Abstract
Background:
Viral hepatitis is an infection that has been reported to be present throughout the year, but some particular months are associated with higher incidences. The primary objective was to review and report on the current knowledge and evidence that existed on seasonality of different type of acute viral hepatitis worldwide in order to develop recommendations for future research, prevention and control.
Materials and Methods:
A systematic literature review was performed to identify all the primary reports and studies published during 1970-2013 on acute hepatitis A, B, C and E (AHA, AHB, AHC and AHE) in human subjects by searching PubMed, reference lists of major articles and correspondence with scientific experts. For each report or study included, the following information was extracted (as applicable to study): Location (country and setting), study population (number of cases, patients), seasonal or monthly rate and study duration.
Results:
There is no definite and consistent seasonal pattern has been observed on AHA; AHB; AHE and AHC, although evidence points towards spring and summer peak for hepatitis A, B, C and E. Multiple source of transmission such as; summer travel to an endemic area, swimming habits of the population in hot months, increase sexual contact, tattoo, poor hygiene and environmental sanitation and food habits (feco-oral transmission of viral hepatitis) probably exists and should be further investigated through analytical and epidemiological.
Keywords: Hepatitis, periodicity, seasons
Introduction
Acute viral hepatitis is a systemic infection affecting the liver predominantly. Almost all the cases of acute viral hepatitis are caused by one of five viral agents: Hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus and hepatitis E virus (HEV). Hepatitis may occur with limited or no symptoms, but often leads to jaundice, anorexia and malaise. Clinically classified the hepatitis in two form acute and chronic; hepatitis is acute when it lasts <6 months and chronic when it persists longer.
Hepatitis A virus and HEV are acquired by consuming food and water contaminated by the virus (excreted in-patient's stool) by way of the fecal-oral. HBV, HCV are contagious by blood and blood products, also may be transmitted by sexual contact. Vaccine for hepatitis A and B is available, but no vaccine is available for hepatitis C or E.
Viral hepatitis infection has been reported to be present throughout the year, but some particular months are associated with higher incidences in most of the countries around the world. The exact reasons why hepatitis infection cases present in one season more than other are not completely understand. However, several researchers have suggested that the climatic and behavioral factors such as, summer travel to endemic area, swimming habits of the population in hot months, increase in sexual contact, tattoo, poor hygiene and environmental sanitation and food habits (feco-oral transmission of viral hepatitis) may play a significant role in the seasonal appearance of diseases.[1,2,3]
However, none have documented a systematic review process or specifically focused on global seasonal patterns of viral hepatitis. Our objectives were to determine the seasonal pattern of viral hepatitis and possible seasonal trigger factors. We systematically reviewed the scientific literature to give an overview of the evidence available from the last 42 years and make recommendations for future research and prevention and control.
Materials and Methods
A systematic review of the literature was performed to identify all the primary reports and studies that have been published between January 1970 and September 2013, conducted on the prevalence and incidence of HAV, HBV, HCV and HEV infections in human subjects. The primary objective was to review and report on the current knowledge and evidence that exists on seasonality of different type of hepatitis worldwide. Studies were included if (i) they had been conducted continuously for a minimum of a full-year to cover all seasons, (ii) the primary outcome was a clinical and laboratory confirmed diagnosis of the diseases of interest in human subjects, (iii) the study reported case data temporally (day, week, month) (iv) the study was written in English and published in a peer reviewed journal, (v) study design did not include intervention trials or deals with chronic hepatitis and (vi) diagnosis of viral hepatitis infection was laboratory-confirmed using recognized tests. If one of the four criteria was not met, the report was excluded. For each report or study included, the following information was extracted (as applicable to study): Location (country and setting), study population (number of cases, patients), seasonal or monthly rate and study duration.
The studies were identified using electronic search of the United States National Library of Medicine and the National Institutes of Health Medical Database (PubMed) from its inception was conducted in September 2012. Search words used were: “Periodicity” and “Seasons” and “hepatitis.” This list was extended by including also references from a recent review of hepatitis epidemiology and correspondence with scientific experts.
As a majority of the data in this review came from countries in the northern hemisphere, seasons were defined based on their occurrence in the Northern hemisphere: Winter (December-February), spring (March-May), summer (June-August) and autumn (September-November). For the countries located in the southern hemisphere like Brazil, data for December, January, February comprised the summer season; data for March, April and May the fall season; June, July and August the winter season; and September, October and November the spring season.[3] Due to lack of consistency among the statistical methods used in most of the studies and limitation of data provide. Therefore, it was not possible to do statistical analysis to integrate the results.
Results
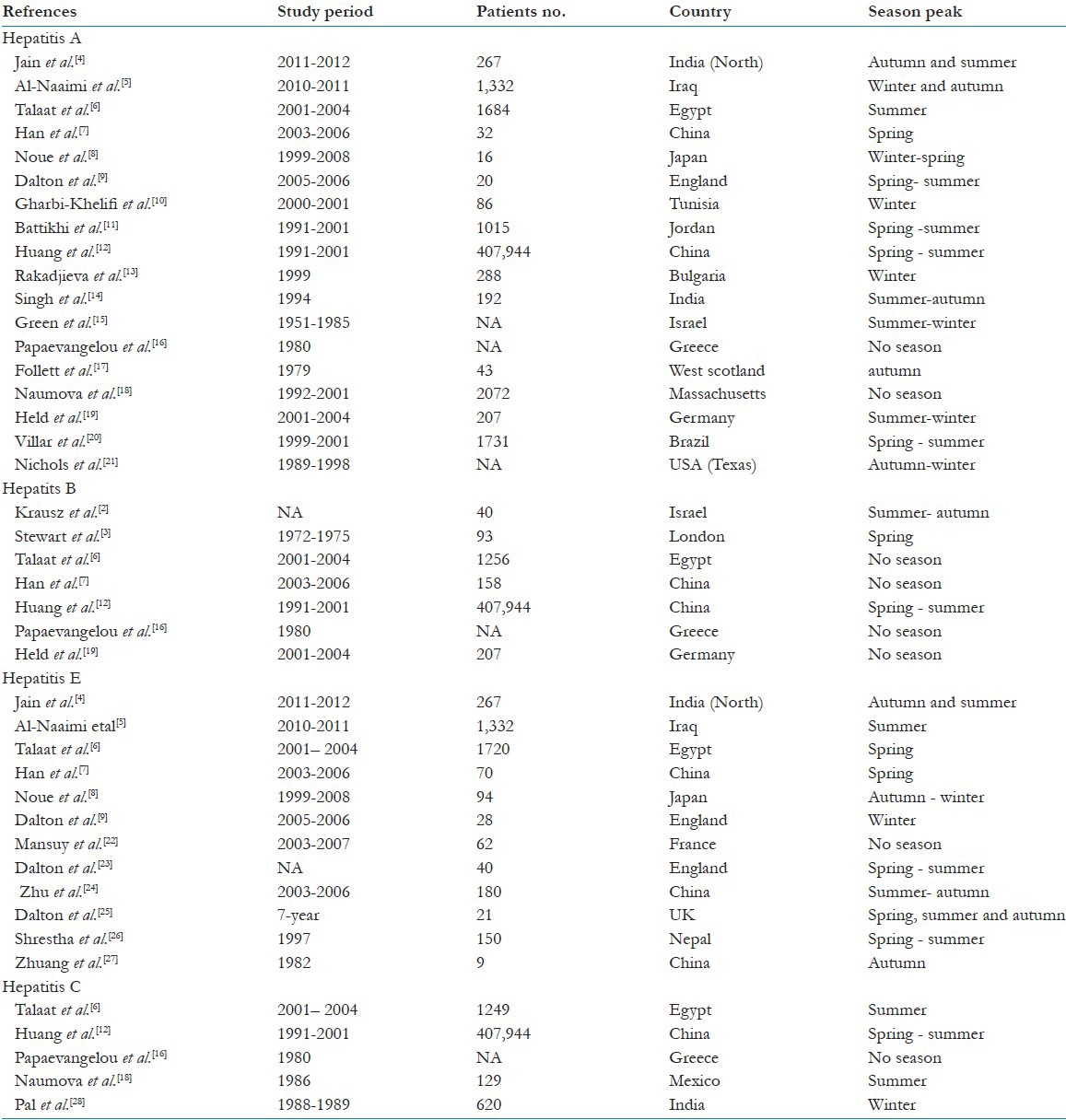
Across four types of viral hepatitis infection (HAV, HBV, HCV and HEV), a total of 389 titles and abstracts was screened for eligibility. Of these, 28 studies from 18 countries conducted across 1970–2013 met the selection criteria and contributed to the systematic review. Key data from these studies are summarized in [Table 1].[4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] Three studies were included in this review does not contain information about the number of participants and the duration of studies were not available in three selected studies. Form a total of studies included in this review, 18 articles included results from patients with acute hepatitis A, eight studies reported on acute hepatitis B, 12 studies on acute hepatitis E (AHE) and five studies on acute hepatitis C (AHC). The seasonal distribution of viral hepatitis A, B, C and E showed a variable peak around the year with most prominent peak in the spring and summer in most of the countries subjects. For the viral hepatitis B infection, found no evidence of seasonality in half of studies included in this review.
Table 1.
Global seasonality of acute viral hepatitis
Discussion
Based on data from 28 studies, irregular, cyclical patterns were observed for acute viral hepatitis, with most prominent peaks were shown in spring and summer for hepatitis A; B; C and E in some of the countries subjects. These findings have been explained by some authors as due to summer travel to an endemic area, swimming habits of the population in hot months, increase sexual contact, tattoo, poor hygiene and environmental sanitation, and food habits (feco-oral transmission of viral hepatitis).[5,20] HAV and HEV are communicable by way of the fecal-oral. On the other hand, HBV, HCV are contagious by blood and blood products, also may be transmitted by sexual intercourse and household exposure to an infected contact, exposure to multiple partners and perinatal exposure, particularly for hepatitis C, but the efficiency of transmission in these settings appears to be low.
Hepatitis A virus is an extremely stable virus and can survive for 12 weeks to 10 months in water.[29,30] This stability accounts for the frequent occurrence of waterborne and shellfish-transmitted outbreaks.[31,32,33] In this regard, the virus is relatively resistant to heat or chemical inactivation and this situation allow the dissemination of HAV infection. Therefore, disruption of sanitation and water supplies was the most likely contributing factor for the seasonal occurrence of hepatitis A and E. A number of case reports has described water exposure as a possible risk factor for acquiring HEV infection: Swimming in a lake,[34] through exposure to water from a high-pressure hose and water sourced from a stream which ran adjacent to a free-range pig farm.[35] In The Netherlands 2/12 (17%) of monthly samples of river water used for drinking water production and recreation were found positive for HEV ribonucleic acid in two separate years.[36] In south India, HEV strains isolated from sewage showed 94% to 100% nucleotide sequence similarity with the HEV strains isolated from the sporadic hepatitis cases. HEV RNA in sewage was identified more often during the summer (81.2%) than the monsoon season (14.5%) (P < 0.001).[37]
Furthermore, the peak incidence of HAV infection in the coast of Rio de Janeiro in Brazil was found during the rainy season. These findings indicate that the HAV infections transmitted indirectly through rainfall because these rains usually fill up the rivers so they could overflow and the persons could be contaminated with these waters. Other possible explanation is the swim habits of the population studied. All of them usually frequented the beaches of the Rio de Janeiro. The coast of Rio de Janeiro is very large and the city has a lot of rivers and waterfalls. Persons that lived at Rio de Janeiro usually swim at the beaches and rivers of the city. During the rainy season, the heavy runoff could cause contamination of these waters leading to excess of HAV infection during this season.[20] The observation in some countries that the peak of the epidemic was during the winter months suggests that transmission of HAV and HEV is related to shellfish consumption. HAV infection is the most serious viral infection linked to shellfish consumption, causing a debilitating disease and occasionally, death. The first documented outbreak of “infectious hepatitis” occurred in Sweden in 1955, when 629 cases were associated with raw oyster consumption.[1] Subsequently, many HAV-associated outbreaks have been reported worldwide. The most demonstrative one was the outbreak of HAV infection in Shanghai, China, in 1988, in which almost 300,000 cases were linked to the consumption of clams harvested from a sewage-polluted area.[38,39] The fairly protracted incubation period (mean duration, 4 weeks) of HAV infection makes it very difficult to determine the association with a particular food vehicle in sporadic cases. Normally, the food will not be available for testing at the time of diagnosis. Thus, it can be safely assumed that HAV infection associated with shellfish consumption is probably underreported. In addition, as shellfish consumption was found to be possibly one of the risk factors involved in AHE AHC it is now suspected that exposure to sewage or wastewater may contribute to HEV endemicity.[40,41]
Travel history and sexual activity, which is supposed to be higher in summer, may give us explanation regarding to the summer seasonal peak of acute viral hepatitis. In a study conducted in UK found that the higher sexual activity and unsafe sex coinciding with the summer vacation. Data relating to sexually transmitted infections and attendances for human immunodeficiency virus tests similarly show an increase in the months following Christmas and in late summer.[42]
Injection drug use is the primary mode of transmission for HCV and HBV infection in the developed world. In one study conducted in USA, seroprevalence of HCV and HBV infection among long-term injection drug users were 85.0% for HCV and 77.4% for HBV and 64.7%, 49.8%, among those who had injected for 1 year or less.[43] Interestingly, seasonality of illicit drug has been reported to be higher in winter than in summer among the USA teens (12–17 years of age). In another study conducted in Australia using arrest data found evidence of higher arrest rates for drug possession and use during the Australian fall and winter (April through July) and lower rates during the Australian spring (November and December).[44] Additionally, a recent longitudinal study of cocaine and cocaine metabolites in wastewater reveal a clear seasonal difference indicative of human seasonal cocaine use patterns.[45] This observation could be a help to explaining seasonal occurrence of HCV or HBV.
Regarding seasonal variation of the occurrence of hepatitis B, some authors detected a peak in either spring or summer, whereas, others found no seasonal peak. Vaccination against hepatitis B has been recommended in most of the countries for all newborns, infants and particular risk groups. This gives us an explanation for the reduction in the incidence of hepatitis B infection and consequently, reduce seasonal incidence peak in general. Furthermore, seasonal exposure to dietary aflatoxin has been reported to be associated with acutely and chronically HBV-infected patients with most prominent during April-July.[46,47]
Finally, the incubation period from time of exposure to the onset of symptoms can range from 2 weeks to 6 months and infection can be self-limited or chronic. Therefore, theoretically people who are infected in autumn or winter can develop active hepatitis shortly after infections are on average diagnosed 1–6 months after the first onset of symptom, resulting in a higher number of notifications in spring and summer. Furthermore, the number of unnotified or viral carrier cases is not known. These factors are potential confounders in the study of viral hepatitis seasonality variation.
In summary, this first systematic review on the seasonal variability of human viral hepatitis found that there is no definite and consistent seasonal pattern has been observed on viral hepatitis, although evidence points toward spring and summer peak. Multiple sources of transmission are likely to exist and these need to be further defined so that specific and appropriate preventive measures can be implemented.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Roos B. Hepatitis epidemic transmitted by oysters. Sven Lakartidn. 1956;53:989–1003. [PubMed] [Google Scholar]
- 2.Krausz Y, Melamed S, Sandler SG, Eliakim M. A comparative study of hepatitis B and non-B in hospitalized adults in an endemic area. Isr J Med Sci. 1977;13:9–14. [PubMed] [Google Scholar]
- 3.Stewart JS, Farrow LJ, Clifford RE, Lamb SG, Coghill NF, Lindon RL, et al. A three-year survey of viral hepatitis in West London. Q J Med. 1978;47:365–84. [PubMed] [Google Scholar]
- 4.Jain P, Prakash S, Gupta S, Singh KP, Shrivastava S, Singh DD, et al. Prevalence of hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus and hepatitis E virus as causes of acute viral hepatitis in North India: A hospital based study. Indian J Med Microbiol. 2013;31:261–5. doi: 10.4103/0255-0857.115631. [DOI] [PubMed] [Google Scholar]
- 5.Al-Naaimi AS, Turky AM, Khaleel HA, Jalil RW, Mekhlef OA, Kareem SA, et al. Predicting acute viral hepatitis serum markers (A and E) in patients with suspected acute viral hepatitis attending primary health care centers in Baghdad: A one year cross-sectional study. Glob J Health Sci. 2012;4:172–83. doi: 10.5539/gjhs.v4n5p172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talaat M, El-Sayed N, Kandeel A, Azab MA, Afifi S, Youssef FG, et al. Sentinel surveillance for patients with acute hepatitis in Egypt, 2001-04. East Mediterr Health J. 2010;16:134–40. [PubMed] [Google Scholar]
- 7.Han YN. Identification of acute self-limited hepatitis B among patients presenting with hepatitis B virus-related acute hepatitis: A hospital-based epidemiological and clinical study. J Int Med Res. 2009;37:1952–60. doi: 10.1177/147323000903700633. [DOI] [PubMed] [Google Scholar]
- 8.Inoue J, Ueno Y, Nagasaki F, Akahane T, Fukushima K, Kogure T, et al. Sporadic acute hepatitis E occurred constantly during the last decade in northeast Japan. J Gastroenterol. 2009;44:329–37. doi: 10.1007/s00535-009-0012-3. [DOI] [PubMed] [Google Scholar]
- 9.Dalton HR, Stableforth W, Hazeldine S, Thurairajah P, Ramnarace R, Warshow U, et al. Autochthonous hepatitis E in Southwest England: A comparison with hepatitis A. Eur J Clin Microbiol Infect Dis. 2008;27:579–85. doi: 10.1007/s10096-008-0480-z. [DOI] [PubMed] [Google Scholar]
- 10.Gharbi-Khelifi H, Sdiri K, Ferre V, Harrath R, Berthome M, Billaudel S, et al. A 1-year study of the epidemiology of hepatitis A virus in Tunisia. Clin Microbiol Infect. 2007;13:25–32. doi: 10.1111/j.1469-0691.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- 11.Battikhi MN, Battikhi EG. The seroepidemiology of Hepatitis A virus in Amman, Jordan. New Microbiol. 2004;27:215–20. [PubMed] [Google Scholar]
- 12.Huang P, Ye G, Zhong J, Sha Q. Assessment of current epidemiological status of viral hepatitis in Guangdong Province, China. Southeast Asian J Trop Med Public Health. 2002;33:832–6. [PubMed] [Google Scholar]
- 13.Rakadjieva T, Stoilova J, Petrov A. A study on the basic epidemiological parameters of viral hepatitis a in the region of Plovdiv, Bulgaria. Folia Med (Plovdiv) 2002;44:11–4. [PubMed] [Google Scholar]
- 14.Singh J, Prakash C, Gupta RS, Bora D, Jain DC, Datta KK. Epidemiology of endemic viral hepatitis in an urban area of India: A retrospective community study in Alwar. Bull World Health Organ. 1997;75:463–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Green MS, Block C, Slater PE. Rise in the incidence of viral hepatitis in Israel despite improved socioeconomic conditions. Rev Infect Dis. 1989;11:464–9. doi: 10.1093/clinids/11.3.464. [DOI] [PubMed] [Google Scholar]
- 16.Papaevangelou G, Roumeliotou-Karayannis A, Contoyannis P. Changing epidemiological characteristics of acute viral hepatitis in Greece. Infection. 1982;10:1–4. doi: 10.1007/BF01640826. [DOI] [PubMed] [Google Scholar]
- 17.Follett EA, McMichael S. Acute hepatitis A infection in West Scotland. Scott Med J. 1981;26:135–7. doi: 10.1177/003693308102600209. [DOI] [PubMed] [Google Scholar]
- 18.Naumova EN, Jagai JS, Matyas B, DeMaria A, Jr, MacNeill IB, Griffiths JK. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect. 2007;135:281–92. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Held L, Hofmann M, Höhle M, Schmid V. A two-component model for counts of infectious diseases. Biostatistics. 2006;7:422–37. doi: 10.1093/biostatistics/kxj016. [DOI] [PubMed] [Google Scholar]
- 20.Villar LM, De Paula VS, Gaspar AM. Seasonal variation of hepatitis A virus infection in the city of Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo. 2002;44:289–92. doi: 10.1590/s0036-46652002000500011. [DOI] [PubMed] [Google Scholar]
- 21.Nichols K, Brender J. Seasonality and urban/rural differences in hepatitis a incidence, Texas, 1989-1998. Tex Public Health Assoc J. 2005;56:3. [Google Scholar]
- 22.Mansuy JM, Abravanel F, Miedouge M, Mengelle C, Merviel C, Dubois M, et al. Acute hepatitis E in south-west France over a 5-year period. J Clin Virol. 2009;44:74–7. doi: 10.1016/j.jcv.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, et al. Autochthonous hepatitis E in Southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–90. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- 24.Zhu G, Qu Y, Jin N, Sun Z, Liu T, Lee H, et al. Seroepidemiology and molecular characterization of hepatitis E virus in Jilin, China. Infection. 2008;36:140–6. doi: 10.1007/s15010-007-7130-8. [DOI] [PubMed] [Google Scholar]
- 25.Dalton HR, Thurairajah PH, Fellows HJ, Hussaini HS, Mitchell J, Bendall R, et al. Autochthonous hepatitis E in southwest England. J Viral Hepat. 2007;14:304–9. doi: 10.1111/j.1365-2893.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 26.Shrestha SM, Shrestha S, Tsuda F, Nishizawa T, Gotanda Y, Takeda N, et al. Molecular investigation of hepatitis E virus infection in patients with acute hepatitis in Kathmandu, Nepal. J Med Virol. 2003;69:207–14. doi: 10.1002/jmv.10276. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang H, Cao XY, Liu CB, Wang GM. Epidemiology of hepatitis E in China. Gastroenterol Jpn. 1991;26(Suppl 3):135–8. doi: 10.1007/BF02779283. [DOI] [PubMed] [Google Scholar]
- 28.Pal PK, Haldar A, Bhattacharya SK. An outbreak of viral hepatitis in a housing complex of north Calcutta. J Commun Dis. 1994;26:88–91. [PubMed] [Google Scholar]
- 29.Biziagos E, Passagot J, Crance JM, Deloince R. Long-term survival of hepatitis A virus and poliovirus type 1 in mineral water. Appl Environ Microbiol. 1988;54:2705–10. doi: 10.1128/aem.54.11.2705-2710.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattar SA, Jason T, Bidawid S, Farber J. Foodborne spread of hepatitis A: Recent studies on virus survival, transfer and inactivation. Can J Infect Dis. 2000;11:159–63. doi: 10.1155/2000/805156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velázquez O, Stetler HC, Avila C, Ornelas G, Alvarez C, Hadler SC, et al. Epidemic transmission of enterically transmitted non-A, non-B hepatitis in Mexico, 1986-1987. JAMA. 1990;263:3281–5. [PubMed] [Google Scholar]
- 32.Leoni E, Bevini C, Degli Esposti S, Graziano A. An outbreak of intrafamiliar hepatitis A associated with clam consumption: Epidemic transmission to a school community. Eur J Epidemiol. 1998;14:187–92. doi: 10.1023/a:1007441106534. [DOI] [PubMed] [Google Scholar]
- 33.Yao G. Clinical spectrum and natural history of viral hepatitis A in a 1988 Shangai epidemic. In: Hollinger BN, Lemon SM, Margolis HS, editors. Viral Hepatitis and Liver Disease. Baltimore: Williams and Wilkins; 1991. pp. 76–8. [Google Scholar]
- 34.Moucari R, Bernuau J, Nicand E, Cazals-hatem D, Valla D, Marcellin P, et al. Acute hepatitis E with severe jaundice: Report of three cases. Eur J Gastroenterol Hepatol. 2007;19:1012–5. doi: 10.1097/MEG.0b013e328209414d. [DOI] [PubMed] [Google Scholar]
- 35.Lockwood GL, Fernandez-Barredo S, Bendall R, Banks M, Ijaz S, Dalton HR. Hepatitis E autochthonous infection in chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:800–3. doi: 10.1097/MEG.0b013e3282f1cbff. [DOI] [PubMed] [Google Scholar]
- 36.Bouwknegt M, Rutjes S, de Roda Husman AM. Med-Vet-Net Annual Scientific Meeting Abstract Book. 4th. St. Malo, France: 2008. Jun 11-14, Hepatits E virus genotype 3 sources in the Netherlands and potential transmission routes to humans; p. 37. (Abstract NEZ05) [Google Scholar]
- 37.Vivek R, Zachariah UG, Ramachandran J, Eapen CE, Rajan DP, Kang G. Characterization of hepatitis E virus from sporadic hepatitis cases and sewage samples from Vellore, south India. Trans R Soc Trop Med Hyg. 2013;107:363–7. doi: 10.1093/trstmh/trt030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang YW, Wang JX, Xu ZY, Guo YF, Qian WH, Xu JX. A serologically confirmed, case-control study, of a large outbreak of hepatitis A in China, associated with consumption of clams. Epidemiol Infect. 1991;107:651–7. doi: 10.1017/s0950268800049347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halliday ML, Kang LY, Zhou TK, Hu MD, Pan QC, Fu TY, et al. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J Infect Dis. 1991;164:852–9. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- 40.Renou C, Moreau X, Pariente A, Cadranel JF, Maringe E, Morin T, et al. A national survey of acute hepatitis E in France. Aliment Pharmacol Ther. 2008;27:1086–93. doi: 10.1111/j.1365-2036.2008.03679.x. [DOI] [PubMed] [Google Scholar]
- 41.Potasman I, Paz A, Odeh M. Infectious outbreaks associated with bivalve shellfish consumption: A worldwide perspective. Clin Infect Dis. 2002;35:921–8. doi: 10.1086/342330. [DOI] [PubMed] [Google Scholar]
- 42.Wellings K, Macdowall W, Catchpole M, Goodrich J. Seasonal variations in sexual activity and their implications for sexual health promotion. J R Soc Med. 1999;92:60–4. doi: 10.1177/014107689909200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: The prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–61. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robert HL, Alan RM. Anchorage, Alaska: University of Alaska Anchorage; 2005. Seasonal Use of Marijuana and Cocaine by Arrestees in Anchorage, Alaska. [Google Scholar]
- 45.Mari F, Politi L, Biggeri A, Accetta G, Trignano C, Di Padua M, et al. Cocaine and heroin in waste water plants: A 1-year study in the city of Florence, Italy. Forensic Sci Int. 2009;189:88–92. doi: 10.1016/j.forsciint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Villar S, Le Roux-Goglin E, Gouas DA, Plymoth A, Ferro G, Boniol M, et al. Seasonal variation in TP53 R249S-mutated serum DNA with aflatoxin exposure and hepatitis B virus infection. Environ Health Perspect. 2011;119:1635–40. doi: 10.1289/ehp.1103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wild CP, Yin F, Turner PC, Chemin I, Chapot B, Mendy M, et al. Environmental and genetic determinants of aflatoxin-albumin adducts in the Gambia. Int J Cancer. 2000;86:1–7. doi: 10.1002/(sici)1097-0215(20000401)86:1<1::aid-ijc1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


