Abstract
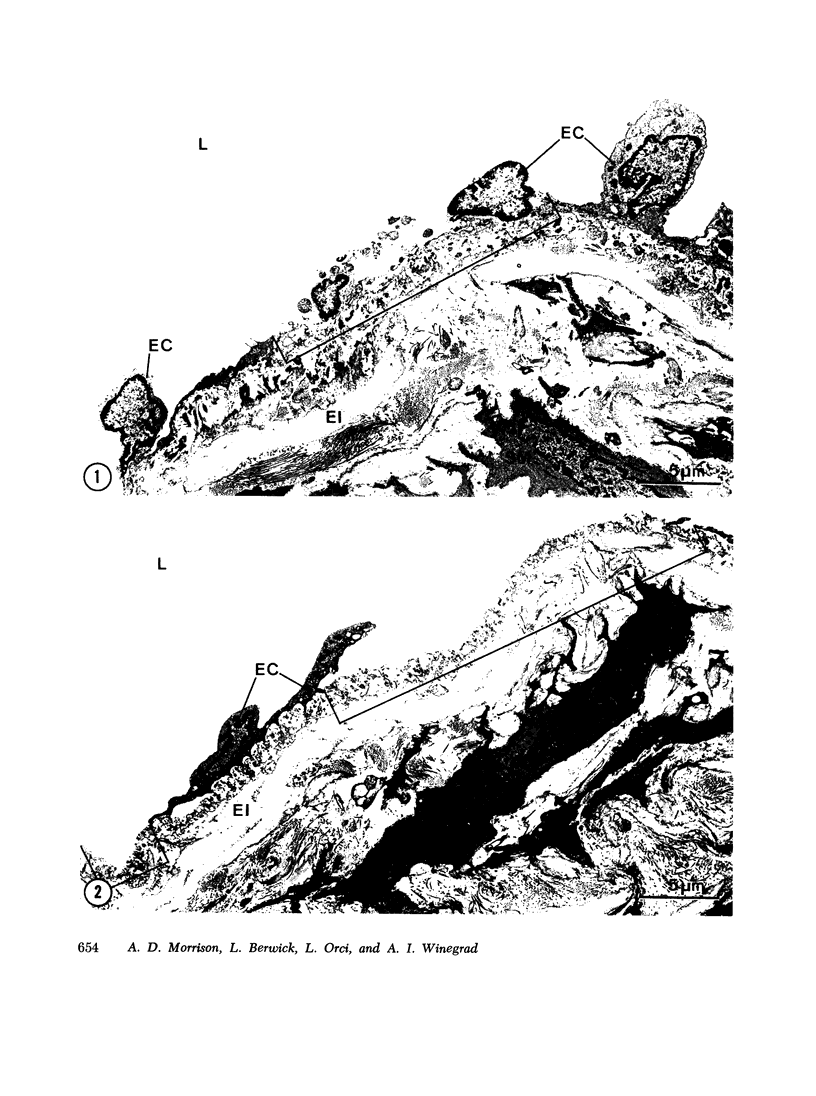
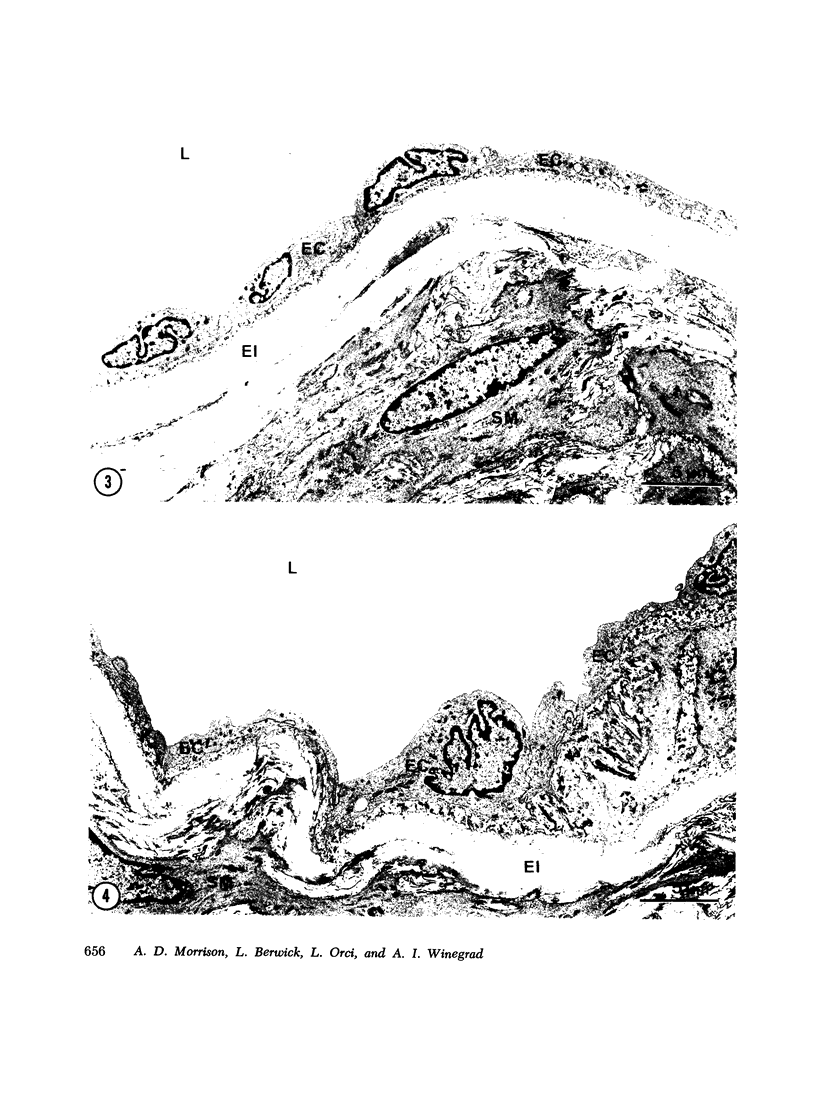
An in vitro preparation of rabbit aortic "intima-media" previously shown to exhibit stable rates of respiration and glucose metabolism and the high rate of aerobic glycolysis considered characteristic of the metabolism of this tissue was subjected to electron microscopic examination. In samples examined immediately after the aortae were dissected free of adipose tissue and adventitia, under conditions similar to those now in common use, marked and widespread alterations in endothelial cell structure were present, including loss of cell integrity. The vascular smooth muscle cells retained a normal electron microscopic (EM) appearance. During subsequent incubation with 5 mM glucose in Krebs-Ringer bicarbonate (KRB), pH 7.4, under the conditions usually employed in studies of this preparation, large zones of the luminal surface were rapidly denuded of endothelium, and the remaining endothelial cells exhibited a wide range of ultrastructural alterations. The smooth muscle cells, however, continued to maintain a normal EM appearance. A method was developed to prepare segments of rabbit aortic intima-media which retained an intact layer of endothelium resembling that observed in tissue fixed in situ. During a 1-h incubation with 5 mM glucose in KRB, pH 7.4, gas phase 5% CO2/95% O2, containing 6% bovine serum albumin, the intact aortic intima-media preparation retains an essentially unmodified EM appearance and exhibits linear rates of respiration. Under these conditions the intact aortic intima-media preparation exhibits significantly higher rates of O2 uptake and glucose uptake than those observed in our previous preparation or in other reported aortic intima-media preparations. The intact aortic intima-media does not exhibit the high rate of aerobic glycolysis during in vitro incubation that has been considered characteristic of the metabolism of rabbit, rat, and swine aortic intima-media. In addition, the magnitude of the Pasteur effect was far greater than that observed in other aortic intima-media preparations. The data suggest that component cells of the aortic intima-media may derive a major fraction of their energy requirements from respiration; they raise further questions concerning the significance of the high rate of aerobic glycolysis observed when aortic intima-media preparations are incubated in vitro, and they suggest that documentation of the EM appearance of the endothelium in such preparations is desirable.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnqvist H. J. Studies of monosaccharide permeability of arterial tissue and intestinal smooth muscle; effects of insulin. Acta Physiol Scand. 1971 Oct;83(2):247–256. doi: 10.1111/j.1748-1716.1971.tb05074.x. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Morrison A. D., Winegrad A. I. Polyol pathway in aorta: regulation by hormones. Science. 1969 Nov 21;166(3908):1007–1008. doi: 10.1126/science.166.3908.1007. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- Morrison A. D., Clements R. S., Jr, Winegrad A. I. Effects of elevated glucose concentrations on the metabolism of the aortic wall. J Clin Invest. 1972 Dec;51(12):3114–3123. doi: 10.1172/JCI107138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. D., Goodman D. B., Rasmussen H., Winegrad A. I. Gluconeogenesis in toad urinary bladder. Biochim Biophys Acta. 1972 Jun 26;273(1):122–131. doi: 10.1016/0304-4165(72)90199-7. [DOI] [PubMed] [Google Scholar]
- Morrison E. S., Scott R. F., Kroms M., Frick J. Glucose degradation in normal and atherosclerotic aortic intima-media. Atherosclerosis. 1972 Sep-Oct;16(2):175–184. doi: 10.1016/0021-9150(72)90051-2. [DOI] [PubMed] [Google Scholar]
- Morrison E. S., Scott R. F., Kroms M., Frick J. Uptake, oxidation, and esterification of free fatty acids in normal and atherosclerotic rabbit aorta. Biochem Med. 1974 Oct;11(2):153–164. doi: 10.1016/0006-2944(74)90108-2. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Scott R. F., Morrison E. S., Kroms M. Aortic respiration and glycolysis in the pre-proliferative phase of diet-induced atherosclerosis in swine. J Atheroscler Res. 1969 Jan-Feb;9(1):5–16. doi: 10.1016/s0368-1319(69)80061-x. [DOI] [PubMed] [Google Scholar]
- Scott R. F., Morrison E. S., Kroms M. Effect of cold shock on respiration and glycolysis in swine arterial tissue. Am J Physiol. 1970 Nov;219(5):1363–1365. doi: 10.1152/ajplegacy.1970.219.5.1363. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. II. Pharmacology of normal and hypotensive vessels. Pharmacol Rev. 1970 Jun;22(2):249–353. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- URRUTIA G., BEAVAN D. W., CAHILL G. F., Jr Metabolism of glucose-U-C-14 in rat aorta in vitro. Metabolism. 1962 May;11:530–534. [PubMed] [Google Scholar]
- Vost A. Lipid accretion in the perfused rabbit aorta. J Atheroscler Res. 1969 May-Jun;9(3):221–238. doi: 10.1016/s0368-1319(69)80018-9. [DOI] [PubMed] [Google Scholar]
- WERTHEIMER H. E., BENTOR V. Influence of diabetes on carbohydrate metabolism of aortic tissue. Diabetes. 1962 Sep-Oct;11:422–425. [PubMed] [Google Scholar]
- WHEREAT A. F. Oxygen consumption of normal and atherosclerotic intima. Circ Res. 1961 May;9:571–575. doi: 10.1161/01.res.9.3.571. [DOI] [PubMed] [Google Scholar]
- Whereat A. F. Recent advances in experimental and molecular pathology. Atherosclerosis and metabolic disorder in the arterial wall. Exp Mol Pathol. 1967 Oct;7(2):233–247. doi: 10.1016/0014-4800(67)90032-9. [DOI] [PubMed] [Google Scholar]
- YALCIN S., WINEGRAD A. I. DEFECT IN GLUCOSE METABOLISM IN AORTIC TISSUE FROM ALLOXAN DIABETIC RABBITS. Am J Physiol. 1963 Dec;205:1253–1259. doi: 10.1152/ajplegacy.1963.205.6.1253. [DOI] [PubMed] [Google Scholar]







