Abstract
Fox transcription factors play a critical role in the regulation of a variety of biological processes. While FoxM1 behaves like the oncogenic transcription factor, FoxO3a is known as a tumor suppressor by inhibiting FoxM1. This study aimed to investigate the clinicopathological significance of FoxM1 and FoxO3a expression in breast cancer. Expression of FoxM1 and FoxO3a were analyzed by immunohistochemical staining on tissue microarray sections from 236 breast cancer patients, and correlated with various clinicopathological characteristics. Overexpression of FoxM1 correlated with adverse clinicopathological features, such as larger tumor size, lymph node metastasis, advanced tumor stage, and lymphovascular invasion. The Kaplan-Meier survival curves revealed no prognostic significance of FoxM1 expression. However, in subgroup analyses with patients of estrogen receptor (ER) positive breast cancers, FoxM1 overexpression associated with poor disease free and overall survival. No association was found between FoxO3a and FoxM1 expression. Regarding clinicopathological variables, the only association between histologic grade and FoxO3a was observed. In conclusion, FoxM1 overexpression was significantly associated with aggressive phenotypes and poor prognosis of ER-positive breast cancer. These findings suggest the possible role of FoxM1 as a prognostic biomarker and putative target of anti-cancer therapy.
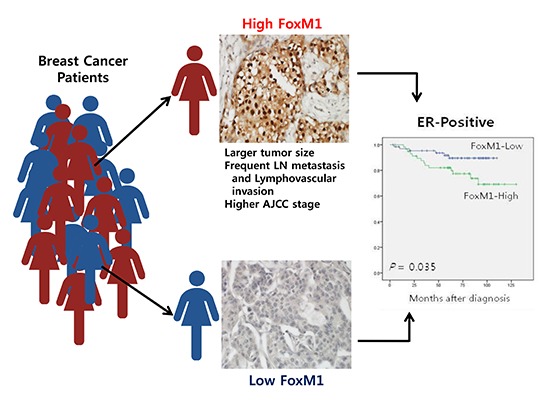
Graphical Abstract
Keywords: Breast Neoplasms, Forkhead Transcription Factor, Foxhead Box M1, Foxhead Box O3a, Prognosis
INTRODUCTION
Breast cancer is the most frequently diagnosed cancer in women and its incidence has increased by more than 20% since the 2008 estimates. While death rates for breast cancer continue to decrease since 1990, it has been estimated that breast cancer still accounts for the second highest morbidity rate of cancer in women worldwide (1). In Korea, breast cancer is the most common cancer in females except for thyroid cancer, and incidence was continuousely increased (2). It is known that breast cancer is a heterogeneous disease characterized by diverse clinical behavior and outcomes (3). Although previous studies have shown the various biomarkers which could predict the tumor behavior and be associated with the prognostic significance (4), the identification of new biomarkers and therapeutic targets is still important for treatment of patients (5).
Forkhead box (Fox) proteins are a large superfamily of transcription factor defined by an evolutionary conserved 'winged-helix' DNA-binding domain (6). The Fox family has since been categorized into 19 subgroups (FoxA to FoxS) including 50 genes in the human genome (7). Several key subfamilies of the FoxM, FoxO, FoxA, FoxC, and FoxP proteins are important factors in oncogenic and tumor suppressive pathways (6). Fox protein family plays a critical role in the regulation of a broad variety of biological processes, including cell cycle progression, proliferation, metabolism, differentiation, migration, DNA damage repair, and apoptosis. The activity of Fox proteins is regulated at the transcriptional level through genetic events and post-translational modifications including phosphorylation, acetylation, and ubiquitination (8).
Previous studies have demonstrated that overexpression of FoxM1 is associated with tumorigenesis and progression in a variety of human cancers, including the breast, colorectum, lung, prostate, liver, pancreas, cervix, blood, and nervous system. FoxM1 also has been implicated in tumor angiogenesis, cancer cell invasion, metastasis, and chemotherapy resistance (9). While FoxM1 behaves like the oncogenic transcription factor, FoxO3a is known as a tumor suppressor which mediate cell cycle arrest, DNA repair, and apoptosis (10). FoxO3a is a downstream target of the PI3K-Akt pathway and negatively regulated by several upstream kinases including Akt, IKK and ERK (6). Deregulation of these kinases induces loss of FoxO3a activity by promoting the nuclear export which retains it as inactive protein in the cytoplasm (6). There is increasing evidence to suggest that FoxO3a is important for the maintenance of tissue homeostasis and is frequently deregulated in human malignancies.
FoxM1 is a transcriptional target of PI3K-Akt-FoxO signaling pathway and repressed by FoxO proteins (11). As a result, FoxO3a negatively regulates the transcriptional output of FoxM1, which promote tumorigenesis and cancer progression. Many genotoxic and cytotoxic chemotherapeutic drugs are mediated through inhibition of the PI3K-Akt signaling pathway, which is a commonly deregulated cascade in cancer. The FoxO3a-FoxM1 axis is a vital downstream of the PI3K-Akt oncogenic pathway and contribute to chemotherapy resistance (10). Moreover, FoxO3a and FoxM1 interact functionally with estrogen receptor-alpha (ERα) and play an important role in estrogen-dependent breast cancer (12). Previous studies have identified a crucial mechanism of estrogen regulation through the activity of forkhead transcription factor (13). The FoxM1 and FoxO3a interaction with ER proteins and regulation of the functional gene networks are likely that they contribute to determine anti-estrogen sensitivity and resistance. Additionally, HER2 overexpression is known to be negatively related to ER expression and has been found to regulate FoxO3a and FoxM1 through PI3K-Akt signaling axis. Previous report has shown that a strong link between FoxM1 and HER2, thus FoxM1 is a potential target in HER2 amplified breast cancer resistance to HER2 targeted therapy (14). Furthermore, FoxO3a has also been suggested as an important intermediary in controlling the inverse expression between HER2 and ER levels and a possible target in therapeutic intervention for HER2 amplified antiestrogen-refractory breast cancers (15).
In this study, we analyzed the expression of FoxM1 and FoxO3a by immunohistochemistry in human breast cancer specimens and compared with various clinicopathological features and prognosis in breast cancer patients. Also, we examined the presence of an association between biomarkers, including FoxM1, FoxO3a, ER, and HER2 to validate their relationship comparing with the results of previous research.
MATERIALS AND METHODS
Patients and specimens
Two hundred and thirty six breast cancer patients who underwent curative surgery between 2001 and 2008 at Hanyang University Hospital were enrolled. Archived hematoxylin-eosin (H&E) and immunohistochemical staining slides, pathology reports, and other medical records were reviewed to confirm the diagnoses as well as to establish the clinicopathologic characteristics of the tumors, such as age, histologic grade, tumor size, lymph node metastasis, American Joint Committee on Cancer (AJCC) tumor stage, lymphovascular invasion, perineural invasion, and patient follow-up information.
Construction of tissue microarray (TMA)
H&E-stained slides were used to define the most morphologically representative, well fixed, and non-necrotic areas. Single tissue cores (2 mm in diameter) were sampled from each paraffin block and assembled into a recipient paraffin block using a TMA instrument (AccuMax Array, ISU ABXIS, Seoul, Korea).
Immunohistochemistry
The expression of FoxM1 and FoxO3a was evaluated by immunohistochemical staining with 4-µm-thick sections from TMA blocks. The sections were first deparaffinized in xylene and then rehydrated through graded ethanol. For antigen retrieval, we performed autoclave heating at 100℃ for 30 min in sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with peroxidase blocking solution (S2023, DakoCytomation, Carpinteria, CA,USA). TMA slides were incubated with primary antibodies at 4℃ overnight, and then incubated with labeled polymer (DAKO REAL EnVision/HRP, K5007, DakoCytomation) for 30 min at room temperature. The primary antibodies were rabbit anti-human FoxM1 (sc-502; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and FoxO3a (sc-11351; Santa Cruz Biotechnology) polyclonal antibody used in 1:100 dilution. 3,3'-diaminobenzidine was used as a chromogen for detection, and Mayer's hematoxylin counterstain was applied. Immunostaining for ER and HER2 was performed using the Bond-Max automated immunostainer (Leica Biosystems, Wetzlar, Germany) using the following antibodies: anti-ER antibody (Clone 6F11, Novocastra, Newcastle upon Tyne, UK) and anti-HER2 antibody (Clone 10A7, Novocastra).
Interpretation of immunohistochemical staining
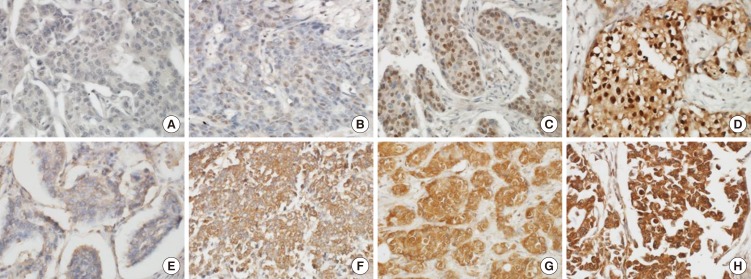
All slides were evaluated independently by two experienced pathologists in a blinded fashion without any knowledge of the patient data. The percentage of positive tumor cells and intensity of staining were assessed using a semi-quantitative scoring system. The intensity score was assigned as follows: 0 (negative), 1 (weak staining), 2 (intermediate staining), and 3 (strong staining) (Fig. 1). The proportion score was as follows: 0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). For FoxM1 and FoxO3a, immunoreactivity was defined as cells showing nuclear staining pattern with or without cytoplasmic staining in the tumor cells with minimal background staining. The expression of FoxM1 and FoxO3a were scored by multiplying the intensity score and proportion score. For survival analyses, the tumors with a final score of ≥6 were considered as high expression, and <6 as low expression. The ASCO/CAP guidelines were used for interpreting the ER and HER2 immunostaining.
Fig. 1. Representative sections of the immunohistochemistry of FoxM1 and FoxO3a in breast cancer tissue (magnification, × 400). (A) Negative FoxM1 staining. (B) Weak FoxM1 staining. (C) Intermediate FoxM1 staining. (D) Strong FoxM1 staining. (E) Negative FoxO3a staining. (F) Weak FoxO3a staining. (G) Intermediate FoxO3a staining. (H) Strong FoxO3a staining.
Statistical analyses
The SPSS 21.0 software package was used for the statistical analysis. The Student's t-test and one-way ANOVA were used to analyze the associations between the mean FoxM1 or FoxO3a expression and various clinicopathological factors. The Kaplan-Meier method was used to make disease-free and overall survival curves, and the log-rank test was used to determine the level of significance. The significant predictors in the univariate survival analysis of the Cox proportional hazard model were going into multivariate survival analysis to explore the independent effects of survival. For the correlation between FoxM1, FoxO3a, ER, and HER2 biomarkers, Pearson's correlation test was used.
Ethics statement
This study, along with the waiver of informed consent, was approved by the institutional review board of The Hanyang University Hospital (HYUH 2013-11-004-001).
RESULTS
Correlation between FoxM1, FoxO3a expression and the clinicopathological features
The FoxM1 and FoxO3a expression were correlated with the conventional prognostic factors to determine the clinicopathologic significance (Table 1). The nuclear FoxM1 overexpression was associated with aggressive phenotypes of breast cancer, such as larger tumor size (t-test, P=0.003), lymph node metastasis (t-test, P<0.001), advanced AJCC stages (one-way ANOVA, P<0.001) and lymphovascular invasion (t-test, P<0.001). Nuclear FoxO3a expression was not associated with any clinicopathological features, except for histologic grade (one-way ANOVA, P=0.016).
Table 1. Association between FoxM1, FoxO3a expression and clinicopathological parameters in breast cancer patients.
| Clinicopathological features | No. | FoxM1 (Immunoreactive score) | P value (t-test) | FoxO3a (Immunoreactive score) | P value (t-test) |
|---|---|---|---|---|---|
| Histologic grade | |||||
| Grade 1 | 40 | 4.70 ± 3.252 | 0.172† | 3.70 ± 2.319 | 0.016* |
| Grade 2 | 96 | 5.19 ± 3.089 | 3.80 ± 2.569 | ||
| Grade 3 | 100 | 4.35 ± 3.089 | 4.77 ± 2.853 | ||
| Primary tumor size | |||||
| < 2 cm | 113 | 4.12 ± 3.049 | 0.003 | 4.02 ± 2.731 | 0.379 |
| ≥ 2 cm | 123 | 5.33 ± 3.096 | 4.32 ± 2.638 | ||
| Lymph node metastasis | |||||
| Absent | 131 | 4.08 ± 3.312 | < 0.001 | 4.08 ± 2.640 | 0.528 |
| Present | 105 | 5.58 ± 2.670 | 4.30 ± 2.741 | ||
| AJCC stage | |||||
| Stage I | 82 | 3.60 ± 3.181 | < 0.001† | 3.94 ± 2.659 | 0.766† |
| Stage II | 97 | 5.33 ± 3.009 | 4.36 ± 2.739 | ||
| Stage III | 57 | 5.53 ± 2.788 | 4.20 ± 2.652 | ||
| Lymphovascular invasion | |||||
| Absent | 124 | 4.06 ± 3.342 | < 0.001 | 4.04 ± 2.604 | 0.393 |
| Present | 112 | 5.51 ± 2.688 | 4.33 ± 2.770 | ||
| Perineural invasion | |||||
| Absent | 184 | 4.80 ± 3.217 | 0.597 | 4.20 ± 2.793 | 0.909 |
| Present | 51 | 4.53 ± 2.880 | 4.16 ± 2.257 | ||
| ER expression | |||||
| Negative | 112 | 4.85 ± 3.208 | 0.648 | 3.91 ± 2.745 | 0.157 |
| Positive | 124 | 4.66 ± 3.063 | 4.40 ± 2.617 | ||
| HER2 amplification | |||||
| Absent | 188 | 4.79 ± 3.053 | 0.878 | 4.02 ± 2.641 | 0.059 |
| Present | 48 | 4.71 ± 3.396 | 4.83 ± 2.801 |
*One-way ANOVA.
The effect of FoxM1 and FoxO3a expression on prognosis in breast cancer
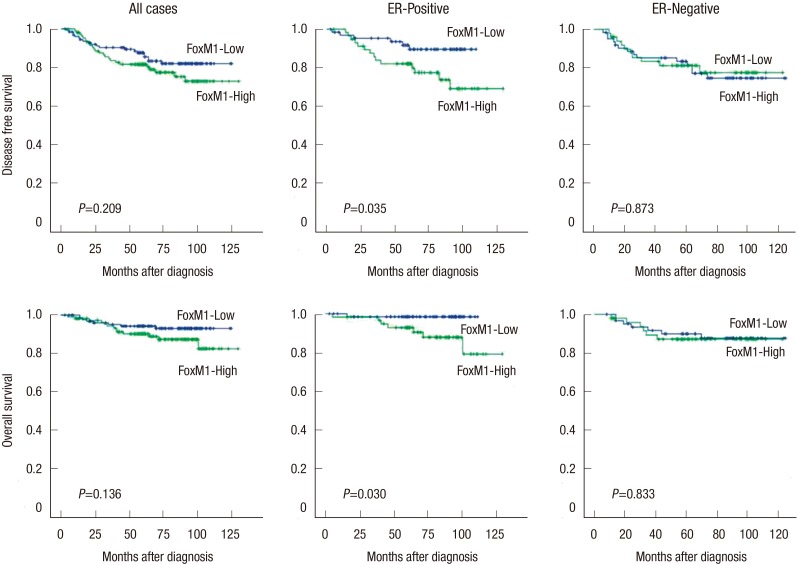
The association between conventional clinicopathological features and prognosis in breast cancer patients were evaluated. As we expected, univariate analyses revealed primary tumor size (P<0.001), lymph node metastasis (P<0.001), AJCC tumor stage (P<0.001), and lymphovascular invasion (P<0.001) as predictors of poor disease-free survival (DFS) in breast cancer patients in Cox's proportional hazard model (Table 2). The Kaplan-Meier survival curves demonstrated no significant association between FoxM1 or FoxO3a expression and clinical outcome. In addition, survival analyses stratified by tumor stage revealed no significant differences. However, the Kaplan-Meier survival curves stratified by ER status revealed that high FoxM1 expression showed significant poor DFS (log-rank test, P=0.035) and overall survival (OS) (log-rank test, P=0.030) in ER-positive breast cancers (Fig. 2). Additionally, FoxM1 was demonstrated as a predictor of poor DFS in ER positive breast cancer patients on univariate Cox regression analyses (P=0.042) (Table 3). However, multivariate analyses failed to detect an independent prognostic significance of FoxM1.
Table 2. Univariate and multivariate Cox regression analyses of various prognostic parameters for survival in breast cancer patients.
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Disease free survival | ||||||
| Histologic grade (G1 vs. G2 & 3) | 0.707 | 0.410-1.219 | 0.212 | |||
| Primary tumor size (≤ 2 cm vs. > 2 cm) | 2.954 | 1.684-5.182 | < 0.001 | 1.841 | 1.009-3.358 | 0.047 |
| Lymph node metastasis (absent vs. present) | 4.344 | 2.421-7.793 | < 0.001 | 2.755 | 1.339-5.668 | 0.006 |
| AJCC stage (I & II vs. III & IV) | 4.081 | 2.458-6.774 | < 0.001 | 3.851 | 1.158-4.047 | 0.016 |
| Lymphovascular invasion (absent vs. present) | 3.885 | 2.135-7.066 | < 0.001 | NS | ||
| FoxM1 expression (low vs. high) | 1.383 | 0.781-2.451 | 0.266 | |||
| FoxO3a expression (low vs. high) | 1.127 | 0.611-2.078 | 0.702 | |||
| ER expression (negative vs. positive) | 0.773 | 0.466-1.283 | 0.302 | |||
| HER2 amplification (absent vs. present) | 1.035 | 0.538-1.991 | 0.917 | |||
| Overall survival | ||||||
| Histologic grade (G1 vs. G2 & 3) | 0.983 | 0.477-2.027 | 0.964 | |||
| Primary tumor size (≤ 2 cm vs. > 2 cm) | 3.253 | 1.454-7.278 | 0.004 | NS | ||
| Lymph node metastasis (absent vs. present) | 3.320 | 1.528-7.210 | 0.002 | NS | ||
| AJCC stage (I & II vs. III & IV) | 3.851 | 1.903-7.792 | < 0.001 | 3.851 | 1.903-7.792 | < 0.001 |
| Lymphovascular invasion (absent vs. present) | 2.722 | 1.253-5.912 | 0.011 | NS | ||
| FoxM1 expression (low vs. high) | 1.932 | 0.801-4.664 | 0.143 | |||
| FoxO3a expression (low vs. high) | 0.862 | 0.347-2.138 | 0.748 | |||
| ER expression (negative vs. positive) | 0.465 | 0.226-0.958 | 0.038 | NS | ||
| HER2 amplification (absent vs. present) | 1.117 | 0.458-2.725 | 0.807 | |||
HR, harzard ratio; CI, confidence interval; NS, not significant.
Fig. 2. Kaplan-Meier curves for disease-free and overall survival for breast cancer according to FoxM1 expression.
Table 3. Univariate and multivariate analyses of various prognostic parameters for survival in ER-positive breast cancer patients.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Disease free survival | ||||||
| Histologic grade (G1 vs. G2 & 3) | 0.097 | 0.013-0.709 | 0.022 | NS | ||
| Primary tumor size (≤ 2 cm vs. > 2 cm) | 2.979 | 1.425-6.226 | 0.004 | NS | ||
| Lymph node metastasis (absent vs. present) | 4.855 | 2.091-11.27 | < 0.001 | 3.676 | 1.412-9.572 | 0.008 |
| AJCC stage (I & II vs. III & IV) | 4.672 | 2.309-9.454 | < 0.001 | NS | ||
| Lymphovascular invasion (absent vs. present) | 4.798 | 1.968-11.70 | 0.001 | NS | ||
| FoxM1 expression (low vs. high) | 2.694 | 1.035-7.014 | 0.042 | NS | ||
| FoxO3a expression (low vs. high) | 1.261 | 0.511-3.111 | 0.614 | |||
| HER2 amplification (absent vs. present) | 1.312 | 0.949-1.814 | 0.101 | |||
| Overall survival | ||||||
| Histologic grade (G1 vs. G2 & 3) | 0.281 | 0.036-2.176 | 0.224 | |||
| Primary tumor size (≤ 2 cm vs. > 2 cm) | 3.195 | 0.960-10.64 | 0.058 | |||
| Lymph node metastasis (absent vs. present) | 3.873 | 1.047-14.32 | 0.042 | NS | ||
| AJCC stage (I & II vs. III & IV) | 4.041 | 1.301-12.55 | 0.016 | 4.041 | 1.301-12.550 | 0.016 |
| Lymphovascular invasion (absent vs. present) | 3.127 | 0.845-11.57 | 0.088 | |||
| FoxM1 expression (low vs. high) | 7.304 | 0.897-59.45 | 0.063 | |||
| FoxO3a expression (low vs. high) | 0.941 | 0.223-3.980 | 0.934 | |||
| HER2 amplification (absent vs. present) | 1.314 | 0.769-2.244 | 0.318 | |||
HR, harzard ratio; CI, confidence interval; NS, not significant.
Assessment of the relationship between breast cancer biomarkers
Since previous studies have found the inverse correlation of FoxM1 and FoxO3a, and possible regulatory network between FoxM1 and ER or HER2 expression, we examined the relationship between FoxM1, FoxO3a, ER, and HER2 expression. However, no significant relationship was observed each of them. The mean expression score of FoxM1 and FoxO3a were not associated with ER expression and HER2 amplification. Also, the relationship between FoxM1 and FoxO3a expression was not identified by Pearson's correlation analysis (P=0.935, Pearson correlation coefficient=0.005).
DISCUSSION
The overexpression of transcription factor FoxM1 and its oncogenic properties have been identified in various human malignancies, including pulmonary squamous cell carcinoma (16), clear renal cell carcinoma (17), hepatocellular carcinoma (18), pancreatic ductal adenocarcinoma (19), uterine cervical cancer (20), and gastric cancer (21, 22). In breast cancer, previous studies have suggested that FoxM1 overexpression is an indicator of poor patient outcome and correlated with the expression pattern of ERα (23). In our study, FoxM1 overexpression correlated with adverse clinicopathological features, including larger tumor size, lymph node metastasis, advanced AJCC stage, and lymphovascular invasion.
There is increasing evidence that FoxM1 and ERα interact with transcription factors to either activate or repress the transcription of target genes. FoxM1 regulates ERα expression by binding directly to Forkhead response elements (FHREs) at the proximal region of ERα promoter and also activated ER modulates FoxM1 expression by binding to the estrogen responsive element of the FoxM1 promoter (24). Our results did not show a significant association between FoxM1 and ERα, but the FoxM1 expression in only ER-positive breast cancer group was related to patient clinical outcome, not in all patients or ER negative group. This represents that a link between FoxM1 and ERα is important in the ERα signaling pathway and overexpression of FoxM1 may contribute to the resistance in anti-estrogen therapy. Actually, Sanders et al. (25) have demonstrated by molecular study that FoxM1 shows different binding pattern according to ERα status and regulates a gene signature related with poor prognosis in ER-positive breast cancer patients. In addition, Yau et al. (26) demonstrated that FoxM1 is associated with the higher risk for metastatic relapse in ER-positive breast cancer.
FoxO3a is a vital downstream effector of the PI3k-Akt signalling pathway and when phosphorylated by Akt, translocated from the nucleus to the cytoplasm and results in inactivation (15). However, the subcellular localization and functional role of FoxO3a is controversial. Habashy et al. (27) have shown that nuclear FoxO3a expression is associated with favorable outcome in ER-positive breast cancer specific survival and distant metastasis free interval. The other study has shown that nuclear FoxO3a expression is a favorable prognostic marker for long-term survival in breast cancer (28). On the contrary, Chen et al. (29) have found that nuclear accumulation of FoxO3a contributes to the increased PI3K-Akt activity resulting in uncoupling of the Akt-FoxO3a axis in chemotherapy resistant breast cancer cells and is associated with poor survival in breast cancer patients. Moreover, FoxO3a has emerged as an important mediator which interacts with FoxM1 on the ERα promoter and regulates ERα expression. Zou et al. (30) have reported using gene expression profiling with a DNA microarray that FoxO3a interacts with ERs and inhibits the expression of their target genes. Another study has confirmed FoxO3a and FoxM1 bind to the same responsive elements in the ERα promoter, but failed to demonstrate that FoxO3a can lead to an up-regulation of ERα expression, suggesting FoxO3a is not the primary biological regulator of ERα transcription (24). The regulatory mechanism, subcellular localization and functional activity of FoxO3a with other transcription factors and proteins remain unclear. In this study, nuclear expression FoxO3a did not show significant associations with FoxM1, ER expression, and the patient's prognosis. The possible explanation of these discrepancies may arise due to the different antibodies used, or different breast cancer cohort. Further investigation and larger study are required to understand the exact molecular mechanism of FoxO3a and to establish the value of FoxO3a expression as a prognostic biomarker or ER regulator.
FoxO3a and FoxM1 have transcriptionally antagonistic functions by competing to bind to the FHREs, the same sequences of target gene promoters. FoxO3a can disturb FoxM1 transcriptional activity by displacing from the FHRE and inducing chromatin condensation of target promoters (11). Furthermore, FoxO3a can repress FoxM1 expression through binding to the FHRE of FoxM1 gene (11). Therefore, FoxM1 and FoxO3a are considered to be that they antagonize each other's activity through various pathways and targeting the FoxO-FoxM1 axis could be a strategy for chemotherapy and overcoming drug resistance (31). However, no studies have shown their inverse relationship in human breast cancer tissues using immunohistochemistry. In this study, we analyzed whether a correlation exists between the immunohistochemical expression of FoxM1 and FoxO3a in human breast cancer tissues, however there was no significant association.
In conclusion, our data demonstrated that high FoxM1 expression was correlated with several adverse prognostic factors, such as tumor size, regional lymph node metastasis, AJCC stage and lymphovascular invasion. Additionally, the high FoxM1 level was associated with poor DFS and OS in ER-positive breast cancer patients. Further investigations and understanding of FoxM1 protein in breast cancer progression and chemotherapy resistance could provide opportunities for the development of potential target therapy.
Footnotes
This study was supported by a grant from the research fund of Hanyang University (HY-2011-MC) to Kiseok Jang.
The authors have no relevant financial relationships or conflicts of interest to disclose.
Conception and coordination of the study: Jang K. Design of ethical issues: Ahn H, Jang K, Chung MS. Acquisition of clinicopathological and experimental data: Ahn H, Sim J, Abdul R, Chung MS, Paik SS, Jang K. Analysis and interpretation of data: Ahn H, Jang K. Manuscript preparation: Ahn H, Jang K. Critical review of manuscript: Oh YH, Park CK. Manuscript approval: all authors.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadoo KA, Fornier MN, Morris PG. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging. 2013;57:312–321. [PubMed] [Google Scholar]
- 4.Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17:R245–R262. doi: 10.1677/ERC-10-0136. [DOI] [PubMed] [Google Scholar]
- 5.Banin Hirata BK, Oda JM, Losi Guembarovski R, Ariza CB, de Oliveira CE, Watanabe MA. Molecular markers for breast cancer: prediction on tumor behavior. Dis Markers. 2014;2014:513158. doi: 10.1155/2014/513158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 7.Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Hum Genomics. 2010;4:345–352. doi: 10.1186/1479-7364-4-5-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–4333. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Koo CY, Muir KW, Lam EW. FOXM1: from cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Gomes AR, Zhao F, Lam EW. Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin J Cancer. 2013;32:365–370. doi: 10.5732/cjc.012.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karadedou CT. Regulation of the FOXM1 transcription factor by the estrogen receptor alpha at the protein level, in breast cancer. Hippokratia. 2006;10:128–132. [PMC free article] [PubMed] [Google Scholar]
- 14.Francis RE, Myatt SS, Krol J, Hartman J, Peck B, McGovern UB, Wang J, Guest SK, Filipovic A, Gojis O, et al. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer. Int J Oncol. 2009;35:57–68. doi: 10.3892/ijo_00000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. 2004;24:8681–8690. doi: 10.1128/MCB.24.19.8681-8690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang DK, Son CH, Lee SK, Choi PJ, Lee KE, Roh MS. Forkhead box M1 expression in pulmonary squamous cell carcinoma: correlation with clinicopathologic features and its prognostic significance. Hum Pathol. 2009;40:464–470. doi: 10.1016/j.humpath.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Wu XR, Chen YH, Liu DM, Sha JJ, Xuan HQ, Bo JJ, Huang YR. Increased expression of forkhead box M1 protein is associated with poor prognosis in clear cell renal cell carcinoma. Med Oncol. 2013;30:346. doi: 10.1007/s12032-012-0346-1. [DOI] [PubMed] [Google Scholar]
- 18.Sun HC, Li M, Lu JL, Yan DW, Zhou CZ, Fan JW, Qin XB, Tang HM, Peng ZH. Overexpression of Forkhead box M1 protein associates with aggressive tumor features and poor prognosis of hepatocellular carcinoma. Oncol Rep. 2011;25:1533–1539. doi: 10.3892/or.2011.1230. [DOI] [PubMed] [Google Scholar]
- 19.Xia JT, Wang H, Liang LJ, Peng BG, Wu ZF, Chen LZ, Xue L, Li Z, Li W. Overexpression of FOXM1 is associated with poor prognosis and clinicopathologic stage of pancreatic ductal adenocarcinoma. Pancreas. 2012;41:629–635. doi: 10.1097/MPA.0b013e31823bcef2. [DOI] [PubMed] [Google Scholar]
- 20.Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW, Cheung AN, Ngan HY. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis. J Pathol. 2008;215:245–252. doi: 10.1002/path.2355. [DOI] [PubMed] [Google Scholar]
- 21.Okada K, Fujiwara Y, Takahashi T, Nakamura Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori M, et al. Overexpression of forkhead box M1 transcription factor (FOXM1) is a potential prognostic marker and enhances chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol. 2013;20:1035–1043. doi: 10.1245/s10434-012-2680-0. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Qi W, Yao R, Tang D, Liang J. Overexpressed transcription factor FOXM1 is a potential diagnostic and adverse prognostic factor in postoperational gastric cancer patients. Clin Transl Oncol. 2014;16:307–314. doi: 10.1007/s12094-013-1076-3. [DOI] [PubMed] [Google Scholar]
- 23.Millour J, Constantinidou D, Stavropoulou AV, Wilson MS, Myatt SS, Kwok JM, Sivanandan K, Coombes RC, Medema RH, Hartman J, et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–2995. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madureira PA, Varshochi R, Constantinidou D, Francis RE, Coombes RC, Yao KM, Lam EW. The Forkhead box M1 protein regulates the transcription of the estrogen receptor alpha in breast cancer cells. J Biol Chem. 2006;281:25167–25176. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 25.Sanders DA, Ross-Innes CS, Beraldi D, Carroll JS, Balasubramanian S. Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol. 2013;14:R6. doi: 10.1186/gb-2013-14-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau C, Wang Y, Zhang Y, Foekens JA, Benz CC. Young age, increased tumor proliferation and FOXM1 expression predict early metastatic relapse only for endocrine-dependent breast cancers. Breast Cancer Res Treat. 2011;126:803–810. doi: 10.1007/s10549-011-1345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habashy HO, Rakha EA, Aleskandarany M, Ahmed MA, Green AR, Ellis IO, Powe DG. FOXO3a nuclear localisation is associated with good prognosis in luminal-like breast cancer. Breast Cancer Res Treat. 2011;129:11–21. doi: 10.1007/s10549-010-1161-z. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Zou L, Lu WQ, Zhang Y, Shen AG. Foxo3a expression is a prognostic marker in breast cancer. PLoS One. 2013;8:e70746. doi: 10.1371/journal.pone.0070746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Gomes AR, Monteiro LJ, Wong SY, Wu LH, Ng TT, Karadedou CT, Millour J, Ip YC, Cheung YN, et al. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One. 2010;5:e12293. doi: 10.1371/journal.pone.0012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC, Lin SH, Hu MC. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:R21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson MS, Brosens JJ, Schwenen HD, Lam EW. FOXO and FOXM1 in cancer: the FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr Drug Targets. 2011;12:1256–1266. doi: 10.2174/138945011796150244. [DOI] [PubMed] [Google Scholar]