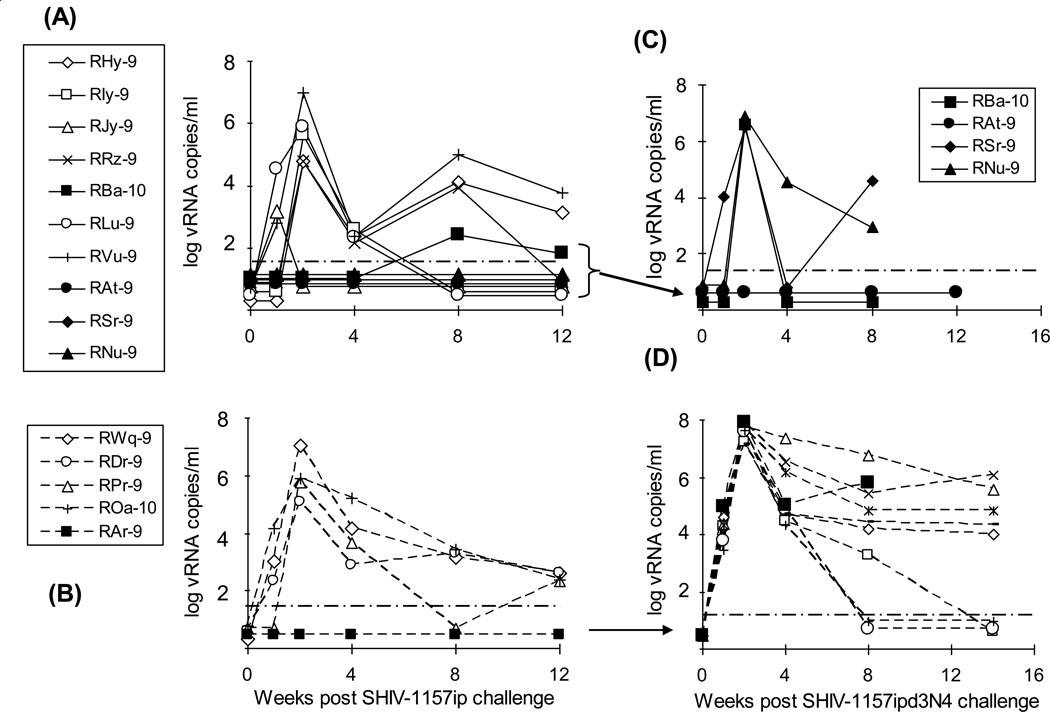
Fig. 3. Plasma viremia post-SHIV-1157ip and SHIV-1157ipd3N4 challenges.
Viral loads after oral challenge with SHIV-1157ip in plasma of animals of Groups 1 and 2 (A) and 3 (B) and after intrarectal rechallenge with SHIV-1157ipd3N4 of animals of Groups 1 and 2 (C) and 3 (D); viral loads of 8 additional SHIV-1157ipd3N4 challenged control animals are also shown (D). Solid symbols in (A) show viremia levels in those animals of Groups 1 and 2 that were later rechallenged with SHIV-1157ipd3N4. Solid square symbols in (B) and (D) show viremia levels in Group 3 control animal, RAr-9, after each challenge, respectively. The solid circle symbols in (C) highlight undetectable viremia levels of animal RAt-9 after rechallenge. The assay's limit of detection (50 copies/ml plasma) is denoted by dashed, horizontal lines (adapted from [13]).