD/TD groups (inclusive of the normative cohort). The conversion equations were derived using data from a random sample of half the participants in the available SD/TD groups for each of the four equations; the second half of each sample was used to validate the formulas. Each of the four conversion equations was built using repeated measures models with generalized estimating equations to account for the correlation in participants with two study eyes, with the Stratus measurement as the dependent variable and SD measurement as the independent variable. Transformations, including log and log-log, were explored for each model; ultimately the Cirrus to Stratus volume equation was the only analysis in which a transformation was used to improve the model. The Cirrus machine calculates macular volume based on the entire 6mm by 6mm square area; for this analysis, Cirrus volume was recalculated (transformed) limiting it to the 6mm diameter circle using a weighted average of the 9 subfields, as footnoted in Table 1. Validity of each equation was evaluated by comparing predicted versus observed metrics computed from the second half of the data that was not used to build the model.
Applicability of conversion equations for making clinically relevant decisions on the individual patient level was evaluated via the Bland-Altman limits of agreement and the 95% CI on the relative differences between the observed and predicted automated Stratus values, using the validation half sample of scan 1 values, computed using linear mixed models.
All reported P values were 2-sided and unadjusted for multiple testing. In view of the number of analyses, only P values <.01 were considered to be unlikely due to chance. SAS version 9.3 (SAS Institute, Cary, NC) was used for all analyses.
Results
The Cirrus/Stratus group included 540 eyes from 292 participants and the Spectralis/Stratus group had 758 eyes from 400 participants (468 eyes [242 participants] from the primary cohort; 290 eyes [158 participants] normative cohort). Ninety-four eyes were excluded because the investigator identified an ocular pathology that could affect the OCT image. No participant was in both SD/TD groups.
The participant and ocular characteristics of individuals in each SD/TD group are in eTable2. The Stratus median (interquartile range) CST was 290 μm in the Cirrus/Stratus group and 239 μm in the Spectralis/Stratus group (including the normative cohort).
Replicate scans on both SD and TD machines were completed in 531 (98%) and 717 (95%) eyes in the Cirrus/Stratus and Spectralis/Stratus group, respectively. In each SD/TD group the same OCT operator obtained nearly all (96% to 98%) scan pairs for a given individual.
Reproducibility
Reproducibility of the two metrics was evaluated within each OCT instrument and within each group and cohort separately (CST Figure 1 and Table 2, and volume eFigure1 and eTable3). The CR and median relative absolute difference was smaller for Spectralis (CR=7%, RAD=1%) than for either Cirrus (CR=14%, RAD=1%) or Stratus (CR=12%/15%, RAD=2%/2% in the Cirrus/Stratus and Spectralis/Stratus groups, respectively) (P<0.0001). Although the CR was greater on Cirrus compared with Stratus, the relative absolute difference in Cirrus was less than that of Stratus (P=0.008). Figure 1 reflects this graphically, with a tighter cluster of CST differences around zero on Cirrus compared with Stratus, yet more observations falling far from the mean. Within all machines, the relative absolute difference shows less variation than the differences on the absolute scale. For macular volume, the reproducibility for the Spectralis was better than the Cirrus or Stratus.
Figure 1.
Bland-Altman plots of the differences versus the means of the automated central subfield thickness (CST) test-retest values, within each machine and each Spectral Domain/Time Domain group
Solid reference line indicates mean difference; dashed lines indicate the limits of agreement.
(A: Stratus results within Cirrus/Stratus group, B: Cirrus results within Cirrus/Stratus group, C: Stratus results within Spectralis/Stratus primary cohort, D: Spectralis results within Spectralis/Stratus primary cohort, E: Stratus results within Spectralis/Stratus normative cohort, F: Spectralis results within Spectralis/Stratus normative cohort)
Table 2.
Central Subfield Thickness Reproducibility Data
| Cohort | Comparison | Stratified by Stratus CST | Comparison of reproducibility among machines‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | <250 | 250–300 | 301–400 | 401–500 | >=500 | Within cohorts | Combining data from cohorts | |||
| Cirrus/Stratus | Stratus vs Stratus | N | 531 | 139 | 141 | 148 | 64 | 39 | Stratus RAD vs Cirrus RAD P=0.008 |
Stratus RAD vs Cirrus RAD P=0.0003 Stratus RAD vs Spectralis RAD P<0.0001 Cirrus RAD vs Spectralis RAD P<0.0001 |
| Median Absolute Difference | 7 | 5 | 7 | 9 | 12 | 11 | ||||
| Median RAD† | 2% | 2% | 3% | 3% | 3% | 2% | ||||
| CR* on micron scale (95% CI) | 40 (37, 42) | 31 | 25 | 44 | 50 | 68 | ||||
| CR* on relative scale (95% CI) | 12% (11%,13%) | 13% | 9% | 13% | 11% | 12% | ||||
| Cirrus vs Cirrus | N | 531 | 139 | 141 | 148 | 64 | 39 | |||
| Median Absolute Difference | 3 | 2 | 3 | 4 | 6 | 7 | ||||
| Median RAD† | 1% | 1% | 1% | 1% | 1% | 1% | ||||
| CR* on micron scale (95% CI) | 54 (50,57) | 33 | 23 | 56 | 77 | 108 | ||||
| CR* on relative scale (95% CI) | 14% (13%,15%) | 12% | 8% | 18% | 18% | 17% | ||||
| Spectralis/Stratus Primary Cohort | Stratus vs Stratus | N | 429 | 112 | 130 | 118 | 46 | 23 | Stratus RAD vs Spectralis RAD P<0.0001 |
|
| Median Absolute Difference | 7 | 5 | 6 | 9 | 11 | 10 | ||||
| Median RAD† | 2% | 3% | 2% | 3% | 3% | 2% | ||||
| CR* on micron scale (95% CI) | 47 (43,50) | 36 | 28 | 51 | 86 | 40 | ||||
| CR* on relative scale (95% CI) | 15% (14%,16%) | 17% | 10% | 15% | 20% | 6% | ||||
| Spectralis vs Spectralis | N | 429 | 112 | 130 | 118 | 46 | 23 | |||
| Median Absolute Difference | 2 | 2 | 2 | 2 | 2 | 7 | ||||
| Median RAD† | 1% | 1% | 1% | 1% | 0% | 1% | ||||
| CR* on micron scale (95% CI) | 24 (22,25) | 30 | 26 | 15 | 16 | 20 | ||||
| CR* on relative scale (95% CI) | 7% (7%,8%) | 10% | 7% | 4% | 3% | 3% | ||||
| Spectralis/Stratus Normative Cohort | Stratus vs Stratus | N | 288 | Stratus RAD vs Spectralis RAD P<0.0001 |
||||||
| Median Absolute Difference | 3 | |||||||||
| Median RAD† | 2% | |||||||||
| CR* on micron scale (95% CI) | 16 (15,18) | |||||||||
| CR* on relative scale (95% CI) | 8% (8%,9%) | |||||||||
| Spectralis vs Spectralis | N | 288 | ||||||||
| Median Absolute Difference | 1 | |||||||||
| Median RAD† | 0% | |||||||||
| CR* on micron scale (95% CI) | 17 (16,19) | |||||||||
| CR* on relative scale (95% CI) | 6% (6%,7%) | |||||||||
CR = Bland – Altman coefficient of repeatability (computed as 1.96*√2*√MSE, where MSE is the mean squared error from repeated measures regression models, with the dependent variable being the given measure and the independent variables participant and eye nested within participant).
RAD= relative absolute difference (computed as absolute value of measurement 1 minus measurement 2, divided by mean of measurement 1 and measurement 2).
Using automated scan values, taking the mean of measurement 1 and measurement 2.
Linear mixed models of the RAD versus the machine, accounting for correlation within participants (eyes and machines)
CST = Central Subfield Thickness, CI = Confidence Interval
Reproducibility of the measurements is in part operator dependent as evident in a secondary analysis restricted to the technicians that performed test-retest scans on at least 20 eyes within each SD/TD group. For the Cirrus/Stratus group, the individual technicians’ (N=7) CST CRs ranged from 8 μm to 95 μm in Cirrus and 25 μm to 54 μm in Stratus. For the Spectralis/Stratus group, primary cohort, the individual technicians’(N=4) CST CRs ranged from 8 μm to 22 μm in Spectralis and 28 μm to 48 μm in Stratus.
Conversion Equation
Table 1 summarizes which values were used for this analysis. In the Cirrus/Stratus group there were 479 and 420 eyes for the CST and volume analyses, respectively, whereas there were 689 and 632 eyes in the Spectralis/Stratus group CST and volume analyses respectively.
The median (5th and 95th percentile) difference in CST measurement between Cirrus and Stratus was +43 μm (5 μm, 70 μm) and +67 μm (29 μm, 86μm) between Spectralis and Stratus (Table 1). Likewise, the difference in the macular volume measurement between Cirrus and Stratus was +1.1mm3 (0.5 mm3, 1.5 mm3) and 1.6mm3 (1.1 mm3, 1.9 mm3) between Spectralis and Stratus (Table 1). Conversion equations to translate Cirrus and Spectralis CST or macular volume into Stratus “equivalents” (Table 1) were derived from half samples of the full datasets depicted in the Bland-Altman plots in Figure 2.
Figure 2.
Bland-Altman plots of the differences between values on machines (Spectral Domain minus Stratus) versus the means of the automated Stratus test-retest values, for each measurement and within each Spectral Domain/Time Domain group.
Solid reference line indicates mean difference; dashed lines indicate the limits of agreement.
(A: Central subfield thickness (CST) results within Cirrus/Stratus group, B: CST results within Spectralis/Stratus group, C: Retinal volume results within Cirrus/Stratus group, D: Retinal volume results within Spectralis/Stratus group.)
The models used to develop the conversion equations were validated by calculating the predicted Stratus thickness and volume for each machine from the observed SD CST and macular volume measurements obtained in the remaining half of the data set and comparing this predicted value to the actual observed Stratus value (eTable4). The predicted CST values fell within 10% of the observed Stratus measurement in 86% of the Cirrus/Stratuspairs and 89% of the Spectralis/Stratus pairs. When comparing the calculated group mean CST to the observed group mean the difference was 1 μm or less. When the database was used to categorize eyes by “normal” thickness, meaning a Stratus OCT CST of <250 μm, a discordance rate of <10% was identified when assigning eyes by the predicted as compared to observed values without any favored directionality to the discordance. By comparison, the same statistics computed using Stratus test versus retest values demonstrated similar or only slightly better agreement (eTable4). Validation for the volume equations yielded even greater agreement between the predicted and observed values for each of these analyses.
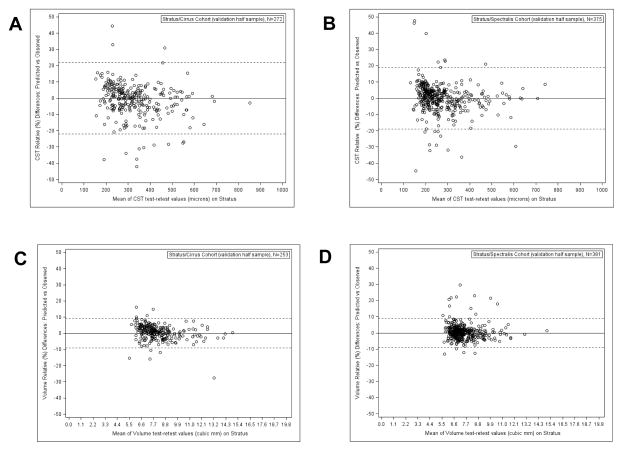
We applied the conversion equations to automated spectral domain values from the validation half sample dataset to calculate converted Stratus values; the relative differences between the observed and predicted values are depicted in Bland-Altman plots in Figure 3. Limits of agreement on the relative difference scale for Cirrus/Stratus and Spectralis/Stratus CST were 21% (95% CI [19%,22%]) and 19% (95% CI [17%,20%]), respectively; for volume the limits of agreement were 9% (95% CI [8%,10%]) and 9%(95% CI [9%,10%]), respectively.
Figure 3.
Bland-Altman plots of the relative (%) differences between the predicted Stratus value (based on the given Spectral Domain value and using the conversion equation) minus the corresponding observed Stratus value, for each measurement and within each Spectral Domain/Time Domain group.
Solid reference line indicates mean difference; dashed lines indicate the limits of agreement.
(A: Central subfield thickness (CST) results within Cirrus/Stratus group, B: CST results within Spectralis/Stratus group, C: Retinal volume results within Cirrus/Stratus group, D: Retinal volume results within Spectralis/Stratus group.)
Discussion
Clinical practice and clinical research rely heavily on OCT images to detect the presence and severity of diabetic macular edema. Technologic advancements have led to rapid adoption of spectral domain OCT instruments, which provide high image resolution, dense image sampling, and the ability to register images between visits. Clinical research studies engaged in analyzing OCT data have had to rapidly evaluate these instruments to establish normative databases, assess reproducibility in the measurements, and develop means of integrating data from a variety of instruments within a trial.
To determine whether changes in software-generated retinal thickness measurements are more likely to indicate real changes in thickness status, rather than change due to measurement variability (measurement error), it is essential to evaluate retinal thickness measurement reproducibility. The DRCR.net accepts up to 10% change in CST between visits, as measured by Stratus at each time point, as within measurement error, based on a previous DRCR.net study.5 In that study, following reading center review of all images (and adjustment of values when indicated), the CR for absolute and relative change in CST was 38 μm and 11%. The corresponding data for macular volume was 0.27mm3 or 3%. The present study confirms the Network’s previous observations while utilizing a much larger number of eyes with broad representation of macular thickness values, a different group of clinical centers and OCT operators, and analysis limited to the raw software generated metrics. The last design feature was meant to provide the CR that would be most applicable to clinical practice where measurements are not reviewed or corrected by a reading center.
Based on the reproducibility data generated with the Cirrus and Spectralis instruments in this study the DRCR. net requires at least a 10% change in CST between visits as the amount of change necessary to indicate a “real” change in macular thickness, when the measurements are made consistently with these respective machines. Other investigators have reported smaller values for coefficient of repeatability; however, these studies have involved a more limited number of participants some of whom have non-central DME or normal macular architecture, very few OCT technicians, and they frequently exclude scans with imperfect quality; (particularly eyes with segmentation errors) thereby limiting the variability introduced by the population, the spectrum of operators, and the machine itself.6–10 The present study also confirms an earlier observation made when evaluating measurement reproducibility with the Stratus instrument and extends this observation to Cirrus and Spectralis, namely that absolute value for measurement reproducibility varies according to the absolute thickness value being evaluated; whereas assessments made as relative change are more constant over a range of thickness values.
The test-retest differences in CST measurements were generally smaller for both SD instruments than for Stratus, and Spectralis was superior to Cirrus. Although Cirrus has a smaller median absolute and relative difference between scan 1 and 2 in comparison with Stratus, there were also more eyes with large discrepancies, leading to a coefficient of repeatability that was larger than Stratus. Although no difference in reproducibility was identified between Stratus and Cirrus for the volume measurement, Spectralis showed significantly less test-retest variation in this measurement than either Stratus or Cirrus. Although operator technique could be a source of variability in replicate measurements, this was minimized by having the same operator perform nearly all of the SD/TD scan pairs in the current study. These reproducibility findings, which favor the Spectralis instrument, are more likely the result of the real time image registration function that was only available and utilized with the Spectralis instrument. We did not test the post-hoc image registration feature available in the Cirrus instrument software; had we used this the measurement variability between the two replicate Cirrus scans may have been reduced. However, this would have required an investigator to review every Cirrus scan pair for post-hoc registration quality and perform manual registration when the software failed to accomplish the task. These methods were not utilized as they have not been adopted within DRCR.net studies and our goal was to report measurement variability replicating the methods used by the network in our trials.
As anticipated, Spectralis and Cirrus generate higher retinal thickness values relative to a TD-OCT machine, since the SD segmentation algorithms measure retinal thickness starting from more posterior structures, such as the retinal pigment epithelium apical surface or Bruch’s membrane as compared with TD algorithms that measure retinal thickness from the ellipsoid zone to the internal limiting membrane. The median difference between Stratus and Cirrus CST was 43 μm while the difference between Stratus and Spectralis was 67 μm. Given the inherent differences in how the instruments measure retinal thickness, it would be incorrect to simply pool the software generated raw data to describe populations that are measured with different instruments. One potential solution to this challenge would be customized software which automatically identifies a common outer retinal boundary in images from all machines, from which thickness values are calculated. Some reading centers have customized software for this purpose, but trials dependent on this solution must accept the time and costs associated with a reading center providing thickness measurements. It is also unlikely that this solution would be available in clinical practice to assist in clinical judgments when patients are scanned with different machines at different time points.
An alternate solution to this problem is development of conversion equations between TD and SD parameters. Other studies have created equations to convert Stratus CST values to either Cirrus or Spectralis, based oncohorts of either healthy subjects or eyes with age-related macular degeneration.11–13 In this study, conversion equations were derived to allow conversion of SD metrics to a common TD or ‘Stratus language’ for studies that permit a variety of OCT machines. This avoids analysis and reporting of trial data within different OCT machine subgroups. These equations were derived independently for Cirrus and Spectralis machines and they transform SD CST and macular volume values into Stratus “equivalent” measurements. Strengths of this analysis include the comprehensive approach taken to obtain the SD and TD measurements, the validation exercise performed on the predicted TD values, and the emphasis on a patient population with diabetes selected for the purposes of generalizing the results to future cohorts with diabetic retinopathy and DME. The findings of the validation exercise demonstrate relatively little difference between the Stratus values predicted based on SD observations and the actual observed Stratus values as compared to the differences observed between test and re-test values on the Stratus machine itself. The conversion equations for both CST and volume are satisfactory to transform values from Cirrus and Spectralis to equivalent Stratus values to report cross sectional population thickness and volume measurements on the same scale across all patients. The network is also utilizing these equations in circumstances in which participants are imaged with Stratus at baseline and switched to SD OCT imaging during follow-up. The SD OCT metrics are converted to Stratus equivalent values to allow change between visits to be evaluated on the same scale. Although a variety of OCT instruments are permitted for data collection, investigators are encouraged to consistently use the same type of OCT machine within individuals over the course of a study to minimize the need for conversion and the increase in measurement variability introduced by this need.
For the purpose of making clinical decisions at an individual patient level when measurements from one machine earlier in a patient’s course are compared to subsequent measurements obtained on an alternate machine, additional variability must be considered by the change in instrumentation, i.e., beyond the approximate 10% threshold for within-machine measurement error alone. A difference between two measurements (after conversion of the SD value to the Stratus scale) increases the threshold to about 20%. This is because the error is associated with both the within-machine measurement error and the variability incurred by changing instrumentation. Therefore, at the individual level, changes <10% within one machine or <20% between multiple machines on the Stratus scale might be “real” or they might be within the measurement error. This creates a potential challenge for clinical decision making. In these situations, monitoring change in values over more than two time points may provide greater certainty as to whether changes of smaller magnitude from one visit to another are real or if they represent measurement variability. Qualitative clinical observations of changes in macular anatomy also may help in the decision as to whether the observed changes in measured thickness are clinically important.
A limitation of this study was the absence of Cirrus and Spectralis OCT scans on study eyes from the same encounter. As such it was not possible to develop conversion equations to translate metrics directly between the Cirrus and Spectralis instruments. In the future, when Stratus becomes increasingly less common, a SD equivalent may become the standard reference value. Alternatively the manufacturers of OCT machines might be persuaded to offer a common outer retinal boundary line for software computations. The conversion equations developed provide a solution to combine data derived from a variety of commonly available OCT machines to report cross sectional observations and to evaluate longitudinal changes when individual patients are imaged initially with time domain OCT and then switched to specific spectral domain instruments within clinical trials.
Supplementary Material
Acknowledgments
Diabetic Retinopathy Clinical Research Network Clinical Sites that participated on this protocol:
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Study Investigator, (C) for Coordinator, (P) for Photographer.
Jacksonville, FL University of Florida College of Med., Department of Ophthalmology, Jacksonville Health Science Cent (125): Kakarla V. Chalam(I); Sandeep Grover(I); Shailesh K. Gupta(I); Vikram S. Brar(I); Tamil M Singh (C,P); Ravi Radhakrishnan(P) Boston, MA Joslin Diabetes Center (106): Jennifer K. Sun(I); Deborah K. Schlossman(I); George S. Sharuk(I); Lloyd Paul Aiello(I); Paul G. Arrigg(I); Paolo S. Silva(I); Sabera T. Shah(I); Timothy J. Murtha(I); Margaret E Stockman(C); Hanna Kwak (C,P); Julie A. Barenholtz (C,P); Leila Bestourous(V); Elizabeth S. Weimann(V); Robert W. Cavicchi(P) West Des Moines, IA Wolfe Eye Clinic (103): Jared S. Nielsen(I); David D. Saggau(I); Kyle J. Alliman(I); Marianne Parker(C); Susan Hallock(C); Jay Rostvold(P) Lakeland, FL Florida Retina Consultants (94): Scott M. Friedman(I); Oren Z. Plous(I); Karen Sjoblom (C,P); Jessica Maldonado (C,P) Austin, TX Retina Research Center (64): Brian B. Berger(I); Ginger J. Manhart(C); Kristen Davis(C); Ben Ostrander(P); Yong Ren(P)
New York, NY Mount Sinai School of Medicine, Dept. of Ophthalmology (43): Patricia J. Pahk(I); Pawan Bhatnagar(I); Robin Nina Ginsburg(I); Steven Allan Teich(I); Farzin Forooghian(I); Roje Oktay Kacmaz(C); Natalie Cheung(C); Anna G. Escuder(C); Barbara A. Galati(P); Eneil Simpson(P) Indianapolis, IN Raj K. Maturi, M.D., P.C. (38): Raj K. Maturi(I); Laura A. Bleau (C,P); Ashley Harless(P); Abby Maple(P); Jama L. Poston(P) Augusta, GA Southeast Retina Center, P.C. (32): Dennis M. Marcus(I); Harinderjit Singh(I); Graciela R. Zapata(C); Heather F. Anderson(C); Kimbi Y. Overton(P); Ken Ivey(P) Baltimore, MD Elman Retina Group, P.A. (31): Michael J. Elman(I); JoAnn Starr(C); Steven M. Disney(C); Theresa M. Butcher(C); Michelle D. Sloan(C); Daniel J. Ketner(P); Terri Cain(P); Peter Sotirakos(P) Milwaukee, WI Medical College of Wisconsin (31): Judy E. Kim(I); William J. Wirostko(I); David V. Weinberg(I); Kimberly E. Stepien(I); Dennis P. Han(I); Vesper V. Williams(C); Kathy J. Selchert(P); Joseph R. Beringer(P); Dennis B. Backes(P); Kristy L. Keller(P) Chicago, IL University of Illinois at Chicago Medical Center (26): Jennifer I. Lim(I); Michael Paul Blair(I); Lawrence Joseph Ulanski(I); Marcia Niec(C); Catherine Carroll(P); Mark Janowicz(P) Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (24): David Browning(I); Andrew N. Antoszyk(I); Ashley A McClain(C); Danielle R. Brooks(C); Angela K. Price(C); Donna McClain(P); Michael D. McOwen(P); Jennifer A. Ballard(P); Loraine M. Clark(P); Susannah J Held(P); Pearl A. Leotaud(P); Uma M. Balasubramaniam(P) Philadelphia, PA University of Pennsylvania Scheie Eye Institute (24): Alexander J. Brucker(I); Sheri Drossner(C); Joan C. DuPont(C); Cheryl Devine(P); Elizabeth A. Windsor(P); Jim M. Berger(P); Laurel Weeney(P); William Nyberg(P) Seattle, WA University of Washington Medical Center (22): James L. Kinyoun(I); Gurunadh Atmaram Vemulakonda(I); Susan A. Rath(C); Brad C. Clifton(P); James D. Leslie(P); Chuck Stephens(P) Portland, OR Retina Northwest, PC (21): Mark A. Peters(I); Stephen Hobbs (C,P); Christophe N. Mallet(P); Joe Logan(P) Lexington, KY Retina and Vitreous Associates of Kentucky (20): Thomas W. Stone(I); Rick D. Isernhagen(I); William J. Wood(I); John W. Kitchens(I); Diana M. Holcomb(C); Terri Kidd(P); Edward A Slade(P) Fort Myers, FL National Ophthalmic Research Institute (19): A. Thomas Ghuman(I); Glenn Wing(I); Paul A. Raskauskas(I); Joseph P. Walker(I); Ashish G. Sharma(I); Eileen Knips (C,P); Crystal Y. Peters(C); Etienne C. Schoeman(P) Baltimore, MD Wilmer Eye Institute at Johns Hopkins (16): Sharon D. Solomon(I); Adrienne Williams Scott(I); Neil M. Bressler(I); Susan Bressler(I); Diana V. Do(I); Mary Frey(C); Sandra West(C); Janis Graul(P); David Emmert(P) Rochester, NY University of Rochester (16): David Allen DiLoreto(I); Mina M. Chung(I); David M. Kleinman(I); George W. O’Gara(C); Peter Macdowell (C); Brandi Deats (P); Rachel Hollar(P) Chicago, IL Northwestern Medical Faculty Foundation (8): Alice T. Lyon(I); Manjot K. Gill(I); Rukhsana G. Mirza(I); Lori Kaminski(C); Lori E. Ackatz(C); Dawn M. Ryan(P); Evica Simjanoski(P) Dallas, TX Texas Retina Associates (8): Gary E. Fish(I); Robert C. Wang(I); Sally Arceneaux(C); Jean Arnwine(C); Kimberly Cummings(P); Michael Mackens(P); Nicholas Hesse(P) Houston, TX Retina Consultants of Houston, PA (8): Eric Chen(I); Matthew S. Benz(I); Tien P. Wong(I); Jennifer E. Hallett(C); Eric N. Kegley(P) Abilene, TX Retina Research Institute of Texas (7): Sunil S. Patel(I); Seong Young Lee(I); Cindy A. Petty(C); Gary L. Rickert(P); Brenda K. Arrington(P) Lawrenceville, NJ Delaware Valley Retina Associates (7): Darmakusuma Ie(I); Susan Geraghty (C,P); Beverly R. Sannazzaro (C,P) New York, NY The New York Eye and Ear Infirmary/Faculty Eye Practice (7): Ronald C. Gentile(I); Estuardo Alfonso Ponce(I); Anita Ou(C); Wanda Carrasquillo-Boyd(P); Robert Masini(P) Chicago, IL Rush University Medical Center (6): Mathew W. MacCumber(I); Elaine M Kernbauer(C); Pamela Hulvey(P) Ft. Lauderdale, FL Retina Group of Florida (6): Mandeep S. Dhalla(I); Jaclyn A. Brady(C); Patricia Aramayo(P); Rita R. Veksler(P) Paducah, KY Paducah Retinal Center (6): Carl W. Baker(I); Tracey M. Caldwell(C); Tana R. Williams(P); Dawn D. Darden(P) Artesia, CA Sall Research Medical Center (5): Joseph B. Michelson(I); Cindy Lee(C); Anabelle Garcia (C,P); Gabriela Suderno(V) Chapel Hill, NC University of North Carolina, Dept of Ophthalmology (5): Seema Garg(I); Odette M. Houghton(I); Cassandra J. Barnhart(C); Rona Lyn Esquejo(P); Debra Cantrell(P) Knoxville, TN Southeastern Retina Associates, P.C. (5): Joseph M. Googe(I); Charity D. Morris(C); David J. Cimino(P); Sarah M. Oelrich(P) Houston, TX Charles A. Garcia, P.A and Associates (4): Charles A. Garcia(I); Edgardo Santisbon(C); Sindya M. Cerda(P) Lubbock, TX Texas Retina Associates (3): Michel Shami(I); Yolanda Saldivar(C); Brenda K. Arrington (C,P) McAllen, TX Valley Retina Institute (3): Victor Hugo Gonzalez(I); Marcos Silva(C); Dina Garcia (C,P); Melody Cruz(C); Santos Garza(P) Aiea, HI Retina Consultants of Hawaii, Inc. (2): Gregg T. Kokame(I); Jacqueline Shen(C); Andrew Yuen(P) Syracuse, NY Retina-Vitreous Surgeons of Central New York, PC (2): G. Robert Hampton(I); Paul F. Torrisi(I); Cindy J. Grinnell(C); Lynn A. Roderick(P) Pittsburgh, PA Retina Vitreous Consultants (1): Karl R. Olsen(I); Pamela P. Rath(I); Heather Carmello(P); Tara Wilson (C)
Financial Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services; grants EY14231 and EY018817.
Footnotes
The members of the DRCR.net who participated in this protocol are listed in eTable 5
Ms. Allison R. Edwards had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Financial Conflicts of Interest: Glenn Jaffe: Heidelberg Engineering, Consultancy
DRCR.net Coordinating Center: Jaeb Center for Health Research, Tampa, FL (staff as of 6/18/13: Adam R. Glassman (Director and Principal Investigator), Roy W. Beck (Executive Director) Talat Almukhtar, Bambi J. Arnold-Bush, Brian B. Dale, Alyssa Baptista, Sharon R. Constantine, Simone S. Dupre, Allison R. Edwards, Meagan L. Huggins, Paula A. Johnson, Brenda L. Loggins, Shannon L. McClellan, Michele Melia, Haijing Qin, Allam Sanchez, Eureca Scott, Cynthia R. Stockdale, Karisse Torres.
Duke Reading Center: Durham, North Carolina: Beth Oakley, Brannon Balsley, John Choong, Cynthia A. Toth, MD, Cynthia Heydary, Garrett Thompson, Glenn J. Jaffe, MD, Katrina Winter, Kelly Shields, Russell Burns.
DRCR.net Operations Center: Johns Hopkins University School of Medicine, Baltimore, MD (staff as of 6/18/13): Neil M. Bressler (Past Network Chair and Principal Investigator), Connie Lawson, Peggy R. Orr, Beth Wellman.
DRCR.net Network Chair: Lee M. Jampol (2013-present)
DRCR.net Vice Chairs: Carl W. Baker (2011-present), Jennifer K. Sun (2012-present), John A. Wells (2013- present), Scott Friedman (2009–2012), Susan B. Bressler (2009–2011), Ingrid U. Scott (2009–2010).
National Eye Institute: Eleanor Schron (2009-current), Donald F. Everett (2003–2006, 2007–2009), Päivi H. Miskala (2006–2007)
Executive Committee: Lloyd Paul Aiello (2002-present; Chair 2002 – 2005), Carl W. Baker (2009-present), Roy W. Beck (2002-present), Neil M. Bressler (2006-present; Chair 2006 – 2008), Susan B. Bressler (2009-Present), Ronald P. Danis (2004-present), Matthew D. Davis (2002-present), Michael J. Elman (2006-present; Chair 2009 and 2012), Frederick L. Ferris III (2002-present), Scott Friedman (2007 –present), Adam R. Glassman (2005-present), Jeffrey G. Gross (2012-present), Glenn J. Jaffe (2012-present), Lee M. Jampol (2012-present; Chair 2013-present), Raj K. Maturi (2009–2011, 2013- Present), Brandi J. Perez (2013- current), Eleanor Schron (2009-present), Jennifer K. Sun (2009-present), John A. Wells (2012-present). Prior Members: Andrew N. Antoszyk (2009), Abdhish Bhavsar (2007–2008, 2010–2012), Alexander J. Brucker (2009–2011), Kakarla V. Chalam (2009–2011), Donald F. Everett (2002–2009), Joan Fish (2008 – 2009), Joseph Googe, Jr. (2009–2011), Diana M. Holcomb (2011–2012), Andreas Lauer (2007–2008), Dennis M. Marcus (2011–2012), Ingrid U. Scott (2009–2010), JoAnn Starr (2009–2011).
Financial Disclosures: A complete list of all DRCR.net financial disclosures is at www.drcr.net. Neil M. Bressler: Grants to investigators at The Johns Hopkins University are negotiated and administered by the institution (such as the School of Medicine) which receives the grants, typically through the Office of Research Administration. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor, but may receive salary or other support from the institution to support their effort on the projects(s). Dr. Neil Bressler is Principal Investigator of grants at The Johns Hopkins University sponsored by the following entities (not including the National Institutes of Health): Abbott Medical Optics, Allergan, Bausch & Lomb, Bristol-Meyer-Squibb, Carl Zeiss Meditec, EMMES Corporation, ForSight Labs, LLC Genentech, Genzyme Corporation, Lumenis, Notal Vision, Novartis, and Regeneron.
References
- 1.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–77. e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema following focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31(6):1009–27. doi: 10.1097/IAE.0b013e318217d739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalam KV, Bressler SB, Edwards AR, et al. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154–61. doi: 10.1167/iovs.12-10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114(8):1520–5. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49(10):4290–6. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comyn O, Heng LZ, Ikeji F, et al. Repeatability of Spectralis OCT measurements of macular thickness and volume in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53(12):7754–9. doi: 10.1167/iovs.12-10895. [DOI] [PubMed] [Google Scholar]
- 8.Domalpally A, Gangaputra S, Peng Q, Danis RP. Repeatability of retinal thickness measurements between spectral-domain and time-domain optical coherence tomography images in macular disease. Ophthalmic Surg Lasers Imaging. 2010;41 (Suppl):S34–41. doi: 10.3928/15428877-20100325-01. [DOI] [PubMed] [Google Scholar]
- 9.Leung CK, Cheung CY, Weinreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49(11):4893–7. doi: 10.1167/iovs.07-1326. [DOI] [PubMed] [Google Scholar]
- 10.Sohn EH, Chen JJ, Lee K, Niemeijer M, Sonka M, Abramoff MD. Reproducibility of diabetic macular edema estimates from SD-OCT is affected by the choice of image analysis algorithm. Invest Ophthalmol Vis Sci. 2013;54(6):4184–8. doi: 10.1167/iovs.12-10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abedi G, Patal P, Doros G, Subramanian ML. Transitioning from stratus OCT to cirrus OCT: a comparison and a proposed equation to convert central subfield macular thickness measurements in healthy subjects. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1353–7. doi: 10.1007/s00417-011-1725-6. [DOI] [PubMed] [Google Scholar]
- 12.Krebs I, Hagen S, Smretschnig E, Womastek I, Brannath W, Binder S. Conversion of Stratus optical coherence tomography (OCT) retinal thickness to Cirrus OCT values in age-related macular degeneration. Br J Ophthalmol. 2011;95(11):1552–4. doi: 10.1136/bjo.2010.194670. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MA, Sepah YJ, Symons RC, et al. Spectral- and time-domain optical coherence tomography measurements of macular thickness in normal eyes and in eyes with diabetic macular edema. Eye (Lond) 2012;26(3):454–62. doi: 10.1038/eye.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.