Abstract
Aim:
The aim of this study was to relate the salivary electrolyte levels with dental caries in children with Down syndrome and to compare salivary electrolyte levels and dental caries of these children with their siblings.
Materials and Methods:
Study population consisted of 30 Down syndrome children (study group) and their 30 healthy siblings (control group). Caries status was determined by dental caries indices in deciduous and permanent dentitions. Un-stimulated saliva from both groups was collected for salivary electrolyte examination.
Results:
In the study group, mean caries experience in primary dentition was 1.00 ± 0.79 and in the control group it was 2.33 ± 1.42, the difference being statistically significant. Mean caries experience in the permanent dentition of the study group (0. 97 ± 0.76) was significantly lower than the control group (2.47 ± 1.25). Salivary electrolyte levels in the study group were significantly higher than the control group.
Conclusion:
There was a significant decrease in dental caries in primary as well as permanent dentition of Down syndrome patients with increase in their salivary electrolyte levels.
Keywords: Dental caries, Down syndrome, salivary electrolytes
INTRODUCTION
Down syndrome is an autosomal disorder caused by an extra chromosome 21. Down syndrome patients show deficiencies, disharmonic, and delay of development in relation to the normal child. Approximately, 1 out of every 800-1,000 births results in an extra chromosome of the 21st group called Trisomy 21 or Down syndrome.[1]
The syndrome is characterized by short stature, characteristic facial features with a protruding tongue, a wide range of learning difficulties, congenital heart disease, gastrointestinal disorders, and other features.[2] Dental characteristics include abnormally rounded labial forms of the tooth crown, partial anodontia, delayed eruption, pronounced periodontal break down, low prevalence of dental caries and malocclusion such as crowding, posterior cross bite, and anterior open bite.[2]
Dental caries development is considered to involve a triad of indispensable factors, which can be concluded as bacteria in dental plaque, carbohydrates in diet and susceptible teeth.[3] Some syndromes, which have chromosomal abnormalities reported to be associated with low caries indices. Down syndrome is an example of this condition;[4] however, the reason of the low incidence of caries in Down syndrome is unclear.[5]
The low caries prevalence had been postulated to be related to delayed eruption, reduced time of exposure to a cariogenic environment, congenitally missing teeth, higher salivary pH and bicarbonate levels, microdontia, spaced dentition, and shallow fissures of the teeth.[2,6] This low caries incidence in children with Down syndrome is in spite of the presence of increased risk factors for caries such as cariogenic diet, decreased salivary flow rate, mouth breathing, imbalanced occlusal forces, and poor access to oral hygiene.[7,8] Several studies have addressed the etiology of low incidence of caries, yet the exact pathogenesis is still unclear.
Recently, it has been shown that a different salivary environment of electrolytes and pH is manifested in Down syndrome children, leading to the lower reported caries rate.[9] Siqueira et al. suggested that there was an alteration in the secretory pathways of the duct and the acinar cells of salivary glands among Down syndrome children due to differences in salivary electrolytes levels.[10]
The purpose of the present study was to better understand the mechanisms of the differences between children with Down syndrome and their unaffected siblings with regard to caries by investigating the salivary electrolyte levels in these populations. Thus, the following study was conducted to estimate salivary electrolyte levels and relate it with dental caries in children with Down syndrome and to compare the salivary electrolyte levels along with dental caries of Down syndrome patients with healthy siblings.
MATERIALS AND METHODS
The subjects of this study were 30 children between the age group of 7 years and 12 years with Down syndrome from Prayas Seva Sansthan, Udaipur City. The study group was formed of only those subjects who were residents of Udaipur City. Ethical Committee approval was obtained from the concerned authorities of the institution. Written consent was obtained from the parents and verbal consent from the children.
The study was carried out by a single examiner to rule out inter-examiner bias. Unaffected siblings (non-Down syndrome) of the study group formed the control group. Subjects in the control group consisted of 30 normal children between 7 years and 12 years age group. Proforma was prepared to gather adequate information of each child of the study group and control group. Part A consisted of general information, i.e., name, age, sex, residential address. Part B consisted of recording the (dental caries indices) index for evaluation of dental caries and estimation of pH and salivary electrolytes. Oral examination was carried out using a mouth mirror and a probe in the study as well as control group. Caries status was determined by recording the number of decayed (d, D), missing (m, M), and filled (f, F) teeth in the primary and permanent dentitions per patient and were referred to as dmft and DMFT scores, respectively.
Un-stimulated saliva was collected and submitted to the laboratory immediately for salivary electrolytes (sodium, chloride, potassium, calcium, and phosphorous ions) examination salivary samples were collected in sterile vials between 10 am and 11 am in order to prevent any bias in the concentration of the saliva due to the circadian rhythm. Children were asked to pool the saliva in the floor of their oral cavity and spit into a sterile vial intermittently. Saliva samples were immediately sent for electrolyte analysis. Concentrations of Na and K ions were measured using the photoelectric flame photometer while that of Cl ion was measured by Van Slyke-Hiller Iodometric method.[11] Ca and P ions were measured using ion chromatography. Statistical analysis was carried out using the Statistical package of Social Science (SPSS Version 15; Chicago Inc., USA). Chi-square test was used to estimate the statistical difference for prevalence of dental caries between the study and control groups. Comparisons between control and test groups for salivary electrolyte levels and caries experience were made using the unpaired t-test. Pearson correlation test was used to assess the relationship between salivary electrolyte levels and dental caries experience.
RESULTS
The study as well as the control group consisted of 16 males and 14 females. Children were affected more in the control group than study group [Table 1]. A greater proportion of Down's syndrome children were found to be caries free (n = 9, 30.0%) than the control group (n = 5, 16.7%).
Table 1.
Prevalence of dental caries in study as well as control group

It was evident from that the mean sodium concentration in saliva (81.63 ± 4.15) of the study group (81.63 ± 4.15) was more than that of the control group (75.47 ± 3.27) [Table 2]. And this difference between the study and control groups was found to be significant (P < 0.05). The mean potassium concentration in saliva of the study group (29.40 ± 3.51) was greater than that of the control group (17.73 ± 3.70) and this difference between the study and control groups was found to be significant (P < 0.05). The mean chloride concentration in saliva of the study group (26.57 ± 3.86) was more than that of the control group (18.83 ± 4.12) and this difference between the study and control groups was found to be significant (P < 0.05). The mean calcium concentration in saliva of the study group (10.93 ± 2.08) was more than that of the control group (6.10 ± 2.11) and this difference between the study and control groups was found to be significant (P < 0.05). The mean phosphorous concentration in saliva of the study group (21.33 ± 1.79) was more than that of the control group (12.17 ± 1.86) and this difference between the study and control groups was found to be significant (P < 0.05). Table 3 shows the comparison of DMFT and dmft in the study and control groups. In study group, mean caries experience in the primary dentition was 1.00 ± 0.79 and in the control group, it was 2.33 ± 1.42, the difference being statistically significant (P < 0.05). Similarly, the mean caries experience in the permanent dentition of the study group (0.90 ± 0.76) was significantly (P < 0.05) less than the control group (2.47 ± 1.25) [Figure 1].
Table 2.
Comparison of salivary electrolytes in mg/dl (sodium, potassium, chloride, calcium, phosphorous) in study and control group
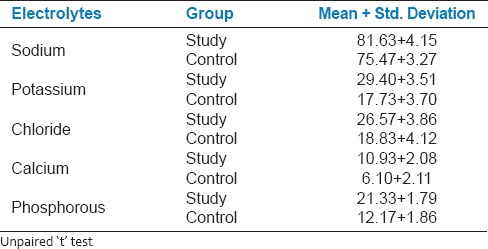
Table 3.
Comparison of DMFT and dmft in study and control groups
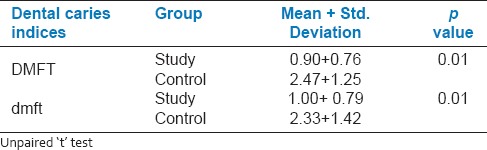
Table 4 shows the correlation between salivary electrolyte levels and DMFT in the study group. There was a negative correlation found between sodium levels and DMFT in the study group. This means that there was a decrease in DMFT with increase in sodium levels. The decrease in DMFT with the increase in sodium levels in the study group was found to be statistically non-significant. However, the decrease in DMFT with the increase in potassium, chloride, calcium, and phosphorous levels in the study group was found to be statistically significant (P < 0.05) [Table 4]. Table 5 shows the correlation between salivary electrolyte levels and dmft in the study group. There was a negative correlation found between sodium levels and dmft in the study group. This means that there was a decrease in dmft with the increase in sodium levels. The decrease in dmft with the increase in sodium levels in the study group was found to be statistically non-significant. However, the decrease in dmft with the increase in potassium, chloride, calcium, and phosphorous levels in the study group was found to be statistically significant (P < 0.05) [Figure 2].
Table 4.
Correlation between salivary electrolytes and DMFT in study group

Table 5.
Correlation between salivary electroytes and dmft in study group

DISCUSSION
There is a little information on the oral health of children with Down's syndrome. Most studies have suggested that the reduction of dental caries in Down syndrome children than that of normal ones may be explained by congenital oligodontia, delayed eruption, a different salivary composition (salivary IgA, salivary pH, buffering capacity, and flow rate) or a difference in eruption times as the teeth of children with Down syndrome often erupts in 1-2 years later than that of the normal child. Often the sequence of eruption of teeth is different than expected.[4] Also sometimes there are missing teeth (usually the upper incisor) or the teeth are peg-shaped or pointed and sometimes are small. This is in accordance with many investigators such as Lee et al. (2004),[11,12] Cogulu et al. (2006)[13] and Castilho et al. (2007).[14] However, the precise cause of the lower prevalence of dental caries in Down syndrome children is still unclear.[15]
There is a general agreement among dental professionals that the salivary secretion and substances secreted within saliva influence to a high degree the strength of individual caries attack, so it has been alleged that salivary composition is a key factor in determining the prevalence of dental caries.[15] Recently, it has been shown that a different salivary environment of electrolytes and pH is manifested in Down syndrome children, leading to the lower reported caries rate.[9] In this study, we have tried to find a relation between salivary electrolyte levels and dental caries in children with Down syndrome and compared it with their siblings.
In spite of Down syndrome children residing in Udaipur City, they were approached through an institution as it was found to be an easy and feasible method. The siblings of Down syndrome children were selected as the control group, considering children with Down syndrome and their siblings have similar dietary pattern, socio-economic status, and oral hygiene habits as they were staying in same homes.
The child was asked to pool the saliva in the floor of the oral cavity and was asked to spit intermittently as this method was easy to obtain the child's cooperation. All samples were collected between 10 am and 11 am, 2 h after any oral or visual exposure to food stuffs. This was carried out to prevent the effect of circadian rhythm on the salivary concentration.[16] Study carried out by Ryberg et al. (1991)[17] showed that low-levels of salivary calcium and phosphate have a modest association with caries susceptibility. Calcium and phosphorous are known to reduce enamel solubility; thus, decreasing incidence of dental caries. In this study, the inverse correlation between DMFT/dmft and salivary calcium ion concentration came in accordance with the results of studies of Kedjarune et al. (1997)[18] and Cornejo et al. (2008).[19]
In the present study, higher levels of chloride were found in the Down syndrome group compared to that of the control group. This is in agreement with studies carried out by Winer and Feller (1972)[20] and Davidovich et al. (2010).[9] It is possible that a disruption in the mechanism of Cl reabsorption that may be part of the trisomy is expressed in this population that modifies the acinar transfer ion mechanism; hence, alters the salivary anion levels.
In the present study, the concentrations of sodium and potassium were elevated in the Down syndrome group. This was in accordance to studies carried out by Winer and Feller (1972)[20] and Davidovich et al. (2010).[9] We hypothesize that these cations act in response to increased Cl (their counter anion) concentration. The higher salivary phosphorous and potassium level in Down syndrome children than those of the control group is also confirmed by the work of Winer and Feller (1972)[20] who found an elevation in the phosphorus and potassium level in mongoloid patients.
In the present study, mean dmft of children with Down syndrome was 1.00 ± 0.79 and it was less than that of siblings (controls). Mean DMFT of children with Down syndrome was 0.90 ± 0.76 and it was lower than that of controls and the difference was found to be statistically significant. This is in accordance with Cutress (1971)[21] and Davidovich et al. (2010)[9] who reported low prevalence of dental caries in both primary and permanent dentitions of Down syndrome individuals.
A significantly greater proportion of Down syndrome children were found to be caries-free (n = 9, 30.0%) than control group (n = 5, 16.66%) in the present study. Stabholz et al. (1991)[8] found that among children with Down syndrome, 84% were caries-free. Orner's study (1975)[22] of the dental caries experience on Down syndrome contrasted with that of their unaffected siblings and showed that Down syndrome individuals had a caries experience less than one-third that of their siblings. This significant decrease in DMT and dmft in the present study could be attributed to increase in salivary electrolyte levels in the study group.
Small quantities of non-specific (total) esterases are present in human parotid saliva. As noted with human plasma, at least three types exist in saliva. These are arylesterases, aliesterases, and cholinesterases, all which are capable of hydrolyzing esters of acetic acid. In addition, it has been shown that the enzyme carbonic anhydrase, which was originally believed to catalyze only the hydration of carbon dioxide and dehydration of carbonic acid or bicarbonate is also a catalyst for the hydrolysis of esters.
Carbonic anhydrase has been demonstrated to be capable of rapidly hydrolyzing beta-naphthyl acetate. Since carbonic anhydrase has been demonstrated in human saliva and has been histochemically localized in human salivary glands, it is likely that it is at least partly responsible for the non-specific esterase activity noted in parotid saliva.[23] It was found that patients with Down syndrome had elevated pH and sodium bicarbonate levels in their parotid saliva. This may be one of the factors responsible for the lowered incidence of dental caries noted in these persons. It has been postulated that glandular carbonic anhydrase influences the conversion of carbon dioxide and water into carbonic acid, which dissociates spontaneously into a hydrogen ion and a bicarbonate ion.
The carbon dioxide and water may enter the salivary glands either from the blood or may be formed in the glands by aerobic respiration.[24] Subsequently, the hydrogen ions are transferred to the blood while sodium ions from the blood are transferred to the salivary glands and secreted with the bicarbonate ions. This series of events may be the mechanism accounting for the rise in pH, sodium, and bicarbonate levels with increases in secretion rate. Thus, an increase in carbonic anhydrase activity could be the factor responsible for the electrolyte increase.
Salivary enzymes also were altered in persons with Down syndrome, and these alterations might provide additional insight into the nature of the metabolic alterations that were responsible for the pH and electrolyte changes. The higher non-specific esterase levels found in the parotid secretion of persons with Down syndrome may actually reflect a change in the amount of carbonic anhydrase present in the cellular and secretory elements of these glands. Thus, an increase in carbonic anhydrase activity could be the factor responsible for the electrolyte and pH rise increase.[24]
We believe that the trisomy in Down syndrome manifests itself in the salivary glands. As a result, a different salivary environment of electrolytes is created that interferes in the caries process, leading to lower caries prevalence among Down syndrome children.
CONCLUSION
Caries experience in both deciduous and permanent dentition of Down syndrome patients was significantly lower than the healthy controls. The salivary electrolyte levels in Down syndrome patients were significantly higher than healthy controls. There was a significant decrease in dental caries in primary and permanent dentition of Down syndrome patients with the increase in their salivary electrolyte levels. These findings also showed that there is a relatively less restorative treatment need than that seen in the normal childhood population due to the presence of less dental caries. Although dental treatment need is not high, these children should receive dental health education, including, oral hygiene instruction, in order to improve their overall oral health.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Dutta S, Nandagopal K, Gangopadhyay PK, Mukhopadhyay K. Molecular aspects of Down syndrome. Indian Pediatr. 2005;42:339–44. [PubMed] [Google Scholar]
- 2.Desai SS. Down syndrome: A review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:279–85. doi: 10.1016/s1079-2104(97)90343-7. [DOI] [PubMed] [Google Scholar]
- 3.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 4.Hennequin M, Faulks D, Veyrune JL, Bourdiol P. Significance of oral health in persons with Down syndrome: A literature review. Dev Med Child Neurol. 1999;41:275–83. doi: 10.1017/s0012162299000596. [DOI] [PubMed] [Google Scholar]
- 5.Takashi N, Toshimasa K, Haruo N. Biochemical characteristic of deciduous enamel before and after the neonatal line in the Down syndrome. Jpn J Pediatr Dent. 2001;39:561–70. [Google Scholar]
- 6.Boyd D, Quick A, Murray C. The Down syndrome patient in dental practice, Part II: Clinical considerations. N Z Dent J. 2004;100:4–9. [PubMed] [Google Scholar]
- 7.Shapira J, Stabholz A, Schurr D, Sela MN, Mann J. Caries levels, Streptococcus mutans counts, salivary pH, and periodontal treatment needs of adult Down syndrome patients. Spec Care Dentist. 1991;11:248–51. doi: 10.1111/j.1754-4505.1991.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 8.Stabholz A, Mann J, Sela M, Schurr D, Steinberg D, Shapira J. Caries experience, periodontal treatment needs, salivary pH, and Streptococcus mutans counts in a preadolescent Down syndrome population. Spec Care Dentist. 1991;11:203–8. doi: 10.1111/j.1754-4505.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 9.Davidovich E, Aframian DJ, Shapira J, Peretz B. A comparison of the sialochemistry, oral pH, and oral health status of Down syndrome children to healthy children. Int J Paediatr Dent. 2010;20:235–41. doi: 10.1111/j.1365-263X.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 10.Siqueira WL, de Oliveira E, Mustacchi Z, Nicolau J. Electrolyte concentrations in saliva of children aged 6-10 years with Down syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:76–9. doi: 10.1016/j.tripleo.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Van Slyke DD, Hiller A. Application of Sendroy's iodometric chloride titration to protein-containing fluids. J Biol Chem. 1947;167:107–24. [PubMed] [Google Scholar]
- 12.Lee SR, Kwon HK, Song KB, Choi YH. Dental caries and salivary immunoglobulin A in Down syndrome children. J Paediatr Child Health. 2004;40:530–3. doi: 10.1111/j.1440-1754.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Cogulu D, Sabah E, Kutukculer N, Ozkinay F. Evaluation of the relationship between caries indices and salivary secretory IgA, salivary pH, buffering capacity and flow rate in children with Down's syndrome. Arch Oral Biol. 2006;51:23–8. doi: 10.1016/j.archoralbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Castilho A, Pardi V, Pereria C. Caries prevalence, level of Mutans streptococci, salivary flow rate and buffering capacity in subjects with Down syndrome. Braz J Oral Sci. 2007;6:1331–6. [Google Scholar]
- 15.Abou El, Yazeed M, Taha S, Shehaby F, Salem G. Relationship between salivary composition and dental caries among a group of Egyptian Down syndrome children. Aust J Basic Appl Sci. 2009;3:720–30. [Google Scholar]
- 16.Lehtonen OP, Gråhn EM, Ståhlberg TH, Laitinen LA. Amount and avidity of salivary and serum antibodies against Streptococcus mutans in two groups of human subjects with different dental caries susceptibility. Infect Immun. 1984;43:308–13. doi: 10.1128/iai.43.1.308-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryberg M, Möller C, Ericson T. Saliva composition and caries development in asthmatic patients treated with beta 2-adrenoceptor agonists: A 4-year follow-up study. Scand J Dent Res. 1991;99:212–8. doi: 10.1111/j.1600-0722.1991.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 18.Kedjarune U, Migasena P, Changbumrung S, Pongpaew P, Tungtrongchitr R. Flow rate and composition of whole saliva in children from rural and urban Thailand with different caries prevalence and dietary intake. Caries Res. 1997;31:148–54. doi: 10.1159/000262390. [DOI] [PubMed] [Google Scholar]
- 19.Cornejo LS, Brunotto M, Hilas E. Salivary factors associated to the prevalence and increase of dental caries in rural school children. Rev Saude Publica. 2008;42:19–25. doi: 10.1590/s0034-89102008000100003. [DOI] [PubMed] [Google Scholar]
- 20.Winer RA, Feller RP. Composition of parotid and submandibular saliva and serum in Down's syndrome. J Dent Res. 1972;51:449–54. doi: 10.1177/00220345720510023401. [DOI] [PubMed] [Google Scholar]
- 21.Cutress TW. Dental caries in trisomy 21. Arch Oral Biol. 1971;16:1329–44. doi: 10.1016/0003-9969(71)90035-5. [DOI] [PubMed] [Google Scholar]
- 22.Orner G. Posteruptive tooth age in children with Down's syndrome and their sibs. J Dent Res. 1975;54:581–7. doi: 10.1177/00220345750540033001. [DOI] [PubMed] [Google Scholar]
- 23.Chauncey HH, Shannon IL. Glandular mechanisms regulating the electrolyte composition of human parotid saliva. Ann N Y Acad Sci. 1965;131:830–8. doi: 10.1111/j.1749-6632.1965.tb34848.x. [DOI] [PubMed] [Google Scholar]
- 24.Winer RA, Chauncey HH. Parotid saliva enzymes in Down's syndrome. J Dent Res. 1975;54:62–4. doi: 10.1177/00220345750540013801. [DOI] [PubMed] [Google Scholar]


