Abstract
Background:
Periodontal disease is of public health concern and hence data on its prevalence rate are necessary. We have documented the prevalence pattern of periodontal disease in a rural population of Belgaum district, India, and identify the optimal treatment needs (TNs).
Materials and Methods:
A cross-sectional study was carried on 1680 dentate adult subjects, examined from 12 villages in Belgaum district, Karnataka, India for prevalence of periodontal status and their TNs (using Community Periodontal Index for Treatment Needs [CPITN]).
Results:
Increase in CPITN score positively correlated with age. Only 4.3% (13) of subjects in the age-group of 20-29 had a CPITN score of 4 indicative of pockets of 6 mm or more when compared to 26% (91) of subjects in the age-group of 45-60 years. 92% (569) of the subjects in the age-group of 30-44 were having a TN score 2 whereas only 5.3% (33) of subjects were having a TN score 0 in the same age-group. Significantly higher need for treatment was observed in males, smokers and subjects using finger and tooth powder. Surprisingly diet of the subjects did not influence TNs.
Conclusion:
Increased prevalence of periodontal diseases and TNs was observed. There is a need for initiating adequate awareness regarding oral hygiene, specifically primary prevention could help in reducing the prevalence of periodontal disease.
Keywords: Community periodontal Index for treatment Needs index, oral hygiene, periodontal health, rural, treatment needs
INTRODUCTION
Periodontitis is a destructive inflammatory disease of the supporting tissues of the teeth and is caused either by group of/specific microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone resulting in periodontal pocket formation and/or gingival recession.[1] The potential of periodontal infections to initiate other systemic pathologies makes it a complex multiphase disease.[2] Periodontal diseases affects the supporting and investing tissues of the teeth and hence is recognized as a major health problem worldwide.[3] About 72.2% of the Indian population resides in rural areas and the dentist to population ratio here can be as high as one dentist for every 250K resident.[4] This high ratio and acute shortages of trained dental manpower have led to severe compromise in dental health. Alarmingly 75-80% of the populations living in rural areas are affected by periodontal disease.[5]
In dental health care delivery system, a dominant preventive approach explains part of the decrease in oral diseases.[6] Direct risk behaviours such as poor oral hygiene practices and dietary habits, tobacco use, and excessive consumption of alcohol are factors that may lead to oral diseases.[7] Of concern, the very high prevalence of chronic inflammatory periodontal disease in rural Indian population are left untreated.[8,9] Socio-cultural factors and systemic infrastructure deficiencies are the major causes of dental diseases in rural India.[10] Nevertheless establishing community programmes to prevent and treat periodontitis is mandatory to know the disease status and treatment needs (TNs) of the target population. Hence, the present study was initiated to:
Estimate the prevalence of periodontal health status and TNs of the rural population of Belgaum, Karnataka, India.
Study the effect of oral hygiene practices and habits of the population on the periodontal TNs.
Identify possible remedial measures if required.
MATERIALS AND METHODS
Study population and sample size
A cross-sectional study was conducted after obtaining ethical clearance from the concerned authorities. Belgaum district is situated in northern Karnataka having 10 Talukas and 1200 villages. 1% of the villages were selected with uniform representation based on lottery method. According to 2001 census, the total population of Belgaum district was 32,00,000 people. In each village, 140 people were randomly selected and examined. The survey was done in nearby schools, and prior information was given to the people through health workers to have free dental checkup and to encourage the maximum number of people to attend. Sample size was calculated using the formula n = 4 (pq/L2) where P = population proportion of positive character, q = 1 − P and L = allowable error. The total study population consisted of 1680 subjects who were selected based on a convenient sample from 12 villages of Belgaum district in the age groups of 15 years and above. Informed consent was taken from each subject prior to recording oral health.
Recording and diagnosis criteria
The Community Periodontal Index of Treatment Needs (CPITN) was developed by the Oral Health Unit of the World Health Organization (WHO) in collaboration with the Federation Dentaire Internationale to assess periodontal health status. It is a simple, time-saving method of assessing the TNs of a specified population group, and has stood the test in a number of major epidemiological studies on the prevalence of periodontal disease.[11,12] The examination of the subjects in this study was conducted according to WHO guidelines using the WHO CPITN–E probe. The teeth examined were 17, 16, 11, 26, 27, 36, 37, 31, 46 and 47. Scoring criteria were followed as previously suggested.[11] The TNs and variables such as age, sex, location, socioeconomic (occupation), oral habits, teeth cleaning habits and diet were also categorized on a printed Performa. To avoid the bias, the author himself examined all the subjects.
Examiner calibration
The examiner was calibrated in the Department of Oral Medicine and Radiology and all the subjects were examined by a single examiner. Intra examiner calibration was undertaken by examining 50 subjects, followed by their re-examination a week later, which resulted in 89% of the diagnostic acceptability with a kappa value of 0.86. Demographic details were filled in the Performa by a trained assistant.
Statistical analysis
Data were analyzed using SPSS package (SPSS Inc., Chicago, IL, version 13.0 for Windows). Qualitative data are summarized using frequencies and percentages. Parametric tests were used for comparisons involving continuous data. One-way analysis of variance was used to determine differences at the 5% significance level (P < 0.05), whereas proportions were compared by the use of Chi-square test. P < 0.05 was selected to denote statistical significance.
RESULTS
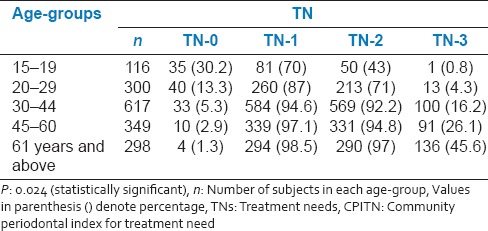
The population was examined and distributed into five age groups according to WHO standard age grouping [Table 1]. The patient details are summarized in Tables 1 and 2. The percentage of dentate persons showing signs of periodontal disease status in all age groups by highest CPITN code number is summarized in Table 3. 30.2% (35) of subjects in the age-group of 15-19 years had a CPITN score 0 indicative of a healthy periodontium as compared to only 3% (10) in the age-group of 45-60 years. Only 4.3% (13) of subjects in the age-group of 20-29 had a CPITN score of 4 indicative of pockets of 6 mm or more as compared to 26% (91) of subjects in the age-group of 45-60 years. The result indicated a positive association of severity of periodontal disease with increasing age.
Table 1.
Distribution of study population on the basis of age and gender
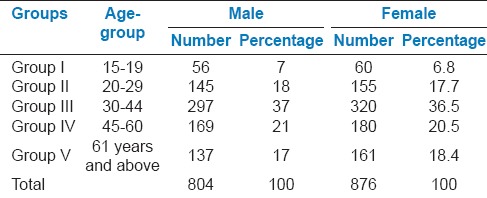
Table 2.
Distribution of study population on the basis of teeth cleaning habits, smoking habits and diet patterns

Table 3.
Distribution of CPITN score according to various age-groups
Periodontal TNs of the study population according to various age groups is presented in Table 4. 92% (569) of the subjects in the age-group of 30-44 were having a TN score 2 indicative of deep scaling and root planning whereas only 5.3% (33) of subjects were having a TN score 0 indicative of no treatment required in the same age-group. Periodontal TNs increased with increase in the age. When gender of the subjects was compared with the periodontal TNs, it was found that males had significantly higher TNs as compared to the females (P < 0.05). Furthermore, statistically higher TNs was observed in the patient group using finger and toothpowder as compared to the group using toothbrush and paste (P = 0.012) [Figure 1]. Comparatively higher TNs was also observed from the subjects who were smoking when compared to nonsmokers (P < 0.05) [Figure 2]. Interestingly diet of the subjects did not influence the TNs [Figure 3].
Table 4.
Periodontal TNs of the study population according to various age groups
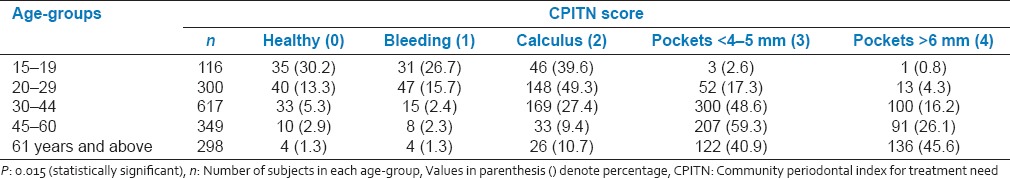
Figure 1.
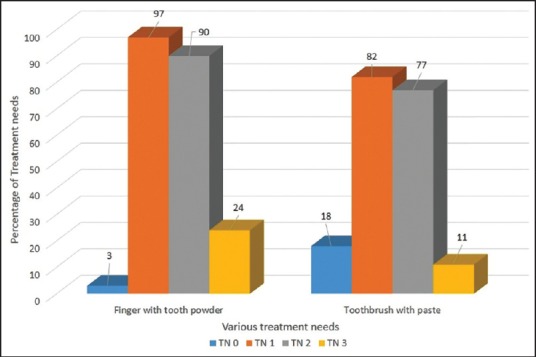
Comparison of treatment needs of the group using toothbrush and paste with finger and tooth powder
Figure 2.
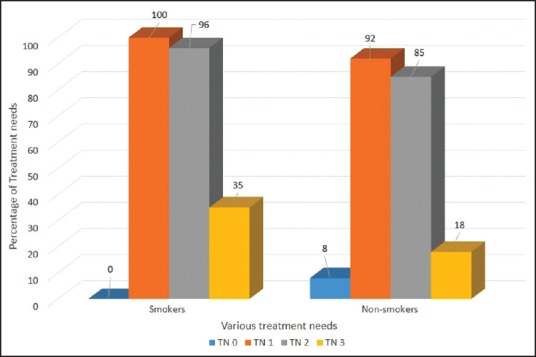
Treatment needs of the study population on the basis of smoking habits
Figure 3.
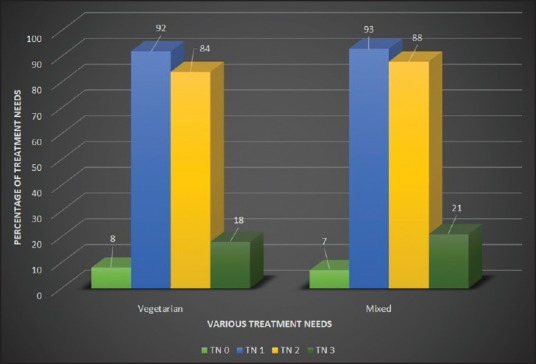
Comparison of treatment needs of subjects on vegetarian diet as compared to mixed diet
DISCUSSION
Several attempts have been made to develop methods for assessing periodontal disease status and TNs on a population basis, which would help in the planning of dental public health services. The CPITN is a functional approach for screening population because it uses accepted clinical criteria, partial mouth scoring and a simple recording procedure, which permits rapid assessment of individuals for periodontal conditions related to TNs.[11] The evaluated data provides information on the magnitude of needs and type of health care personnel required to public health policy makers.
In meeting the objectives of the study we observed a high percentage of periodontal disease status and need for treatment in the population (61%), which is consistent with other studies attributing such observations to lack of dental health care facilities, awareness, motivation and dental health education among the rural population.[13,14]
Although we observed a positive correlation of periodontal disease severity with age, this may be cumulative effects of other disease conditions and prolonged exposure of periodontium to pathogenic factors in bacterial deposits.[15,16] The higher prevalence of periodontal disease severity among men may be due to their higher indulgence in adverse oral habits when compared to women.[17,18] Although, some studies have shown no gender difference on the TNs.[19] While one study has attributed higher periodontal disease rate among females to poor nutrition, hormonal imbalance and frequent child births.[20]
We observed higher prevalence of periodontal disease and TNs among individuals indulging in tobacco smoking habits. Our observations are consistent with other studies[18,21] linking tobacco smoking and chewing to plaque formation as a major cause of initiating periodontal diseases.[21,22] Individuals using toothbrush and toothpaste were less severely affected and had lower TNs than those using finger and toothpowder/other users which may be anticipated as tooth brushing with paste is one of the most effective and available devices for removal of bacterial plaque from the teeth.[23,24]
Interestingly diet pattern did not influence prevalence of periodontal disease or TNs. Although our results are consistent with other studies,[25,26] some studies have indicated a higher prevalence of periodontal disease among vegetarians compared with nonvegetarians.[27] Contrasting this, recent study has reported better periodontal health among vegetarians as compared to people on mixed diets[28] necessitating further clarity on this issue.
CONCLUSION AND RECOMMENDATIONS
Consistently higher prevalence of periodontal disease (61%) and TNs was observed coinciding with lack of awareness among the individuals about periodontal diseases. Oral habits such as smoking and tooth cleaning procedures have a significant effect on the periodontal health and TNs. Hence if awareness of present day knowledge is increased, the prevalence and severity and TNs could be reduced considerably. Studies conducted at regular intervals in the community would produce substantial data to form a baseline from which the planning of the countrywide program could be initiated to meet the future dental health needs.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Saini R. Ozone therapy in dentistry: A strategic review. J Nat Sci Biol Med. 2011;2:151–3. doi: 10.4103/0976-9668.92318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saini R, Saini S, Saini SR. Periodontitis: A risk for delivery of premature labor and low birth weight infants. J Nat Sci Biol Med. 2011;2:50–2. doi: 10.4103/0976-9668.82321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geneva: World Health Organization; 1978. WHO: Technical report series no. 621. Epidemiology, Etiology and Prevention of Periodontal Disease. [PubMed] [Google Scholar]
- 4.Tandon S. Challenges to the oral health workforce in India. J Dent Educ. 2004;68:28–33. [PubMed] [Google Scholar]
- 5.Saparamadu KD. The provision of dental services in the Third World. Int Dent J. 1986;36:194–8. [PubMed] [Google Scholar]
- 6.Gimmestad AL, Holst D, Fylkesnes K. Changes in restorative caries treatment in 15-year-olds in Oslo, Norway, 1979-1996. Community Dent Oral Epidemiol. 2003;31:246–51. doi: 10.1034/j.1600-0528.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 7.Petersen PE. Sociobehavioural risk factors in dental caries — international perspectives. Community Dent Oral Epidemiol. 2005;33:274–9. doi: 10.1111/j.1600-0528.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 8.Madden IM, Stock CA, Holt RD, Bidinger PD, Newman HN. Oral health status and access to care in a rural area of India. J Int Acad Periodontol. 2000;2:110–4. [PubMed] [Google Scholar]
- 9.Bhat M. Oral health status and treatment needs of a rural Indian fishing community. West Indian Med J. 2008;57:414–7. [PubMed] [Google Scholar]
- 10.Singh A, Purohit BM. Addressing oral health disparities, inequity in access and workforce issues in a developing country. Int Dent J. 2013;63:225–9. doi: 10.1111/idj.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN) Int Dent J. 1982;32:281–91. [PubMed] [Google Scholar]
- 12.Pilot T, Barmes DE, Leclercq MH, McCombie BJ, Sardo Infirri J. Periodontal conditions in adolescents, 15-19 years of age: An overview of CPITN data in the WHO Global Oral Data Bank. Community Dent Oral Epidemiol. 1987;15:336–8. doi: 10.1111/j.1600-0528.1987.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanei AS, Nasrabadi AN. Periodontal health status and treatment needs in Iranian adolescent population. Arch Iran Med. 2005;8:290–94. [Google Scholar]
- 14.Singh S, Gupta ND, Sharma AK, Bay A. Periodontal health status of rural and urban population of Aligarh district of Uttar Pradesh state. Indian Soc Periodontol. 2005;9:86–90. [Google Scholar]
- 15.Curtis MA, Slaney JM, Aduse-Opoku J. Critical pathways in microbial virulence. J Clin Periodontol. 2005;32(Suppl 6):28–38. doi: 10.1111/j.1600-051X.2005.00817.x. [DOI] [PubMed] [Google Scholar]
- 16.Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–67. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh SV, Tripathi A, Akbar Z, Chandra S, Tripathi A. Prevalence of dental myths, oral hygiene methods and tobacco habits in an ageing North Indian rural population. Gerodontology. 2012;29:e53–6. doi: 10.1111/j.1741-2358.2010.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan S, Bhat MK. Assessment of periodontal status of rural nepalese population using the community periodontal index. J Nepal Dent Assoc. 2009;10:97–104. [Google Scholar]
- 19.Markkanen H. Periodontal treatment need in a Finnish industrial population. Community Dent Oral Epidemiol. 1978;6:240–4. doi: 10.1111/j.1600-0528.1978.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 20.Reddy J. The WHO Oral Health goals for the year 2000 in South Africa. Int Dent J. 1992;42:150–6. [PubMed] [Google Scholar]
- 21.Tanwir F, Altamash M, Gustafsson A. Influence of betel nut chewing, dental care habits and attitudes on perceived oral health among adult Pakistanis. Oral Health Prev Dent. 2008;6:89–94. [PubMed] [Google Scholar]
- 22.Zeng J, Williams SM, Fletcher DJ, Cameron CM, Broadbent JM, Shearer DM, et al. Re-Examining the Association Between Smoking and Periodontitis in the Dunedin Study With an Enhanced Analytical Approach. J Periodontol. 2014 doi: 10.1902/jop.2014.130577. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Ambjørnsen E. Remaining teeth, periodontal condition, oral hygiene and tooth cleaning habits in dentate old-age subjects. J Clin Periodontol. 1986;13:583–9. doi: 10.1111/j.1600-051x.1986.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 24.Löe H. Oral hygiene in the prevention of caries and periodontal disease. Int Dent J. 2000;50:129–39. doi: 10.1111/j.1875-595x.2000.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 25.Janelle KC, Barr SI. Nutrient intakes and eating behavior scores of vegetarian and nonvegetarian women. J Am Diet Assoc. 1995;95:180–6. doi: 10.1016/s0002-8223(95)00045-3. 189. [DOI] [PubMed] [Google Scholar]
- 26.Key TJ, Appleby PN, Rosell MS. Health effects of vegetarian and vegan diets. Proc Nutr Soc. 2006;65:35–41. doi: 10.1079/pns2005481. [DOI] [PubMed] [Google Scholar]
- 27.Rezy CT, Telly TG. A comparative study of prevalence of periodontal disease among vegetarians and non vegetarians population of Trivandrum. J Ind Dent Assoc. 1976;48:313–16. [Google Scholar]
- 28.Staufenbiel I, Weinspach K, Förster G, Geurtsen W, Günay H. Periodontal conditions in vegetarians: A clinical study. Eur J Clin Nutr. 2013;67:836–40. doi: 10.1038/ejcn.2013.101. [DOI] [PubMed] [Google Scholar]


