Abstract
HIV clade C (HIV-C) strains comprise ~56% of all HIV infections worldwide, and AIDS vaccines intended for global use must protect against this subtype. Our vaccine strategy has been to induce balanced antiviral immunity consisting of both neutralizing antibody and cell-mediated immune responses, an approach we tested in primates. As reported earlier, after isolating recently transmitted HIV-C strains from Zambian infants, we used env from one such virus, HIV1084i, to generate a multimeric gp160 immunogen. From another virus, isolated from a different child of the same mother-infant cohort, we cloned env to generate a recombinant simian-human immunodeficiency virus (SHIV), which was adapted to rhesus monkeys to yield SHIV-1157ip. Infant macaques were immunized with recombinant viral proteins, including multimeric HIV-C Env 1084i. To test whether cross-protection could be achieved, we mismatched HIV-C Env immunogens and challenge virus env. All vaccinated and control monkeys were exposed orally to low-dose SHIV-1157ip. Animals with no or only transient infection were rechallenged intrarectally with a high dose of R5 SHIV-1157ipd3N4, a "late”, animal-evolved variant of SHIV-1157ip. Compared to controls, the vaccinees had significantly lower peak viral RNA loads, and one vaccinee remained completely virus-free, even in lymphoid tissues. Data from our novel heterologous mucosal challenge model and our protein-only immunogens imply that significant protection against heterologous viruses circulating in the local community may be achievable with a strategy that seeks to simultaneously induce cellular immunity as well as neutralizing antibody responses.
Keywords: HIV clade C, vaccine, R5 SHIV, animal model, protein immunization, multimeric gp160
1. Introduction
The development of AIDS vaccines dependent solely upon the induction of cellular immunity has been called into question following the failed STEP human efficacy trial [1]. Those disappointing results supported the general notion that an effective AIDS vaccine will also need to elicit broadly neutralizing antibodies (nAbs). Moreover, the encouraging efficacy data from the recently completed Phase III vaccine trial in Thailand, whereby a combination of cellular immunity-inducing canarypox-based ALVAC vCP1521 plus antibody-inducing AIDSVAX B/E resulted in 31% protection, provides additional new data suggesting that an efficacious vaccine should generate a balanced immune response of both cellular and humoral antiviral immunity [http://news.bbc.co.uk/2/hi/health/8272113.stm].
The simian-human immunodeficiency virus (SHIV)/rhesus macaque model has provided proof-of principle that nAbs alone – given as passive immunization – can protect against immunodeficiency virus infection [reviewed in 2]. To test the efficacy of a vaccine designed to induce both cellular and humoral immunity, the virus challenge should approximate real-life conditions that mimic the most important aspects of HIV transmission among humans [3]. Some of these conditions would include repeated mucosal exposure with an R5 virus that is non-homologous to the strain of virus(es) used for vaccine construction. Until recently, a mucosally transmissible R5 SHIV clade C (SHIV-C) had been unavailable to test vaccine efficacy against HIV clade C (HIV-C). Here, we used two SHIV-C variants, SHIV-1157ip and SHIV-1157ipd3N4 [4], viruses that originated from SHIV-1157i, a chimera containing the SHIV-vpu+ backbone (expressing simian immunodeficiency virus (SIV) mac239 gag and nef [5]) and incorporating most of gp120 plus the entire extracellular and transmembrane domains of gp41 of HIV1157i, a recently transmitted virus isolated from a Zambian infant. SHIV-1157ip, the early biological isolate, was obtained after passage through 5 rhesus macaques during peak viremia, an adaptation strategy we devised to avoid selecting neutralization escape viruses. Three of the five monkeys developed AIDS [6], and a late virus, SHIV-1157ipd, was isolated from one of them. A molecular clone was derived using the 5’ half of SHIV-vpu+ and the 3' half of SHIV-1157ipd with an extra NF-κB binding site in the long terminal repeats. The resulting SHIV-1157ipd3N4 is highly replication competent and exclusively R5-tropic.
We developed these SHIV-C strains to evaluate candidate vaccines for use in infants against mucosal HIV-C transmission. Earlier, we showed strong containment of SHIV clade B (SHIV-B) replication in macaques immunized as infants solely with multimeric clade B gp160 [7–9]. In the studies described here, we incorporated multimeric clade C gp160 into a protein vaccine that also contained SIV Gag-Pol particles and HIV Tat. We deliberately mismatched SHIV-C env and the Env immunogen, which was derived from HIV1084i [10] isolated from a postnatally infected infant who was part of the same patient cohort at Lusaka Hospital as infant 1157i, the source of SHIV-C env. We reasoned that primate vaccine efficacy testing should approximate the real-life situation where human AIDS vaccine recipients will likely encounter HIV strains differing from those used to generate vaccines, although they may be exposed to strains circulating in the local community [3].
Materials and methods
2.1 Animals
Indian-origin rhesus monkeys (Macaca mulatta) were housed at the Yerkes National Primate Research Center (YNPRC), Atlanta, GA, a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All procedures were approved by the Animal Care and Use Committees of Emory University and the Dana-Farber Cancer Institute.
2.2 Immunogens
The pJW-gp160–1084i DNA plasmid encoding env derived from the pediatric isolate, HIV1084i [10], has been described [11]. Codon-optimized gene fragments of SIVmac239 gag-pro (1.8 kb) and SHIV89.6P tat (0.3 kb) were PCR amplified from multiple annealed overlapping oligonucleotide primers (100 bp each) [12]. Each fragment was cloned into the mammalian expression plasmid pJW4303 [13]. Animals receiving DNA were inoculated intradermally with 250 µg of each plasmid (total 750 µg DNA) at each time point; control animals (Groups 3 and 3a) received 750 µg of empty pJW4303 DNA plasmid vector. For protein inoculations, HIV-C gp160 was prepared from recombinant vaccinia virus infected BSC-40 cells as described previously [14]. SIV Gag-Pol particles were prepared as essentially as described [15–17] using an early-late synthetic promoter and HIV Tat was purchased from Advanced Bioscience Laboratories, Inc. (Kensington, MD). Proteins were given i.m. in incomplete Freund’s adjuvant at 100 µg of each per inoculation.
2.3 Humoral immune responses
ELISAs for anti-Env antibody binding were performed as described [13,18]. Antibody titers were calculated as reciprocal serum dilution giving O.D. readings >5 standard deviations above background as calculated using prebleed serum at the same dilutions. Neutralizing antibody titers were measured using a viral infectivity assay of TZM-bl cells (obtained through the AIDS Research and Reference Reagent Program (ARRRP), Division of AIDS, National Institute of Allergy and Infectious Disease, National Institute of Health, Bethesda MD) as described previously [13,18]. Due to the limitations in the amount of blood that could be collected from infant macaques, preimmune sera were not always available. As such, percent neutralization was calculated relative to luciferase activity level in negative control serum samples consisting of virus + pooled sera from 5 naive rhesus macaques to offset potential virus proliferation inhibitors/and or enhancers occasionally present in macaque serum. Neutralizing antibody titers were estimated as the reciprocal serum dilution giving 50% inhibition of virus replication.
2.4 Virus-specific cellular immunity
PBMC were isolated from whole blood by centrifugation in heparinized CPT tubes (BD Biosciences-Pharmigen) and washed 3× prior to assay. For antigen-specific T-cell stimulation, overlapping 20-mer SIVmac239 Gag and 15-mer HIV Tat peptides representing the complete protein sequences were obtained through ARRRP. Interferon (IFN)-γ ELISPOT analysis was done as described [18] using plates coated with purified mouse anti-human IFN-γ (BD Biosciences-Pharmigen, San Diego, CA). Cells were incubated overnight at 105 cells/well in assay medium plus pooled peptides of SIVmac239 Gag (pools #1, #2 and #3 consisting of peptides 1–42, 43–84 and 85–125, respectively) or HIV Tat. Assays were done in duplicate and background levels were determined by incubation of cells without peptides. Intracellular staining after peptide stimulation was performed essentially as described elsewhere [19].
2.5 Viruses and challenges
SHIV-1157ip and SHIV-1157ipd3N4 have been described [4]; the amino acid Env sequences of both viruses share 93.2% homology (Fig 1). The amino acid sequence of the HIV1084i gp160 used for immunization shares 78.8 and 78.4% homology with SHIV-1157ip Env and SHIV-1157ipd3N4 Env, respectively (Fig. 1). Amino acid homology was determined using the ‘SIM-Alignment Tool for protein sequences’ at http://expasy.org/tools/sim-prot.html utilizing the Blosum 62 Comparison matrix with a gap open penalty of 12 and a gap extension penalty of 4. Virus stocks of each were prepared from concanavalin A-stimulated naive rhesus PBMC; TNF-α (10 ng/ml) was included for generating the SHIV-1157ipd3N4 stock only.
Fig. 1. Amino acid sequence alignment of HIV1084i, SHIV-1157ip and SHIV-1157ipd3N4 Envs.
MSD: membrane spanning domain.
2.6 Viral load and statistical measurements
Plasma vRNA loads were assessed by quantitative RT-PCR for SIV gag sequences (QiaAmp RNA Blood Mini-Kit – Qiagen) [20] Peak viremia levels were compared by 2-sided Wilcoxon rank-sum test.
3. Results
3.1 Vaccination and pre-challenge immune responses
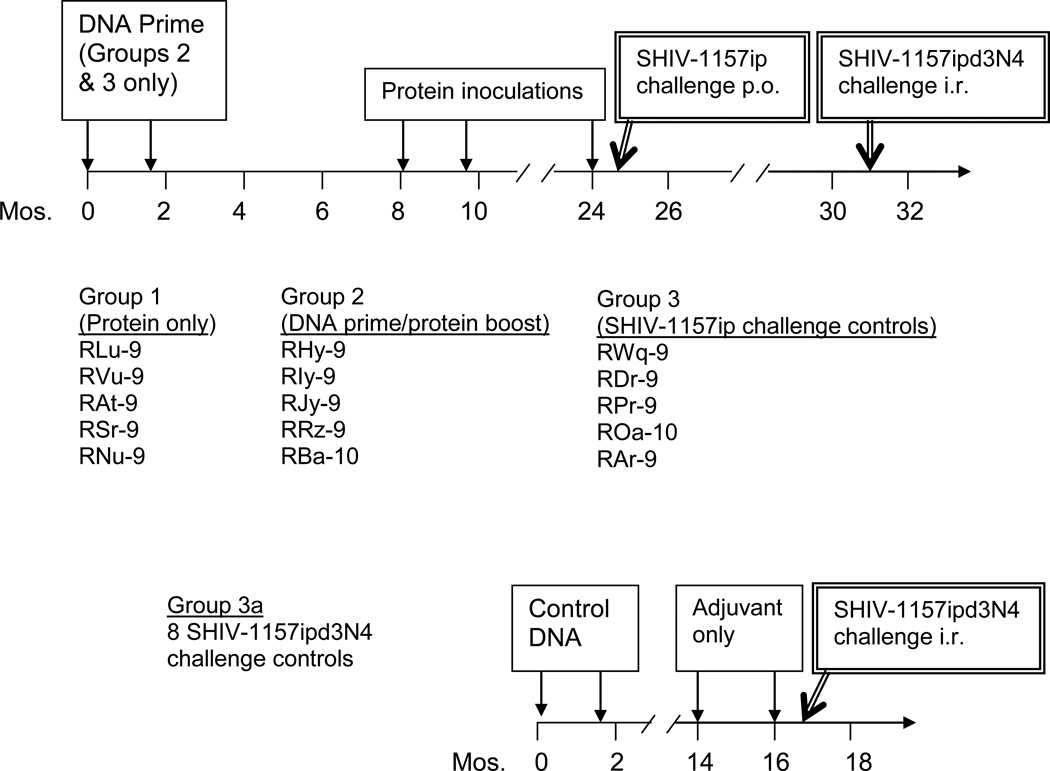
Five macaques were given the multigenic protein-only vaccine (Group 1, Fig. 2), Group 2 (5 animals) received a more conventional bimodal DNA prime/protein boost regimen, and Group 3 (controls) received empty vector DNA. DNA vaccination consisting of codon-optimized plasmids encoding SIV gag-protease, HIV tat and heterologous HIV1084i clade C env started on postnatal d 1–5 (Group 2, Fig. 2); another DNA immunization was given at week 6. Group 2 was rested and then given soluble protein boosts consisting of SIV Gag-Pol particles [15–17], HIV Tat and HIV1084i gp160. At this time, age-matched Group 1 animals were vaccinated with the viral proteins.
Fig. 2. Protein or DNA prime/protein boost vaccination and low-dose/high-dose virus challenge schema in infant macaques.
Animals of Groups 2 and 3 were inoculated with DNA starting between d1–5 of birth. Animals of Group 2 received DNA encoding SIV gag-pro, HIV tat and HIV1084i gp160 env, while Group 3 animals were given control DNA. Protein immunizations, consisting of SIV Gag-Pol particles, HIV Tat and multimeric HIV1084i gp160, were initiated at mo 8 and given to Group 2 animals and age-matched, naïve Group 1 animals. Two weeks after the 3rd protein inoculation of Group 1 and 2 animals, all monkeys were challenged orally with SHIV-1157ip (3.7 50% animal infectious doses (AID50)). Animals with no or only transient infection, as described in the text, were rechallenged 6 mo later with a high dose of SHIV-1157ipd3N4 (20 AID50, intrarectally).
Two weeks after the final protein inoculation, all groups were challenged orally with heterologous SHIV-1157ip. At this time, IFN-γ ELISPOT analysis of peripheral blood mononuclear cells (PBMC) (Table 1) showed strong SIV Gag-specific reactivity in three Group 1 animals (RLu-9, RAt-9 and RSr-9); RSr-9 also had strong HIV Tat-specific ELISPOT responses. Two animals from Group 2 (RHy-9 and RRz-9) demonstrated strong SIV Gag-specific and HIV Tat-specific ELISPOT responses. No specific reactivity was observed in any controls (Group 3). All 10 animals from Groups 1 and 2 had high-titer binding antibodies to homologous HIV1084i gp160, a relatively neutralization resistant virus (Table 1). Only one animal of Group 1 had measurable anti-HIV1084i nAb activity. Importantly, 9 of 10 vaccinees had significant nAb activity to the heterologous challenge virus, SHIV-1157ip. Controls (Group 3) showed no anti-Env-C antibodies. These data demonstrate that multimeric HIV-C gp160 (as part of a multigenic protein vaccine) or DNA priming/protein boosting induced cross-reactive nAb to SHIV-C in macaques.
Table 1.
Prechallenge immune responses in rhesus macaques.
| T cell reactivity1 | nAb titers2 | |||||
|---|---|---|---|---|---|---|
| SIV Gag | HIV Tat | HIV1084i gp160 Ab binding titers |
HIV1084i | SHIV-1157ip | ||
| Group 1 (protein only) | RLu-9 | 4,500 | 310 | 3.1 × 105 | 20 | 130 |
| RNu-9 | 370 | 70 | 3.1 × 104 | <10 | <10 | |
| RVu-9 | 20 | 180 | 6.2 × 105 | <10 | 15 | |
| RSr-9 | 4,400 | 1,560 | 6.2 × 105 | <10 | 160 | |
| RAt-9 | 3,790 | 40 | 6.2 × 104 | <10 | 130 | |
| Group 2 (DNA prime/protein boost) | RHy-9 | 7,320 | 3,600 | 3.1 × 105 | <10 | 50 |
| RIy-9 | 270 | 140 | 6.2 × 105 | <10 | 20 | |
| RJy-9 | 130 | 20 | 3.1 × 104 | <10 | 140 | |
| RRz-9 | 7,130 | 3,100 | 3.1 × 104 | <10 | 640 | |
| RBa-10 | 370 | 450 | 6.2 × 104 | <10 | 120 | |
| Group 3 (control) | RWq-9 | 0 | 0 | <103 | <10 | <10 |
| RAr-9 | 0 | 0 | <103 | <10 | <10 | |
| RDr-9 | 40 | 0 | <103 | <10 | <10 | |
| RPr-9 | 20 | 0 | <103 | <10 | <10 | |
| ROa-10 | 0 | 0 | <103 | <10 | <10 | |
PBMC and sera of individual animals were tested 2 weeks after the final protein inoculations (day of SHIV-1157ip challenge).
T-cell responses in PBMC to peptide pools of the indicated viral antigen were determined by IFN-γ ELISPOT analysis and given as SFU/106 PBMC. Responses to each of three SIV Gag peptide pools have been summed.
Serum neutralizing antibody titers to HIV1084i and SHIV-1157ip were measured using the TZM-bl-based reporter assay and show the reciprocal dilution of sera giving 50% neutralization. adapted from Rasmussen et al.[13]
3.2 Low-dose oral challenge with early SHIV-1157ip
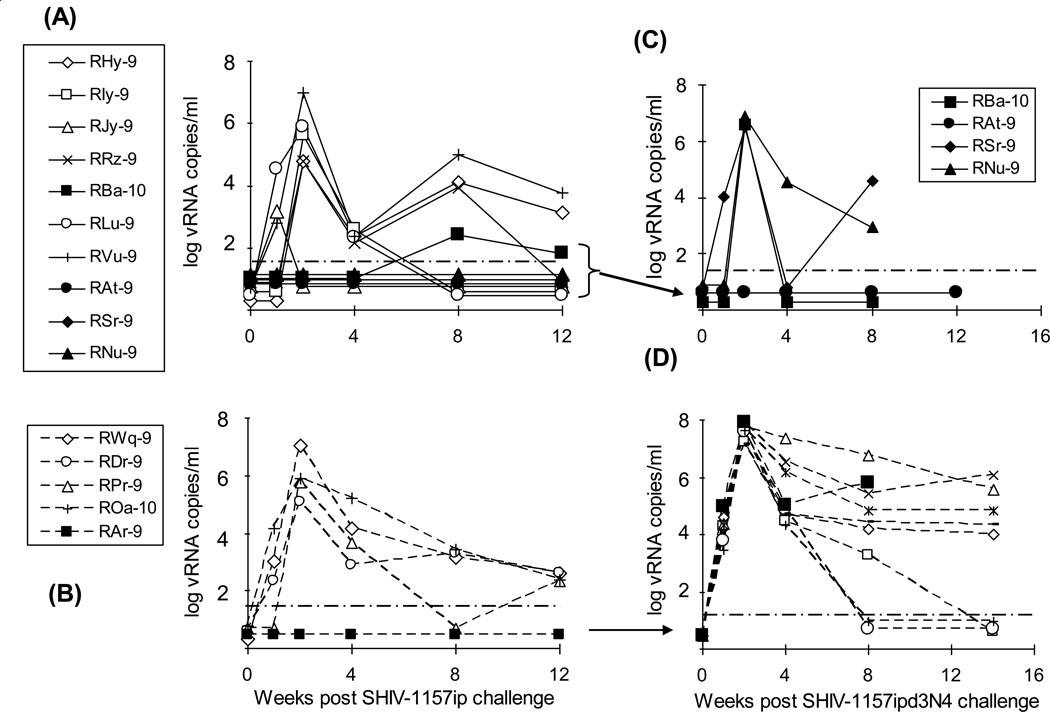
Oral SHIV-1157ip challenge employed a relatively low dose (3.7 50% animal infectious doses (AID50); (Fig. 3A and B) in an attempt to approximate the lower HIV-C inocula that are likely involved in milk-borne HIV-C transmission [21]. Three animals from Group 1 (RAt-9, RSr-9 and RNu-9) remained virus-free and 2 animals from Group 2 (RJy-9 and RBa-10) were only transiently viremic with viral RNA (vRNA) loads of 1,465 and 267 vRNA copies/ml of plasma, respectively (Fig. 3A). At this virus dose, 4 of 5 controls became systemically infected (Fig. 3B).
Fig. 3. Plasma viremia post-SHIV-1157ip and SHIV-1157ipd3N4 challenges.
Viral loads after oral challenge with SHIV-1157ip in plasma of animals of Groups 1 and 2 (A) and 3 (B) and after intrarectal rechallenge with SHIV-1157ipd3N4 of animals of Groups 1 and 2 (C) and 3 (D); viral loads of 8 additional SHIV-1157ipd3N4 challenged control animals are also shown (D). Solid symbols in (A) show viremia levels in those animals of Groups 1 and 2 that were later rechallenged with SHIV-1157ipd3N4. Solid square symbols in (B) and (D) show viremia levels in Group 3 control animal, RAr-9, after each challenge, respectively. The solid circle symbols in (C) highlight undetectable viremia levels of animal RAt-9 after rechallenge. The assay's limit of detection (50 copies/ml plasma) is denoted by dashed, horizontal lines (adapted from [13]).
3.3 High-dose intrarectal challenge with late SHIV-1157ipd3N4
Next, we sought to test whether vaccinees with no or transient infection after low-dose challenge might be protected from high-dose challenge with the more virulent SHIV-1157ipd3N4. Thus, animals that remained uninfected or had <1,000 vRNA copies/ml plasma were rechallenged intrarectally with SHIV-1157ipd3N4. This included 3 animals from Group 1 (RAt-9, RSr-9 and RNu-9), one animal from Group 2 (RBa-10), and one from control Group 3 (RAr-9) (Fig. 3C and D). After rechallenge with SHIV-1157ipd3N4, the non-vaccinated animal, RAr-9, had a peak plasma viremia level of 7.7 × 107 vRNA copies/ml (Fig. 3D); similarly, eight additional age-matched, non-vaccinated controls that received the same dose of SHIV-1175ipd3N4 intrarectally (Group 3a, Fig. 1) had peak plasma viremia levels ranging from 1.3 – 7.1 × 107 vRNA copies/ml (Fig. 3D). All controls, i.e. animals of Group 3a plus RAr-9, rapidly became viremic with vRNA loads ranging from 103 – 105 copies/ml at 1 week post-exposure. In contrast, only 1 of 4 previously vaccinated monkeys had detectable virus by week 1 after SHIV-1157ipd3N4 exposure. Among the vaccinees that became infected, peak plasma viremia levels ranged from 3.3 – 7.4 × 106 copies/ml, i.e. a log lower than controls (Fig. 3C; p=0.003, rechallenged vaccinees versus controls). Importantly, one vaccinee of Group 1 given protein only, RAt-9, had no evidence of infection after rechallenge with high-dose SHIV-1157ipd3N4. RNA isolated from a peripheral lymph node biopsy of RAt-9 at week 10 post SHIV-1157ipd3N4 rechallenge was also negative by RT-PCR analysis. These results indicate that protein immunization alone can confer complete protection against a sequential low-dose/high-dose mucosal challenge with heterologous R5 SHIV-C.
We then sought to delineate the immune parameters that may explain the complete protection of RAt-9 at the time of SHIV-1157ipd3N4 rechallenge. At this time, RAt-9 had much higher virus-specific cellular immune reactivity to both SIV Gag (8,615 spot-forming units (SFU)/106 PBMC for all 3 Gag peptide pools) and HIV Tat (705 SFU/106 PBMC) than the rechallenged vaccinees that had evidence of viral containment, RBa-10 and RNu-9 (240 and 550 anti-Gag IFN-γ ELISPOTs, respectively, and none against Tat). Of note, control monkey RAr-9 that had been exposed orally to SHIV-1157ip without becoming infected had no specific antiviral cellular immunity at the time of rechallenge. We then compared total ELISPOT reactivity against all Gag plus Tat peptide pools at the time of the first and second challenges (Table 2). The protected animal, RAt-9, was the only one with a large increase of total ELISPOT activity. Even the transiently infected animal, RBa-10, had fewer total ELISPOTs at the time of the second challenge compared to the first. Thus, protection in RAt-9 was associated with a strong boosting effect via the initial virus exposure.
Table 2.
Immune parameters and viral outcome of both SHIV challenges in rechallenged RM1.
| total ELISPOTS2 | SHIV-1157ip nAb activity | ||||
|---|---|---|---|---|---|
| SFU | fold change from 1st challenge |
titer | fold change from 1st challenge3 |
vRNA | |
| RAt-9 | |||||
| 1st challenge | 3,830 | 130 | <50 | ||
| 2nd challenge | 9,320 | 2.4× | 160 | 1.2× | <50 |
| RSr-9 | |||||
| 1st challenge | 5,960 | 160 | <50 | ||
| 2nd challenge | 160 | 0.007 | <10 | 0.06 | 3.3 × 106 |
| RNu-9 | |||||
| 1st challenge | 440 | <10 | <50 | ||
| 2nd challenge | 550 | 1.25× | <10 | - | 7.4 × 106 |
| RBa-10 | |||||
| 1st challenge | 820 | 120 | transient – 267 | ||
| 2nd challenge | 240 | 0.29 | <10 | 0.08 | 3.8 × 106 |
| RAr-9 (Control) | |||||
| 1st challenge | 0 | <10 | <50 | ||
| 2nd challenge | 5 | - | <10 | - | 7.7 × 107 |
Comparison of virus-specific ELISPOT reactivity, SHIV-1157ip nAb activity and peak plasma viremia levels at the time of 1st challenge (SHIV-1157ip) and 2nd challenge (SHIV-1157ipd3N4) from animals that were rechallenged with SHIV-1157ipd3N4. Values for the completely protected animal, RAt-9, are underlined.
The sum of all HIV-Tat and SIV Gag-specific ELISPOTS at the time of each challenge.
Where 1st challenge values were <10, the magnitude fold-change was calculated using a value of 9. adapted from Rasmussen et al. [13]
Although the protected animal, RAt-9, had a nAb titer of 1:160 against the first virus, SHIV-1157ip (Table 2), nAb activity against the rechallenge virus was minimal, reflecting the relative neutralization resistance of the late virus, SHIV-1157ipd3N4. However, RAt-9 was the only double-challenged animal that maintained nAb titers during the 6 months between challenges. NAb activity of all the other rechallenged animals was minimal or negative against all viruses tested. These data suggest that virus-specific T-cell immunity was associated with complete protection of RAt-9 from mucosal SHIV-1157ipd3N4 challenge as only low-level nAb activity against the 2nd challenge virus was seen.
3.4 Long-lived immunity in the protected animal, RAt-9
Repeated attempts to detect plasma viremia in the protected animal, RAt-9, were negative. In addition, transfer of blood from RAt-9 to infant macaques that were depleted of CD8+ cells by anti-CD8 mAb treatment also failed to reveal any virus replication that may have been contained by virus-specific immune responses in RAt-9 (data not shown).
A long-term follow up immune assessment of animal RAt-9 revealed that it maintained high nAb levels against the early virus, SHIV-1157ip as late as two years after challenge with the second virus (Table 3). At no time did RAt-9 generate more than minimal nAb activity against the second challenge virus, SHIV-1157ipd3N4, a more difficult to neutralize, tier 2 variant. Gag-specific cellular immune responses in PBMC, as measured by IFN-γ ELISPOT and intracellular cytokine staining analysis also demonstrated that RAt-9 maintained long-lived virus-specific cellular immunity.
Table 3.
Long-term immune reactivity of protected animal, RAt-9.
| NAb reactivity: | |||
|---|---|---|---|
| time post-SHIV-1157ipd3N4 challenge |
SHIV-1157ip | SHIV- 1157ipd3N4 |
|
| 18 mos | 90 | <10 | |
| 24 mos | 110 | <10 | |
| Cellular immune reactivity1: | ||||
|---|---|---|---|---|
| SIV Gag-specific ELISPOTS: | 5,853 ± 910/106 PBMC | |||
| Intracellular staining: | SIV Gag peptide stimulation | |||
| % IFN-γ | %TNF-α | % IL-2 | ||
| CD3+CD8+ | 0.38 | 1.14 | 0.58 | |
| CD3+CD4+ | 0.92 | 2.61 | 2.61 | |
Cellular immune reactivity was tested 41 months post-SHIV-1157ipd3N4 challenge. Intracellular staining values show the percentage of each gated population showing expression of the indicated cytokine. Background values (no stimulation) have been subtracted.
4. Discussion
The course of the protected monkey, RAt-9, sheds light onto the mechanisms responsible for immune protection. The clear-cut boosting of cellular antiviral immunity leads us to postulate that this animal had sub-threshold infection with the live early virus, which was reined in by pre-existing antiviral immunity and thus never became overt. This nevertheless led to strong amplification of cellular immunity and the maintenance of nAb titers against the early virus. When this animal then encountered the high dose of the relatively neutralization-resistant late virus, cellular immunity – probably supported by antibody-dependent cellular cytotoxicity (ADCC), which was demonstrated to be high at the time of challenge (data not shown) – then held SHIV-1157ipd3N4 in check. Of note, this protected animal continues to maintain very high levels of antivirus ELISPOT activity more than 3 years after challenge.
The protection from the initial low-dose SHIV-1157ip challenge in animals immunized solely with soluble viral proteins, including multimeric Env, is consistent with our previous study showing containment of SHIV-B in macaques immunized with multimeric Env only [7–9]; high nAb activity was associated with, but not predictive of virus containment. Several groups have pursued AIDS vaccine strategies based upon multimeric gp140 to maintain conserved, conformational Env determinants in an effort to generate broadly reactive nAbs [22–28]. Our data show that multimeric gp160 derived from a recently transmitted HIV clade C strain is a potent immunogen and can generate protective antiviral immunity when used as part of a more complex protein vaccine. The fact that we observed either viral containment or complete protection is promising, especially when considering the single high-dose (20 AID50) intrarectal challenge with the late virus.
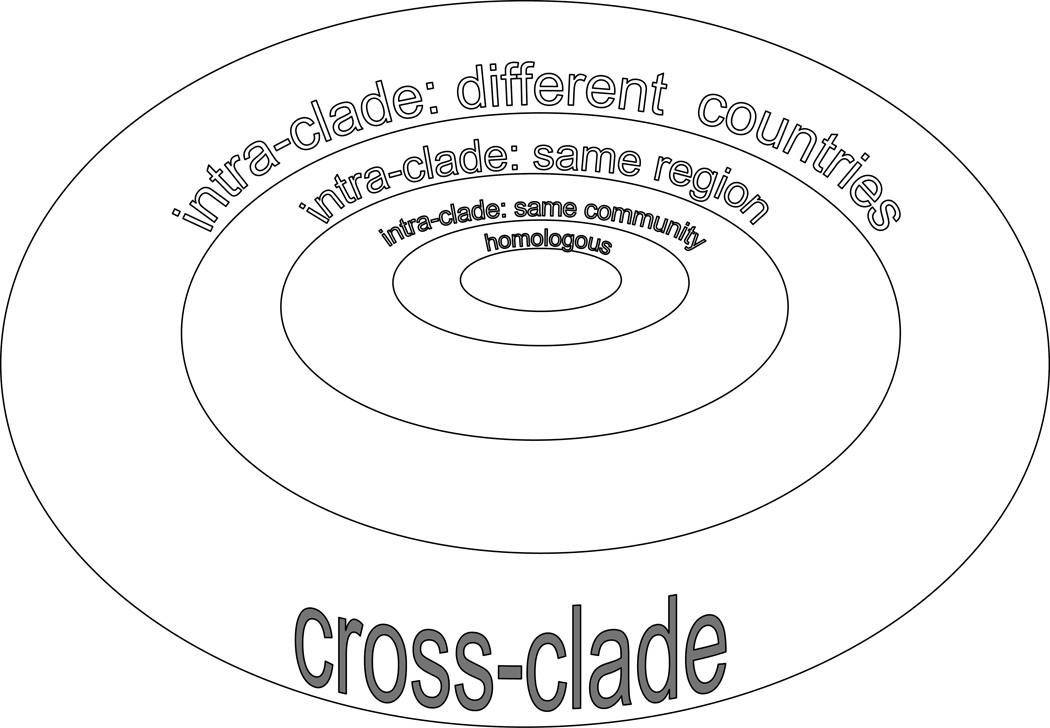
The strain of HIV any individual may be exposed to will be heterologous to the strain (or strains) used for developing the vaccine components. We propose that AIDS vaccines, especially those seeking to induce broad-based humoral immunity, should be tested against SHIV strains that are heterologous to the HIV Env component of the vaccine. The degree of strain diversity for such testing is shown schematically in Fig. 4 as a series of ever expanding concentric circles. In this schema, the diversity between the Envs in the vaccine and the challenge SHIV increases progressively. The smallest degree of Env divergence is represented by intra-clade strains isolated within a local community – as in our case – then expands to regionally divergent intra-clade strains to global intraclade strain differences; cross-clade diversity between the vaccine Env and SHIV challenge Env represents the greatest divergence. Our upfront heterologous challenge study, based upon locally divergent HIV-C env genes, was designed to model repeated human mucosal exposure to evolving strains circulating within the community. This is represented as the second tier of Env diversity between vaccine strain and challenge strain (Fig. 4) and, indeed, vaccination with multimeric gp160 from a recently transmitted local strain was able to induce nAbs against a heterologous challenge virus.
Fig 4. Increasing the diversity between immunogens and Env in challenge SHIV strains.
The degree of heterogeneity between the strain of Env in a potential vaccine and the challenge strain of virus is shown schematically as increasingly larger concentric circles.
Acknowledgments
The authors thank Susan Sharp for assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH grants P01 AI48240, including ARRA funding, to R.M.R and R.A.R. and RR-00165 providing base grant support to the Yerkes National Primate Research Center.
References
- 1.Steinbrook R. One step forward, two steps back--will there ever be an AIDS vaccine? N Engl J Med. 2007 Dec 27;357(26):2653–2655. doi: 10.1056/NEJMp0708117. [DOI] [PubMed] [Google Scholar]
- 2.Kramer VG, Siddappa NB, Ruprecht RM. Passive immunization as tool to identify protective HIV-1 Env epitopes. Curr HIV Res. 2007 Nov;5(6):642–655. doi: 10.2174/157016207782418506. [DOI] [PubMed] [Google Scholar]
- 3.Vlasak J, Ruprecht RM. AIDS vaccine development and challenge viruses: getting real. Aids. 2006 Nov 14;20(17):2135–2140. doi: 10.1097/QAD.0b013e328010beb5. [DOI] [PubMed] [Google Scholar]
- 4.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD, et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. J Virol. 2006 Sep;80(17):8729–8738. doi: 10.1128/JVI.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JT, Halloran M, Lord CI, Watson A, Ranchalis J, Fung M, et al. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69(11):7061–7067. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert M, Rasmussen RA, Song R, Ong H, Sharma P, Chenine AL, et al. SHIV-1157i and passaged progeny viruses encoding R5 HIV-1 clade C env cause AIDS in rhesus monkeys. Retrovirology. 2008;5:94. doi: 10.1186/1742-4690-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen RA, Hofmann-Lehman R, Montefiori DC, Li PL, Liska V, Vlasak J, et al. DNA prime/protein boost vaccine strategy in neonatal macaques against simian human immunodeficiency virus. J Med Primatol. 2002 Feb;31(1):40–60. doi: 10.1034/j.1600-0684.2002.1o019.x. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen RA, Hofmann-Lehmann R, Li PL, Vlasak J, Schmitz JE, Reimann KA, et al. Neutralizing antibodies as a potential secondary protective mechanism during chronic SHIV infection in CD8+ T-cell-depleted macaques. Aids. 2002 Apr 12;16(6):829–838. doi: 10.1097/00002030-200204120-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen RA, Montefiori DC, Robinson HL, McClure HM, Ruprecht RM. Heterologous neutralizing antibody induction in a simian-human immunodeficiency virus primate model: lack of original antigenic sin. J Infect Dis. 2001;184(12):1603–1607. doi: 10.1086/324582. [DOI] [PubMed] [Google Scholar]
- 10.Grisson RD, Chenine AL, Yeh LY, He J, Wood C, Bhat GJ, et al. Infectious molecular clone of a recently transmitted pediatric human immunodeficiency virus clade C isolate from Africa: evidence of intraclade recombination. J Virol. 2004 Dec;78(24):14066–14069. doi: 10.1128/JVI.78.24.14066-14069.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen RA, Ong H, Kittel C, Ruprecht CR, Ferrantelli F, Hu SL, et al. DNA prime/protein boost immunization against HIV clade C: Safety and immunogenicity in mice. Vaccine. 2006 Mar 20;24(13):2324–2332. doi: 10.1016/j.vaccine.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 12.Andre S, Seed B, Eberle J, Schraut W, Bultmann A, Haas J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol. 1998 Feb;72(2):1497–1503. doi: 10.1128/jvi.72.2.1497-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen RA, Ong H, Song R, Chenine AL, Ayash-Rashkovsky M, Hu SL, et al. Efficacy of a multigenic protein vaccine containing multimeric HIV gp160 against heterologous SHIV clade C challenges. Aids. 2007 Sep 12;21(14):1841–1848. doi: 10.1097/QAD.0b013e32828684ea. [DOI] [PubMed] [Google Scholar]
- 14.Chenine AL, Buckley KA, Li PL, Rasmussen RA, Ong H, Jiang S, et al. Schistosoma mansoni infection promotes SHIV clade C replication in rhesus macaques. Aids. 2005 Nov 4;19(16):1793–1797. doi: 10.1097/01.aids.0000189857.51935.0b. [DOI] [PubMed] [Google Scholar]
- 15.Haffar O, Garrigues J, Travis B, Moran P, Zarling J, Hu SL. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990 Jun;64(6):2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu SL, Travis BM, Garrigues J, Zarling JM, Sridhar P, Dykers T, et al. Processing, assembly, and immunogenicity of human immunodeficiency virus core antigens expressed by recombinant vaccinia virus. Virology. 1990 Nov;179(1):321–329. doi: 10.1016/0042-6822(90)90300-g. [DOI] [PubMed] [Google Scholar]
- 17.Polacino PS, Stallard V, Klaniecki JE, Pennathur S, Montefiori DC, Langlois AJ, et al. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques. J Virol. 1999 Oct;73(10):8201–8215. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen RA, Ong H, Kittel C, Ruprecht CR, Ferrantelli F, Hu SL, et al. DNA prime/protein boost immunization against HIV clade C: safety and immunogenicity in mice. Vaccine. 2006 Mar 20;24(13):2324–2332. doi: 10.1016/j.vaccine.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 19.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008 Feb;38(2):350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, et al. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses. 2000 Sep 1;16(13):1247–1257. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- 21.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999 Jul;180(1):93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 22.Barnett SW, Lu S, Srivastava I, Cherpelis S, Gettie A, Blanchard J, et al. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J Virol. 2001;75(12):5526–5540. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beddows S, Schulke N, Kirschner M, Barnes K, Franti M, Michael E, et al. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2005 Jul;79(14):8812–8827. doi: 10.1128/JVI.79.14.8812-8827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, et al. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol. 2002 Jun;76(11):5357–5368. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derby NR, Kraft Z, Kan E, Crooks ET, Barnett SW, Srivastava IK, et al. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J Virol. 2006 Sep;80(17):8745–8762. doi: 10.1128/JVI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffs SA, Goriup S, Kebble B, Crane D, Bolgiano B, Sattentau Q, et al. Expression and characterisation of recombinant oligomeric envelope glycoproteins derived from primary isolates of HIV-1. Vaccine. 2004 Feb 25;22(8):1032–1046. doi: 10.1016/j.vaccine.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Robinson WE, Jr, Kawamura T, Gorny MK, Lake D, Xu JY, Matsumoto Y, et al. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc Natl Acad Sci U S A. 1990;87(8):3185–3189. doi: 10.1073/pnas.87.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Wyatt R, Sodroski J. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J Virol. 2001;75(3):1165–1171. doi: 10.1128/JVI.75.3.1165-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]