Abstract
Primary adenocarcinoma of rete testis is one of the rarest intrascrotal tumors. Very few cases have been reported in the literature. In addition, presence of biphasic component creates difficulty in the diagnosis. We present here a unique third case of rete testis adenocarcinoma having distinct cytologically malignant spindle cell component in a young male who presented with recurrent hydrocele.
Keywords: Adenocarcinoma, biphasic, hydrocele, metaplastic, orchiectomy, rete testis
INTRODUCTION
Primary adenocarcinoma of rete testis is an extremely rare intrascrotal extratesticular neoplasm occurring most frequently in elderly males but has a wide age range of 8-91 years. Although near about 60 cases of rete testis carcinoma have been published till date, less than 30 cases had fulfilled the strict diagnostic criteria required for true rete testis adenocarcinoma.[1,2] The diagnosis of this unusual lesion is based largely on documenting its origin from the rete testis and excluding other primary and metastatic neoplasms.[3] We present a very unique and possibly the third case of primary rete testis adenocarcinoma in a young male, that also featured a malignant spindle cell component predominantly in a biphasic pattern.[3,4]
CASE REPORT
A 32-year-old farmer presented with a gradually enlarging painful right scrotal swelling and heaviness of 1½ year duration. He was treated by a village quack for recurrent right-sided hydrocele. There was no history of cryptorchidism, abnormal sexual development or trauma in the inguinoscrotal region. On examination, the swelling was tender, fluctuant but non-translucent. The overlying skin was indurated but without any visible growth over the same. There was no palpable lymphadenopathy. The clinician suspected an underlying testicular mass for which the patient underwent ultrasonography (USG) of the scrotum and abdominopelvic computed tomography (CT). USG revealed a solid well-delineated echogenic mass in right scrotum with collection of small amount of fluid surrounding the right testicular region. CT scan was negative for retroperitoneal lymph nodes. Serum alpha-fetoprotein, alkaline phosphatase, prostate-specific antigen, carcino-embryonic antigen (CEA) and beta-human chorionic gonadotropin levels were in the normal range. Routine pre-operative investigations were otherwise normal. An 18-Fludeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) scan failed to demonstrate any unknown primary site. A planned right radical orchiectomy was performed. On exploration, there was blood-mixed fluid in the scrotal sac with thickening of the tunica vaginalis. A whitish solid circumscribed mass was observed almost replacing the testis. The surgeon was unsure of the exact site of tumor origin. The resected specimen comprised of the right testicle with its appendages and a 5 cm segment of spermatic cord. On cut section, a whitish-tan solid mass measuring 10.2 cm × 7.4 cm × 5.6 cm with focal cystic and hemorrhagic areas were found which almost replaced the normal testicular tissue [Figure 1a]. A small area of compressed brownish testicular tissue was present peripherally in the upper pole. Histopathological examination displayed a combination of a pure adenocarcinoma, a biphasic pattern consisting of intimate admixture of malignant epithelial and spindle cell components and also predominantly sarcoma-like areas. A distinct but gradual transition from normal rete testicular lining to dysplastic and malignant epithelium was noted [Figure 1b]. The epithelial tumorous part demonstrated varied patterns namely tubules and cords (sertoliform) in desmoplastic stroma, elongated and compressed branching tubules (retiform), solid areas with tiny slit-like channels (kaposiform) and slender papillae with thin fibrovascular cores [Figures 1c, d and 2a]. The epithelial tufts projecting into the large cyst-like dilated epithelium-lined channels of rete testis, morphologically mimicking renal glomeruli was also appreciated [Figure 2b]. The epithelial lining cells were cuboidal to columnar with eosinophilic cytoplasm, focal areas of nuclear stratification and moderate pleomorphism [Figure 2c]. Solid cords of tumor infiltrated into the seminiferous tubules that showed maturation arrest, but appeared to spare the epididymis and ductuli efferentes [Figure 2d]. No focus of Intratubular germ cell neoplasia was detected. The tumor also spared the vas deferens and scrotal skin. Predominantly sarcoma-like areas revealed mostly storiform pattern and short fascicles of moderately atypical spindle cells with brisk mitotic figures, areas of necrosis and metaplastic bone formation [Figure 3a–c]. On immunohistochemical analysis, malignant epithelium expressed diffuse, strong cytoplasmic positivity for cytokeratin (CK) with focal positivity of spindle cells. Vimentin was strongly and diffusely positive in spindle cells of sarcomatoid areas [Figure 3d]. However, calretinin was negative throughout. The patient was well and without any evidence of local recurrence or metastatic disease 6 months after surgery.
Figure 1.
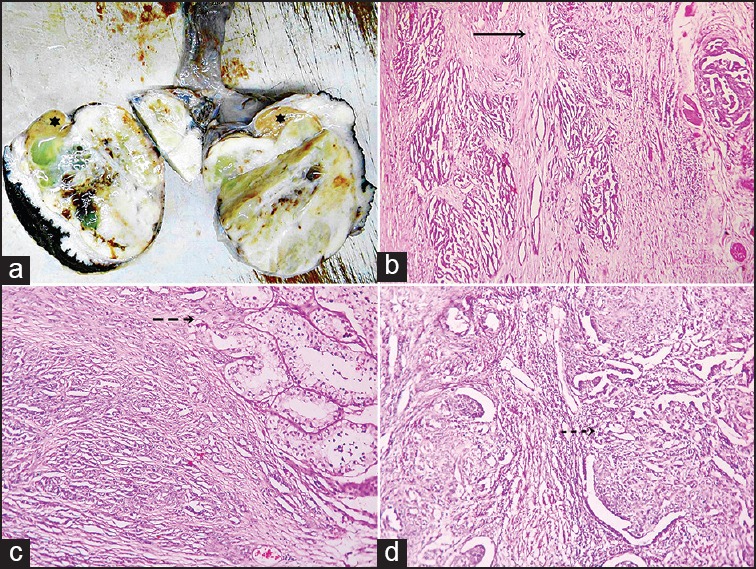
(a) Orchiectomy specimen showing a solid whitish mass with focal cystic and hemorrhagic areas and residual normal testicular tissue in upper pole (star) (b) photomicrograph showing gradual transition from normal rete testicular lining to dysplastic and malignant lining in the direction of arrow (H and E, ×40) (c) photomicrograph showing tubules and cords of malignant cells in desmoplastic stroma infiltrating the seminiferous tubules (broken arrow) (H and E, ×100) (d) photomicrograph showing retiform tubules and solid areas with kaposiform (broken arrow) channels (H and E, ×100)
Figure 2.
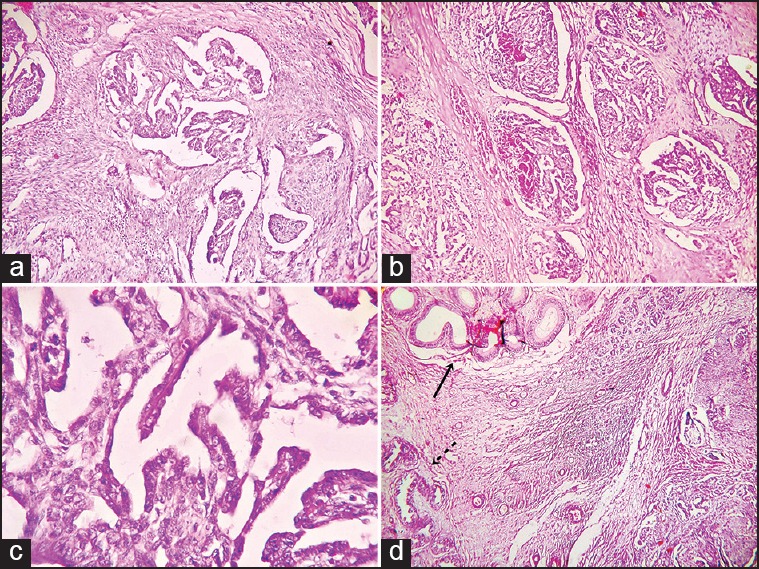
(a) Biphasic area of the adenocarcinoma showing papillary structures with fibrovascular core along with spindle cell component arranged in fascicles (H and E, ×100) (b) photomicrograph showing epithelial tufts projected into the large dilated epithelium-lined channels of rete testis mimicking renal glomeruli (H and E, ×100) (c) photomicrograph of lining cuboidal to columnar epithelial cells showing moderate pleomorphism (H and E, ×400) (d) photomicrograph demonstrating epididymis (solid arrow) and ductuli efferentes (broken arrow) free from infiltrating tumor mass (H and E, ×100)
Figure 3.
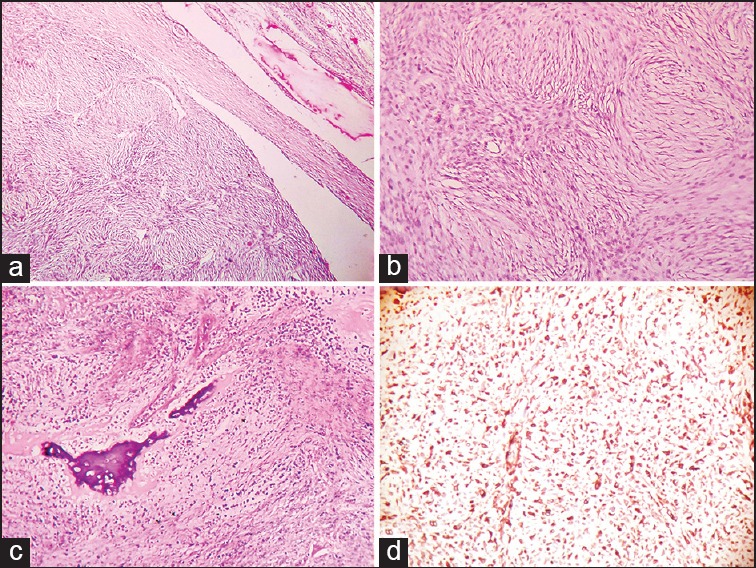
(a) Photomicrograph of sarcoma-like area of the tumor mass displaying spindle cells arranged in storiform pattern and compressed seminiferous tubules (H and E, ×100) (b) photomicrograph showing spindle cells in storiform pattern, brisk mitotic activity and moderate pleomorphism (H and E, ×400) (c) photomicrograph showing metaplastic bone formation and necrosis in sarcoma-like area (H and E, ×100) (d) photomicrograph showing diffuse and strong positivity of vimentin in sarcoma-like area (×100)
DISCUSSION
Most patients with adenocarcinoma of rete testis present with scrotal pain and/or swelling and frequently tumors are masked by a hydrocele, hematocele, inguinoscrotal hernia, epididymitis etc.[2,5] The differential diagnosis includes entities most importantly malignant mesothelioma of tunica vaginalis, ovarian-type (mullerian) tumors of testis and paratestis, metastatic adenocarcinoma, epididymal adenocarcinoma, malignant sertoli cell tumors, etc. Despite remarkable architectural similarity, grossly mesothelioma, as opposed to rete adenocarcinoma, certainly involves the tunica vaginalis and even the scrotal skin in a multifocal fashion; microscopically display typical tubulopapillary pattern, minimal cytological atypia and positivity for mesothelioma — related markers such as calretinin, WT-1, CK5/6 and negativity for adenocarcinoma-related antibodies. Mullerian type tumors are positive for CEA and cancer antigen 125 but negative for CK5/6 and calretinin. Metastatic adenocarcinomas are mostly bilateral, multifocal and most importantly have a detected source of primary.[2,6] The strict criteria of Nochomovitz and Orenstein[2,7] for rete adenocarcinoma includes involvement of testicular hilum by the tumor, demonstrable transition from normal rete testis to neoplastic rete epithelium, absence of histologically similar extra-scrotal tumor as well as testicular or paratesticular tumor, immunohistochemical exclusion of other possibilities, particularly malignant mesothelioma. Demonstration of a transition from normal to neoplastic rete epithelium is perhaps the strongest evidence for the diagnosis but large masses often obliterate the normal anatomy such that a transition is not evident.[2,5] Thus to sum up, the fatally deceptive clinical presentation, plethora of differential diagnosis and the seemingly complex criteria of Nochomovitz and Orenstein[2,7] all together make the diagnosis of this rare entity a real challenge. In our case, the transition from normal to malignant rete epithelium was surely the strongest feature supporting the diagnosis, besides others such as characteristic glomeruloid and other patterns, lack of scrotal skin and epididymal involvement, negative serum biomarkers, negativity for calretinin with positive vimentin and absence of any other primary site of tumor. In the present case, a biphasic and also a predominantly sarcoma-like component harboring metaplastic ossification besides a purely adenocarcinomatous component added to the diagnostic challenge due to extreme scarcity of such findings in literature.[3,4] Thus, the biphasic pattern along with the young age of the patient added to the uniqueness of the case.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Sogni F, Monga G, Terrone C, Gontero P. Primary adenocarcinoma of the rete testis: Diagnostic problems and therapeutic dilemmas. Scand J Urol Nephrol. 2008;42:83–5. doi: 10.1080/00365590701517608. [DOI] [PubMed] [Google Scholar]
- 2.Amin MB. Selected other problematic testicular and paratesticular lesions: Rete testis neoplasms and pseudotumors, mesothelial lesions and secondary tumors. Mod Pathol. 2005;18(Suppl 2):S131–45. doi: 10.1038/modpathol.3800314. [DOI] [PubMed] [Google Scholar]
- 3.Visscher DW, Talerman A, Rivera LR, Mazur MT. Adenocarcinoma of the rete testis with a spindle cell component. A possible metaplastic carcinoma. Cancer. 1989;64:770–5. doi: 10.1002/1097-0142(19890801)64:3<770::aid-cncr2820640334>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Crisp-Lindgren N, Travers H, Wells MM, Cawley LP. Papillary adenocarcinoma of rete testis. Autopsy findings, histochemistry, immunohistochemistry, ultrastructure, and clinical correlations. Am J Surg Pathol. 1988;12:492–501. [PubMed] [Google Scholar]
- 5.Nochomovitz L. Tumours of collecting ducts and rete. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; 2004. pp. 265–6. [Google Scholar]
- 6.Mermershtain W, Vardi N, Gusakova I, Klein J. Serous papillary adenocarcinoma of the rete testis: Unusual ultrasonography and pathological findings. J Cancer Res Ther. 2007;3:37–9. doi: 10.4103/0973-1482.31970. [DOI] [PubMed] [Google Scholar]
- 7.Nochomovitz LE, Orenstein JM. Adenocarcinoma of the rete testis. Case report, ultrastructural observations, and clinicopathologic correlates. Am J Surg Pathol. 1984;8:625–34. [PubMed] [Google Scholar]


