Abstract
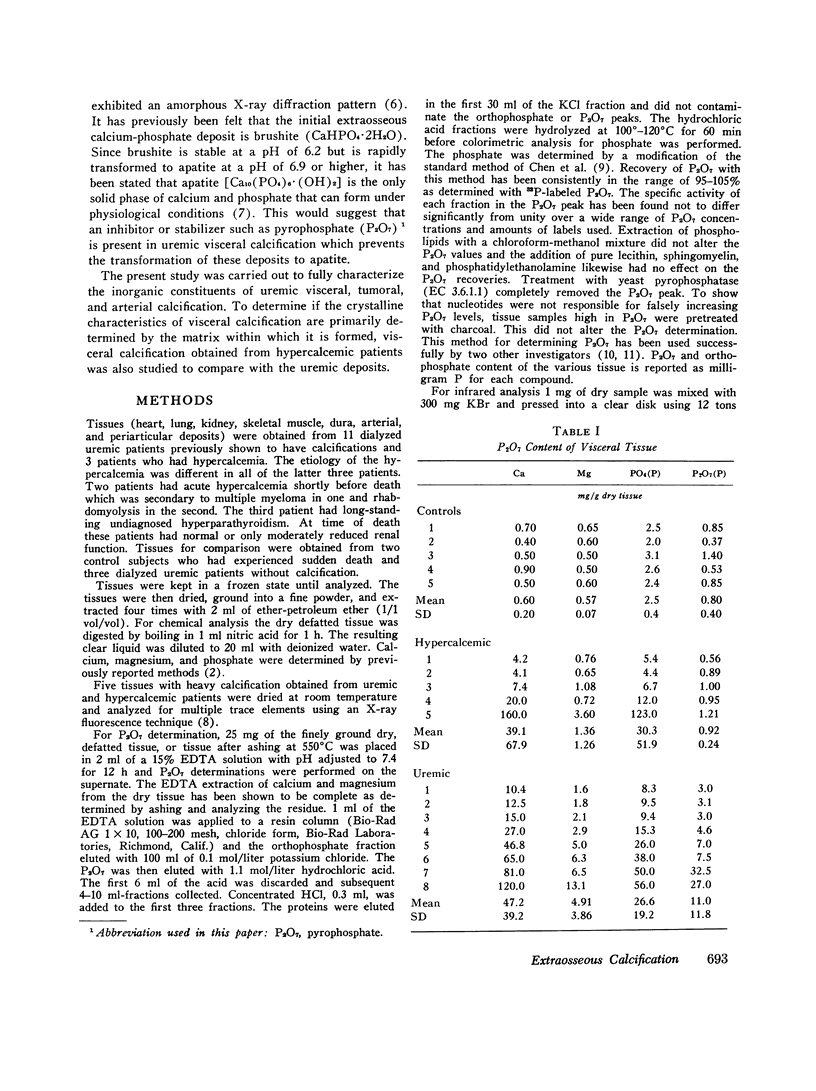
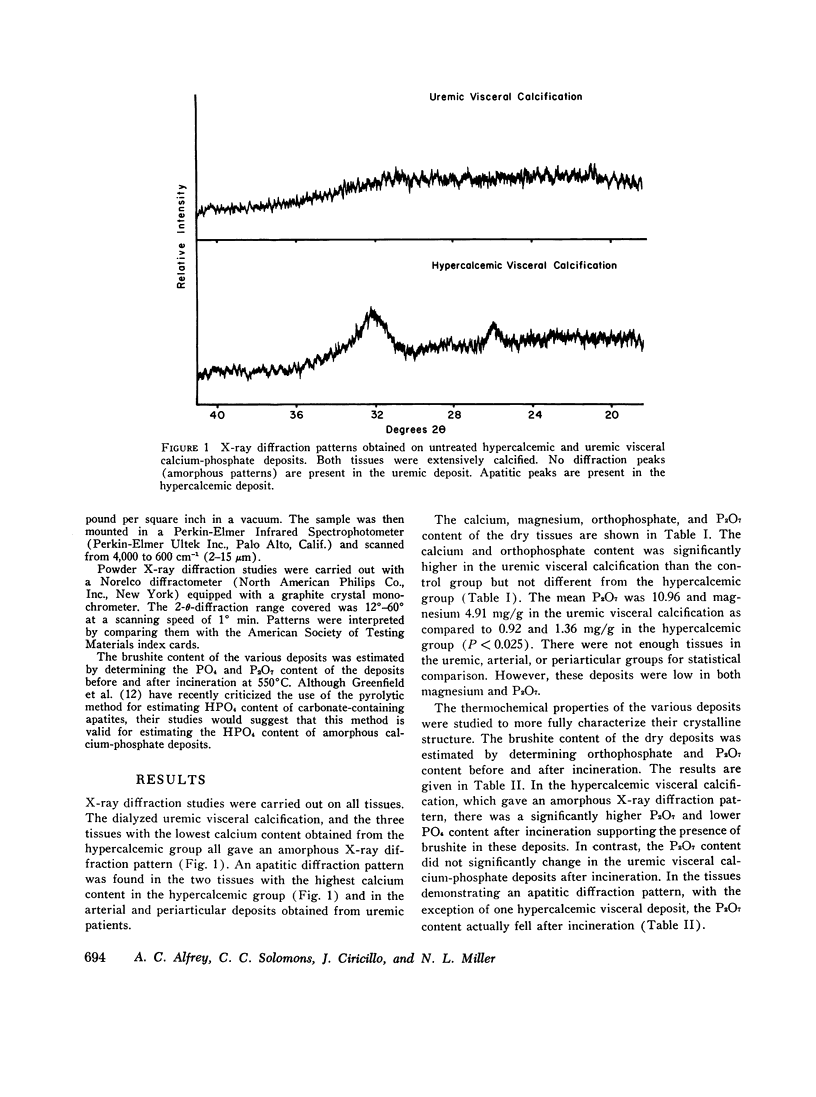
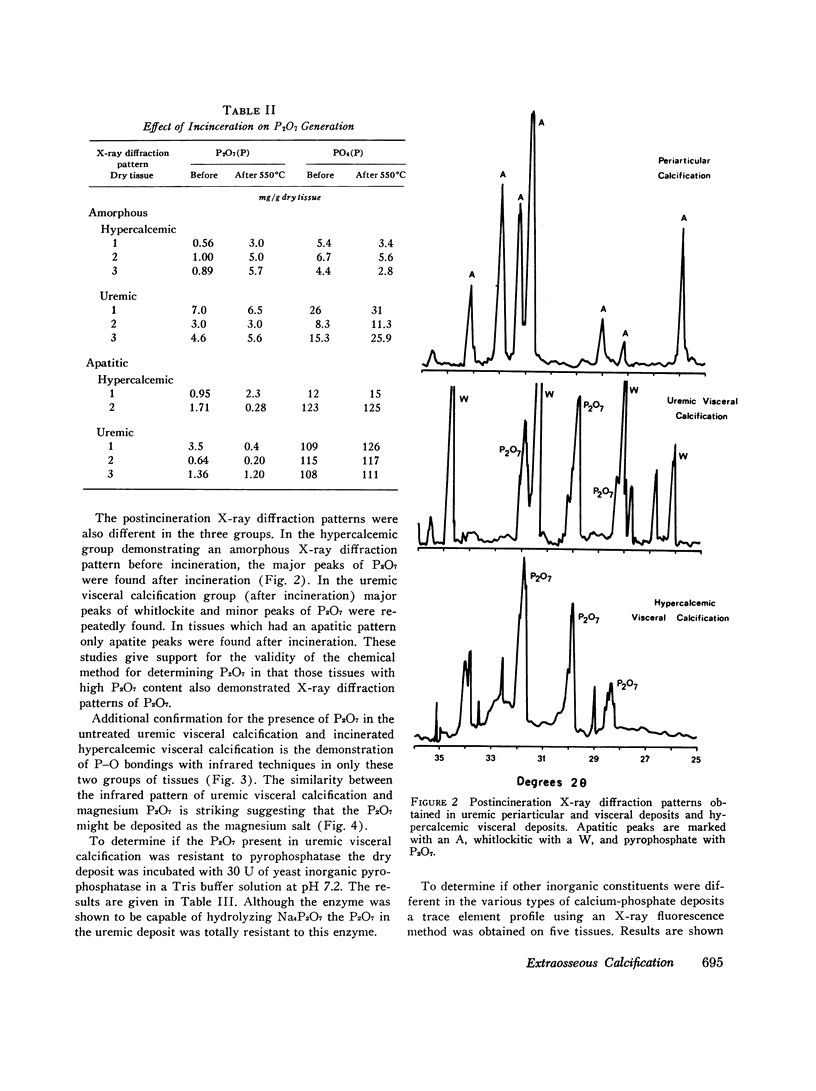
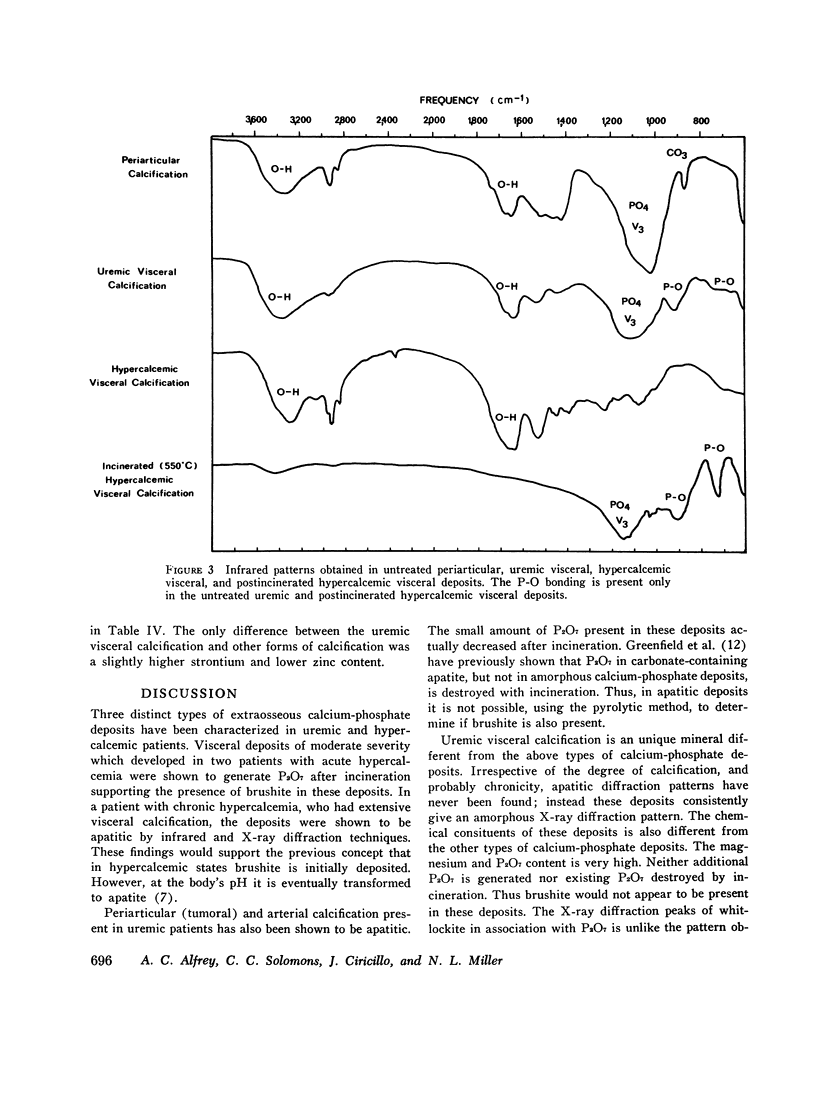
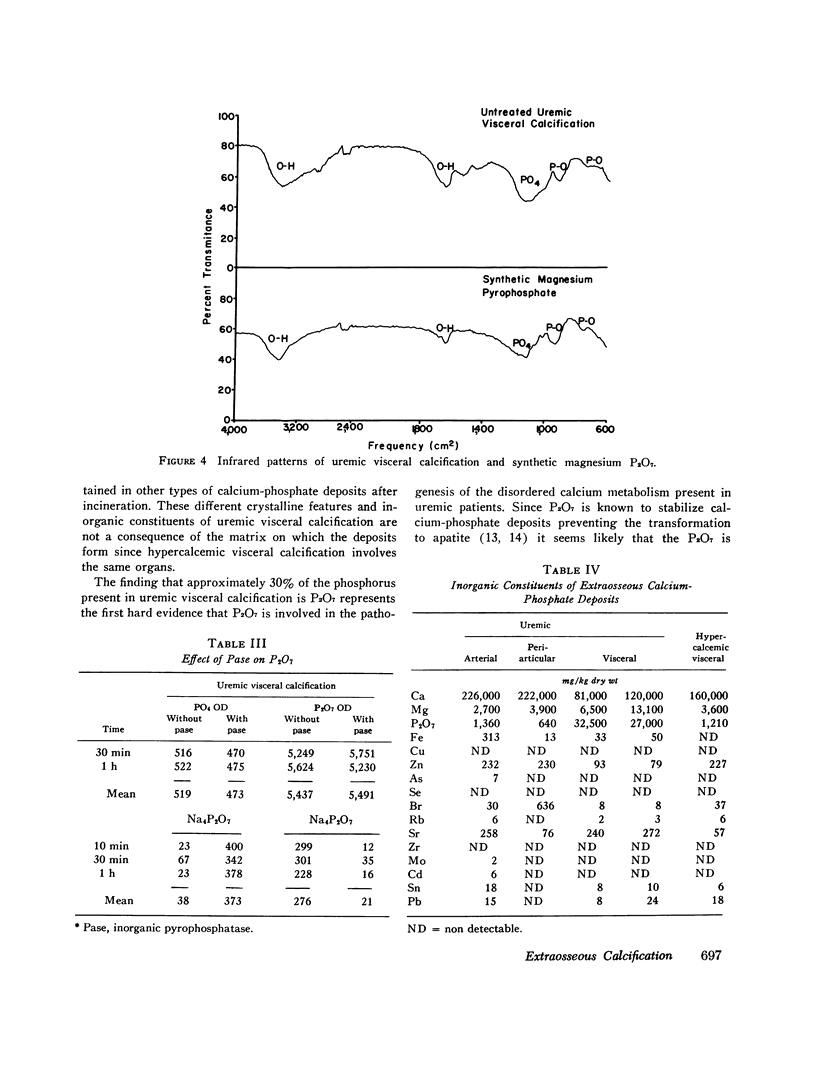
The inorganic constituents and crystalline features of extraosseous calcium-phosphate deposits obtained from dialyzed uremic and hypercalcemic patients were studied. Visceral calcification (heart, lung, and kidney) in hypercalcemic patients exhibited either an amorphous or apatitic X-ray diffraction pattern. Uremic visceral calcification consistently gave an amorphous diffraction pattern. Although the calcium content of uremic and hypercalcemic visceral deposits was similar, other inorganic constituents were different. The mean pyrophosphate was 11 +/- 11.8 and magnesium 4.91 +/- 3.86 mg/g in the uremic group as compared to 0.92 +/- 0.24 and 1.36 +/- 1.26 mg/g in the hypercalcemic group (P less than 0.025). After incineration hypercalcemic visceral deposits having an amorphous diffraction pattern were found to generate pyrophosphate supporting the presence of brushite in these deposits. The small amount of pyrophosphate in apatitic deposits from both uremic and hypercalcemic patients actually decreased after incineration and the pyrophosphate content of uremic visceral deposits was unchanged by incineration. It is concluded that in hypercalcemic patients the initial visceral deposit is brushite which is subsequently transformed to apatite. Arterial and tumoral calcium-phosphate deposits in uremic patients were also apatite. Uremic visceral calcium-phosphate deposits are an unique mineral high in magnesium with approximately 30% of the phosphorus present as pyrophosphate. The high pyrophosphate content of these deposits could alter their crystalline structure and prevent the transformation to apatite. The infrared features, high magnesium content of the deposit, and resistance of pyrophosphate in the deposit to hydrolysis by pyrophosphatase suggests that the pyrophosphate may be deposited as the magnesium salt.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfrey A. C., Jenkins D., Groth C. G., Schorr W. S., Gecelter L., Ogden D. A. Resolution of hyperparathyroidism, renal osteodystrophy and metastatic calcification after renal homotransplantation. N Engl J Med. 1968 Dec 19;279(25):1349–1356. doi: 10.1056/NEJM196812192792501. [DOI] [PubMed] [Google Scholar]
- Alfrey A. C., Miller N. L., Butkus D. Evaluation of body magnesium stores. J Lab Clin Med. 1974 Aug;84(2):153–162. [PubMed] [Google Scholar]
- Armstrong D., VanWormer D., Solomons C. C. Increased inorganic serum pyrophosphate in serum and urine of patients with osteogenesis imperfecta. Clin Chem. 1975 Jan;21(1):104–108. [PubMed] [Google Scholar]
- CANER J. E., DECKER J. L. RECURRENT ACUTE (?GOUTY) ARTHRITIS IN CHRONIC RENAL FAILURE TREATED WITH PERIODIC HEMODIALYSIS. Am J Med. 1964 Apr;36:571–582. doi: 10.1016/0002-9343(64)90105-6. [DOI] [PubMed] [Google Scholar]
- Cathala G., Brunel C. L'activité pyrophosphatasique de la phosphatase alcaline du cerveau. Biochim Biophys Acta. 1973 Jul 5;315(1):73–82. doi: 10.1016/0005-2744(73)90131-9. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Hammond W. S., Alfrey A. C., Contiguglia S. R., Stanford R. E., Huffer W. E. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med. 1975 Sep;83(3):330–336. doi: 10.7326/0003-4819-83-3-330. [DOI] [PubMed] [Google Scholar]
- Contiguglia S. R., Alfrey A. C., Miller N. L., Runnells D. E., Le Geros R. Z. Nature of soft tissue calcification in uremia. Kidney Int. 1973 Sep;4(3):229–235. doi: 10.1038/ki.1973.104. [DOI] [PubMed] [Google Scholar]
- David D. S., Sakai S., Granda J., Cheigh J. S., Riggio R. R., Stenzel K. H., Rubin A. L. Role of pyrophosphate in renal osteodystrophy. Trans Am Soc Artif Intern Organs. 1973;19:440–445. doi: 10.1097/00002480-197301900-00076. [DOI] [PubMed] [Google Scholar]
- Francis M. D. The inhibition of calcium hydroxypatite crystal growth by polyphosphonates and polyphosphates. Calcif Tissue Res. 1969;3(2):151–162. doi: 10.1007/BF02058658. [DOI] [PubMed] [Google Scholar]
- Greenfield D. J., Termine J. D., Eanes E. D. A chemical study of apatites prepared by hydrolysis of amorphous calcium phosphates in carbonate-containing aqueous solutions. Calcif Tissue Res. 1974;14(2):131–138. doi: 10.1007/BF02060289. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Hampers C. L., Merrill J. P. Secondary hyperparathyroidism and renal osteodystrophy in chronic renal failure. Analysis of 195 patients, with observations on the effects of chronic dialysis, kidney transplantation and subtotal parathyroidectomy. Medicine (Baltimore) 1969 Sep;48(5):333–374. [PubMed] [Google Scholar]
- LeGeros R. Z., Contiguglia S. R., Alfrey A. C. Pathological calcifications associated with uremia: two types of calcium phosphate deposits. Calcif Tissue Res. 1973 Oct 23;13(3):173–185. doi: 10.1007/BF02015408. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Popovtzer M. M., Coburn J. W., Makoff D. L., Maxwell M. H., Kleeman C. R. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N Engl J Med. 1968 Sep 26;279(13):697–700. doi: 10.1056/NEJM196809262791308. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Bisaz S., Fleisch H. Pyrophosphate and diphosphonates in calcium metabolism and their possible role in renal failure. Arch Intern Med. 1969 Nov;124(5):571–577. [PubMed] [Google Scholar]
- Russell R. G., Mühlbauer R. C., Bisaz S., Williams D. A., Fleisch H. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif Tissue Res. 1970;6(3):183–196. doi: 10.1007/BF02196199. [DOI] [PubMed] [Google Scholar]
- Terman D. S., Alfrey A. C., Hammond W. S., Donndelinger T., Ogden D. A., Holmes J. H. Cardiac calcification in uremia. A clinical, biochemical and pathologic study. Am J Med. 1971 Jun;50(6):744–755. doi: 10.1016/0002-9343(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Whittier F. C., Freeman R. M. Potentiation of metastatic calcification in vitamin D-treated rats by magnesium. Am J Physiol. 1971 Jan;220(1):209–212. doi: 10.1152/ajplegacy.1971.220.1.209. [DOI] [PubMed] [Google Scholar]
- Wöltgens J. H., Bonting S. L., Bijvoet O. L. Inorganic pyrophosphatase in mineralizing hamster molars. 3. Influence of diphosphonates. Calcif Tissue Res. 1973;13(2):151–157. doi: 10.1007/BF02015405. [DOI] [PubMed] [Google Scholar]
- Wöltgens J., Ahsmann W. Determination of orthophosphate in the presence of inorganic pyrophosphate in the assay of inorganic pyrophosphatase activity. Anal Biochem. 1970 Jun;35(2):526–529. doi: 10.1016/0003-2697(70)90217-4. [DOI] [PubMed] [Google Scholar]



