Abstract
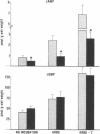
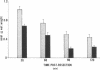
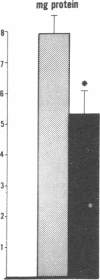
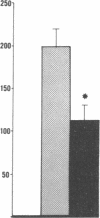
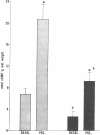
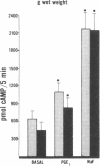
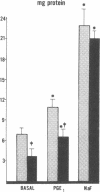
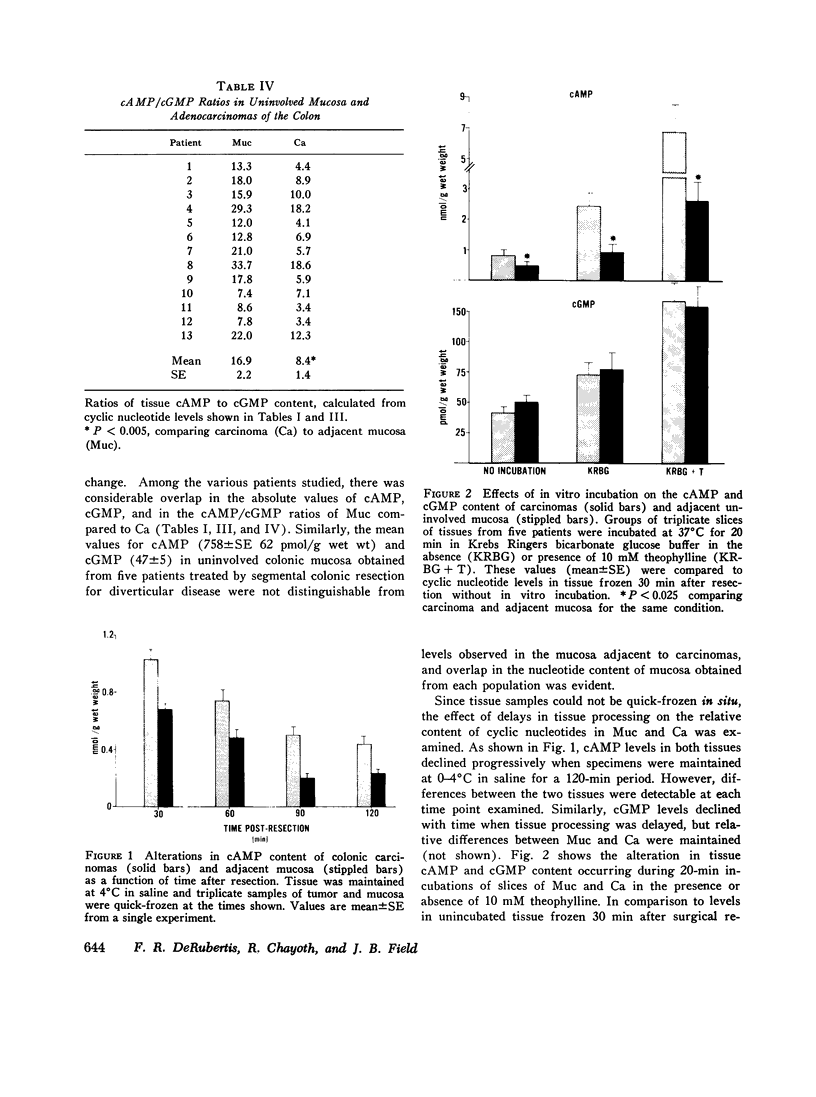
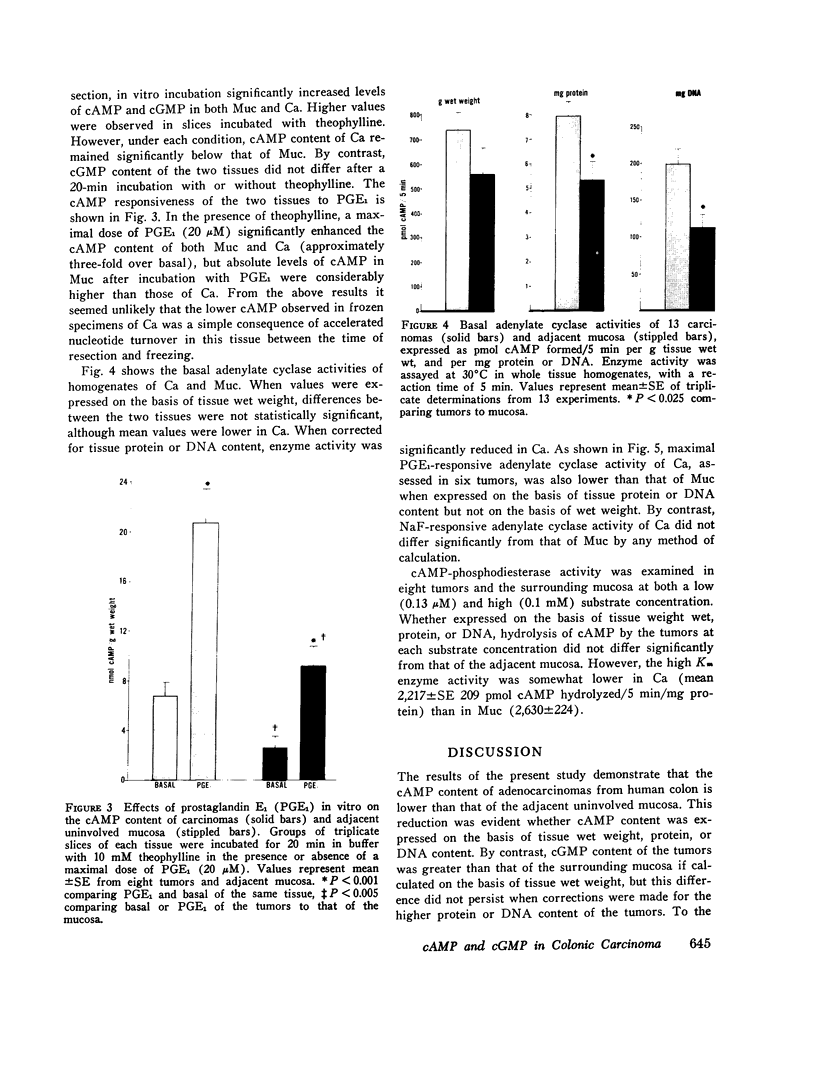
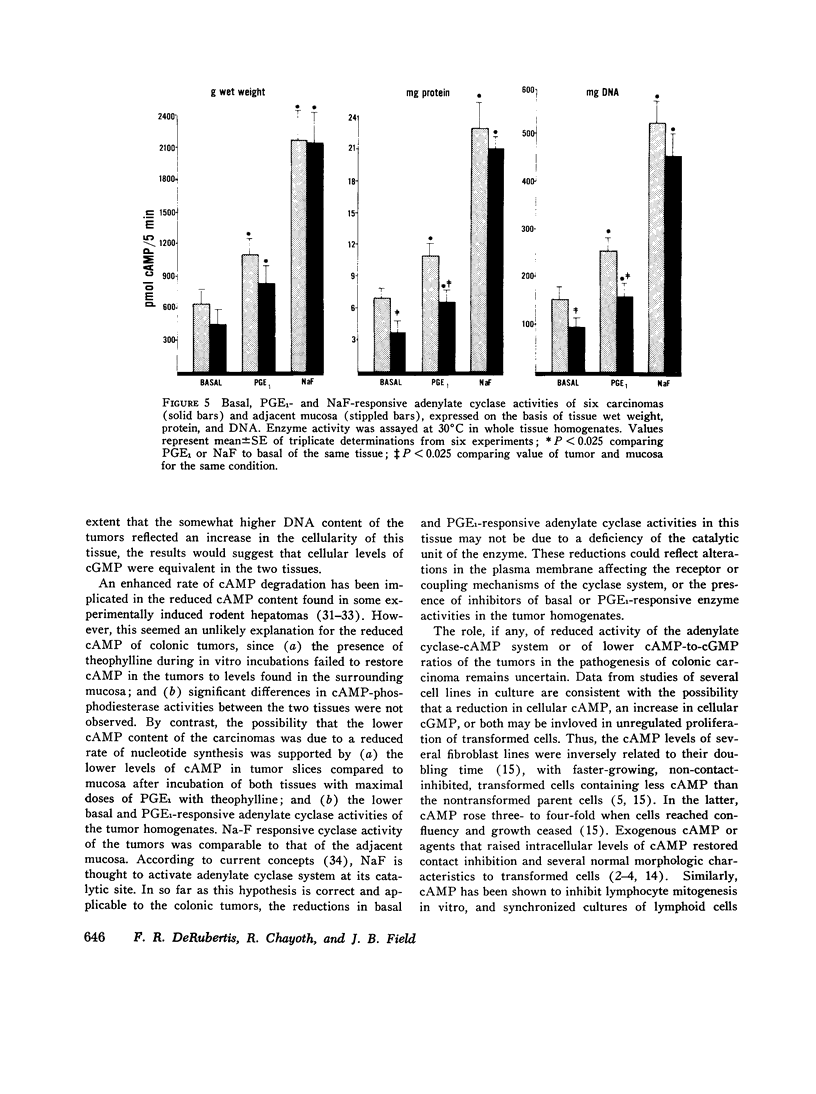
Data from cultured cells have suggested that cyclic AMP and cyclic GMP may be important determinants of cell growth and transformation. However, few studies have examined cyclic nucleotide content and metabolism in naturally occurring tumors of man. Accordingly, in the present study we compared cAMP and cGMP levels and metabolism in carcinomas of the human colon to those of the adjacent uninvolved mucosa after therapeutic resection of these tissues. The cAMP content of the tumors, determined in samples frozen 30 min after excision, was significantly lower than that of the adjacent mucosa, when expressed on the basis of tissue wet weight, protein, or DNA content. By contrast, the cGMP content of the tumors was higher than that of the surrounding mucosa if calculated on the basis of tissue wet weight, but this difference did not persist when correction was made for the higher protein or DNA content of the tumors. Incubation of slices of mucosa or tumor with or without theophylline in vitro increased tissue cAMP and cGMP content above levels observed in frozen samples of the same tissue. However, after such incubations cAMP levels in the tumors remained clearly below that of the mucosa, while cGMP content of the two tissues did not differ. The failure of theophylline to abolish differences in cAMP content and the comparable activities of high and low Km cAMP-phosphodiesterase in homogenates of the two tissues suggested that the lower cAMP content of the tumors was a consequence of diminished cAMP synthesis rather than enhanced degradation. This possibility was supported by the reduction in basal and maximal prostaglandin E1 (PGE1)-responsive adenylate cyclase activity found in tumor homogenates relative to those of mucosa, and the lower levels of cAMP in tumor slices after incubation of the tissues with a maximal dose of PGE1 and theophylline. Since NaF-responsive adenylate cyclase activity was not significantly reduced in the tumors, the lower basal and PGE1 activities may not be related to a deficiency of the catalytic unit of the cyclase complex in this tissue. The role of reduced activity of the adenylate cyclase-cAMP system and/or reduced tissue cAMP-to-cGMP ratios in the pathogenesis of colonic carcinoma is uncertain, but these changes might favor unregulated cellular proliferation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Gallo M., Pastan I. Adenylate cyclase activity in fibroblasts transformed by Kirsten or Moloney sarcoma viruses. Decreased activity and loss of response to prostaglandin E1. J Biol Chem. 1974 Nov 25;249(22):7041–7048. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher F. R., Scott D. F., Potter V. R., Morris H. P. Endocrine control of cyclic adenosine 3',5'-monophosphate levels in several Morris hepatomas. Cancer Res. 1972 Oct;32(10):2135–2140. [PubMed] [Google Scholar]
- Chayroth R., Epstein S., Field J. B. Increased cyclic AMP levels in malignant hepatic nodules of ethionine treated rats. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1663–1670. doi: 10.1016/0006-291x(72)90534-7. [DOI] [PubMed] [Google Scholar]
- Clark J. F., Morris H. P., Weber G. Cyclic adenosine 3',5'-monophosphate phosphodiesterase activity in normal, differentiating, regenerating, and neoplastic liver. Cancer Res. 1973 Feb;33(2):356–361. [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Zor U., Field J. B. Evidence for persistent binding of biologically active thyrotropin to thyroid in vitro. Endocrinology. 1975 Jun;96(6):1579–1586. doi: 10.1210/endo-96-6-1579. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Adler W. H., Hudson T. Role of cyclic adenosine 3',5'-monophosphate in lymphocyte mitogenesis. J Immunol. 1974 Jul;113(1):151–161. [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Curnow R. T. Inhibition of glucagon-mediated increases in hepatic cyclic adenosine 3', 5'-monophosphate by prostaglandin E1 and E2. Endocrinology. 1974 Jul;95(1):93–101. doi: 10.1210/endo-95-1-93. [DOI] [PubMed] [Google Scholar]
- DeRubertis F., Yamashita K., Dekker A., Larsen P. R., Field J. B. Effects of thyroid-stimulating hormone on adenyl cyclase activity and intermediary metabolism of "cold" thyroid nodules and normal human thyroid tissue. J Clin Invest. 1972 May;51(5):1109–1117. doi: 10.1172/JCI106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J. B., Larsen P. R., Yamashita K., Mashiter K., Dekker A. Demonstration of iodide transport defect but normal iodide organification in nonfunctioning nodules of human thyroid glands. J Clin Invest. 1973 Oct;52(10):2404–2410. doi: 10.1172/JCI107430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Burke G. C., Morris H. P. Cyclic AMP and cyclic GMP content and binding in malignancy. Biochem Biophys Res Commun. 1975 Jan 20;62(2):320–327. doi: 10.1016/s0006-291x(75)80141-0. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Granner D. K. Protein kinase: altered regulation in a hepatoma cell line deficient in adenosine 3',5'-cyclic monophosphate-binding protein. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1516–1522. doi: 10.1016/0006-291x(72)90779-6. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie R. A., Walker C. M., Croll G. A. Decreased basal cyclic adenosine 3',5'-monophosphate levels in Morris hepatoma 5123 t.c. (h). Biochem Biophys Res Commun. 1974 Jul 10;59(1):167–173. doi: 10.1016/s0006-291x(74)80189-0. [DOI] [PubMed] [Google Scholar]
- Hickie R. A., Walker C. M., Datta A. Increased activity of low-Km cyclic adenosine 3':5'-monophosphate phosphodiesterase in plasma membranes of Morris hepatoma 5123tc (h). Cancer Res. 1975 Mar;35(3):601–605. [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Murad F. Increased particulate and decreased soluble guanylate cyclase activity in regenerating liver, fetal liver, and hepatoma. Proc Natl Acad Sci U S A. 1975 May;72(5):1965–1969. doi: 10.1073/pnas.72.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Tomkins G. M. Pleiotypic control by cyclic AMP: interaction with cyclic GMP and possible role of microtubules. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1659–1663. doi: 10.1073/pnas.70.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Millis A. J., Forrest G., Pious D. A. Cyclic AMP in cultured human lymphoid cells: relationship to mitosis. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1645–1649. doi: 10.1016/0006-291x(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Moens W., Vokaer A., Kram R. Cyclic AMP and cyclic GMP concentrations in serum- and density-restricted fibroblast cultures. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1063–1067. doi: 10.1073/pnas.72.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Rhoads A. R., Morris H. P., West W. L. Cyclic 3',5'-nucleotide monophosphate phosphodiesterase activity in hepatomas of different growth rates. Cancer Res. 1972 Dec;32(12):2651–2655. [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Ryan W. L., Heidrick M. L. Inhibition of cell growth in vitro by adenosine 3',5'-monophosphate. Science. 1968 Dec 27;162(3861):1484–1485. doi: 10.1126/science.162.3861.1484. [DOI] [PubMed] [Google Scholar]
- Schorr I., Rathnam P., Saxena B. B., Ney R. L. Multiple specific hormone receptors in the adenylate cyclase of an adrenocortical carcinoma. J Biol Chem. 1971 Sep 25;246(18):5806–5811. [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Ware P. Adenylate cyclase in intestinal crypt and villus cells: stimulation by cholera enterotoxin and prostaglandin E1. Gastroenterology. 1975 Jan;68(1):94–104. [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Thomas E. W., Murad F., Looney W. B., Morris H. P. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate: concentrations in Morris hepatomas of different growth rates. Biochim Biophys Acta. 1973 Feb 28;297(2):564–567. doi: 10.1016/0304-4165(73)90106-2. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Watson J. The influence of intracellular levels of cyclic nucleotides on cell proliferation and the induction of antibody synthesis. J Exp Med. 1975 Jan 1;141(1):97–111. doi: 10.1084/jem.141.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Chambers D. A., Bourne H. R., Melmon K. L. Cyclic GMP stimulates lymphocyte nucleic acid synthesis. Nature. 1974 Sep 27;251(5473):352–353. doi: 10.1038/251352a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Johnson G. S., Pastan I. Control of DNA synthesis and mitosis in 3T3 cells by cyclic AMP. Biochem Biophys Res Commun. 1972 Aug 21;48(4):743–748. doi: 10.1016/0006-291x(72)90669-9. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic AMP modulates microvillus formation and agglutinability in transformed and normal mouse fibroblasts. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1263–1267. doi: 10.1073/pnas.72.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk R., Wicks W. D., Bevers M. M., van Rijn J. Rapid arrest of DNA synthesis by N 6 ,O 2' -dibutyryl cyclic adenosine 3',5'-monophosphate in cultured hepatoma cells. Cancer Res. 1973 Jun;33(6):1331–1338. [PubMed] [Google Scholar]