Abstract
Mucormycosis is a rare opportunistic aggressive and fatal infection caused by mucor fungus. Seven types of mucormycosis are identified based on the extension and involvement of the lesion, of which the rhino orbital mucormycosis is most common in the head and neck region. Although it is widely spread in nature, clinical cases are rare and observed only in immunocompromised patients and patients with uncontrolled diabetes mellitus. Early symptoms include fever, nasal ulceration or necrosis, periorbital edema or facial swelling, paresthesia and reduced vision. Involvement of cranial nerves although not common, facial nerve palsy is a rare finding. The infection may spread through cribriform plate to the brain resulting in extensive cerebellar infarctions. Timely diagnosis and early recognition of the signs and symptoms, correction of underlying medical disorders, and aggressive medical and surgical intervention are necessary for successful therapeutic outcome.
Keywords: Diabetes mellitus, facial nerve palsy, mucormycosis
INTRODUCTION
Mucormycosis is a life-threatening opportunistic mycotic infection[1] caused by saprophytic fungi (phycomycetes, zygomycetes. mucoraceae) frequently found in soil, residue plants, spoiled food and upper respiratory tract of healthy people.[2] It becomes pathogenic when associated with predisposing factors such as immunocompromised states most commonly (60-81%) diabetes mellitus.[3,4] Mucormycosis can manifest as one of seven different clinical syndromes such as Rhino cerebral, pulmonary, gastrointestinal, central nervous system, cutaneous, disseminated, miscellaneous (bones, joints, heart, kidney, mediastinum). Rhinocerebral type is the most common and has a characteristic method of spread. The fungal hyphae have high affinity to the internal elastic lumina of arterial blood vessels and are highly angio-invasive ensuing thromboembolism and cause subsequent thrombotic infarction.[5] Cranial nerve findings signify severe infection and signal a grave prognosis. Currently, treatment for the disease is based on three main principles; rapid reversal of underlying predisposing factors, antifungal therapy with amphotericin B and timely surgical intervention.
CASE REPORT
A 75-year-old patient with poorly controlled diabetes reported to the Department of Oral Medicine and Radiology with the chief complaint of swelling and pain in the right side of the face and right eye. On examination, patient was oriented with the normal gait. There were ptosis and complete drooping of his right upper eyelid [Figure 1]. Other significant clinical features observed were, drooping of the corner of the mouth, drooling of saliva and absence of wrinkles in the right half of the forehead (suggestive of lower motor neuron lesion). Cranial nerve examination revealed involvement of III, IV, VI, VII cranial nerves, but there was no altered or loss of taste sensation. Intra-oral examination revealed chronic generalized periodontitis. Patient was referred to the Departments of ENT, Ophthalmology and Neurology for opinion. He was diagnosed of having acute maxillary sinusitis with complete opthalmoplegia, periorbital edema, loss of visual acuity and orbital cellulitis with 7th cranial nerve palsy (level 5). Routine blood investigations were carried out which was found to be normal except for elevated erythrocyte sedimentation rate (>150 mm Hg after 1 h) and hyperglycemia (random blood sugar: 334 mg/dl). Orthopantomogram revealed haziness of right maxillary sinus. Computed tomography (CT) revealed axial proptosis of the globe of the right eye and retro-orbital haziness, maxillary sinus was filled with soft tissue (25 HU) [Figure 2]. Magnetic resonance imaging (MRI) brain revealed bilateral maxillary and ethmoidal inflammatory sinusitis extending into the right orbit causing orbital post septal cellulitis and proptosis [Figure 3]. Patient was operated by functional endoscopic sinus surgery, a polypoidal mass of tissue was recovered as biopsy specimen that was subjected for KOH mounts and fungal cultures to check for mucormycosis. The microscopic examination of the biopsy material and the nasal discharge was done in 10% KOH wet mounts. It showed the characteristic broad, aseptate branched hyphae, which was consistent with mucormycosis [Figure 4]. The patient was managed medically as his fluctuant blood sugar levels contradicted surgical debridement. The patient was started on intravenous insulin infusion and amphotericin B at 0.3 mg/kg/day, and this was gradually increased to 1 mg/kg/day with the monitoring of the serum electrolytes and the renal functions. There was a considerable improvement in the patient, and he is under follow up.
Figure 1.
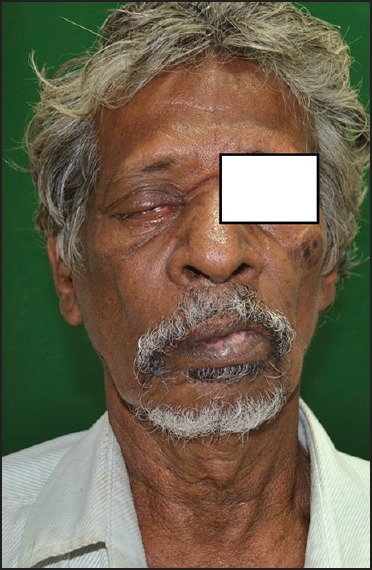
Patient showing ptosis and drooping of the corner of mouth
Figure 2.
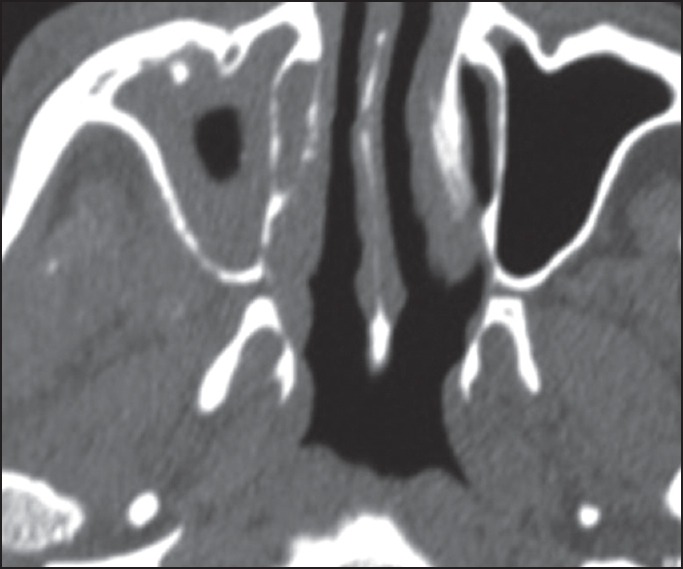
Computed tomography image showing proptosis of the globe of right eye and hyperintense right maxillary sinus
Figure 3.
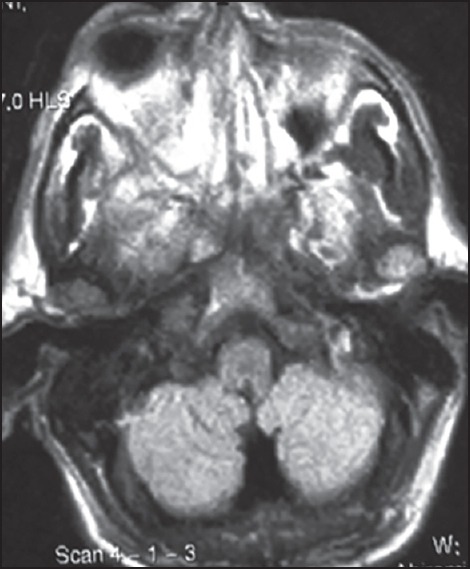
T2-weighted magnetic resonance imaging showing bilateral maxillary and ethmoidal inflammatory sinusitis right orbit proptosis
Figure 4.
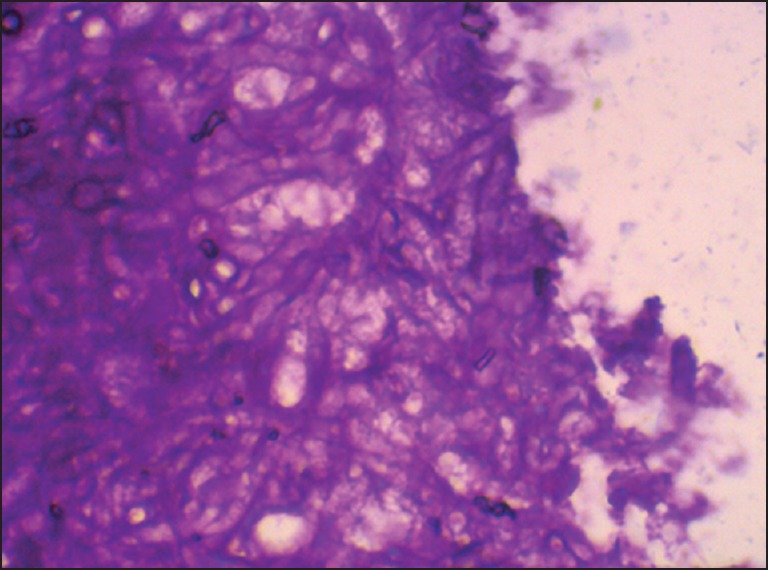
Pictomicrograph showing broad, aseptate, branched hyphae
DISCUSSION
Mucormycosis (mycosis mucorina) was first reported by Paultouf.[6] Cephalic mucormycosis has two forms of which rhino orbito cerebral form is a fatal variety and rhino paranasal sinuses form generally follows a benign clinical course. The cerebral form is the most easily identified by apparent ocular manifestations. The pathway of spread although not clear is believed to initiate from the nasal mucosa, spreading to the ethmoid, maxillary sinuses, orbit and finally the intracranial fossa.[7] However, pterygopalatine fossa is also considered to be a reservoir of mucor from where it spreads to retroglobal space of orbit and infratemporal space.[7] Many theories suggest angioinvasion as a cause and dissemination of the infection. Patients usually present with facial and orbital pain, visual loss and generalized swelling on the affected side. Multiple cranial nerve palsies are also reported in patients with mucormycosis. Frequency of facial nerve paralysis in conjunction with rhino-orbital-cerebral mucormycosis is 11%.[8] Our patient had right side facial nerve palsy. Although pathophysiology for facial nerve paralysis is not known, some reports indicate that the infection can reach from the pterygopalatine fossa to inferior orbital fissure, orbital apex, and infratemporal fossa.[8] Pathology of resistance arteries in diabetic patients may cause edema and localize facial nerve ischemia, which would compromise the blood supply to the nerve leading to facial nerve palsy. In most diabetics with facial nerve palsy the chorda tympani is spared and hence the taste sensation remains unaffected as seen in our case. This is due to location of lesion distal to the bifurcation of chorda tympani where the nerve enters the stylomastoid foramen,[9] unlike in cases of facial nerve palsy with viral or any other etiology where the entire facial nerve is involved. Although the reason for the vulnerability of the distal part of the facial nerve in diabetics cannot be ascertained the high susceptibility of microcirculation distal to the chorda tympani may cause localized facial nerve ischemia. Diagnostic imaging includes CT of the sinuses, which show oedematous mucosa, fluid filling in ethmoid sinuses, and destruction of periorbital tissues and bony margins. MRI is useful in identifying the intradural and intracranial extent of the disease, cavernous sinus thrombosis, or thrombosis of the cavernous portion of the internal carotid artery. As imaging studies carry their own limitations, tissue culture is the gold standard for diagnosis of mucormycosis, unlike any fungal infection.[10] Diagnosis is confirmed histologically by detection of aseptate hyphae with right angled branching is pathognomonic of mucormycosis.[11]
Complications of rhino orbito cerebral mucormycosis include headache, fever, sinusitis, facial swelling, unilateral orbital apex syndrome, convulsions, changes in consciousness, coma and stroke.[2] Blindness may result from central retinal artery occlusion or involvement of the optic nerve via direct orbital extension. Bilateral eye signs are suggestive of cavernous sinus thrombosis. The orbital findings in our case revealed periorbital edema in the right eye leading to closure of eye and proptosis. Significant neurological findings included drooping of the corner of the mouth, drooling of saliva and absence of wrinkles in the right half of the forehead, which were suggestive of lower motor neuron lesion. Initiation of appropriate early therapy (within 5 days) yields better prognosis,[12] nevertheless high mortality of 30-70% is evident. Combined medical and surgical treatment increases the survival rate from 57.5% to 78% when compared to medical management alone. The standard medical therapy is Amphothericin B in a dose of 1-1.5 mg/kg/day for several weeks based on the clinical response and degree of nephrotoxicity.[13] Lipid formulation of amphothericin B can be used at higher doses and for a longer time than amphothericin B due to its reduced nephrotoxicity.[14] As pterygopalatine fossa is considered to be a reservoir of the fungus debridement of the pterygopalatine fossa seems to be the definitive method of managing this infection. In recalcitrant patients and infections resistant to amphotericin B, Posaconazole is an alternate drug of choice. It acts primarily as a cytochrome P-450 3A4 inhibitor.[15] In addition to the standard regimen, the use of 100% oxygen for 90-180 min at pressures from 2 to 2.5 atmospheres with 1 or 2 exposures daily for a total of 40 treatments is necessary.
CONCLUSION
Though incidence of facial nerve palsy in uncontrolled diabetic patients with mucormycosis is only 11% it is an important and disfiguring manifestation, which requires immediate control of diabetic status, surgical debridement and appropriate antifungal therapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Santos Gorjón P, Blanco Pérez P, Batuecas Caletrío A, Muñoz Herrera AM, Sánchez González F, de la Fuente Cañibano R. Rhino-orbito-cerebral mucormycosis, a retrospective study of 7 cases. Acta Otorrinolaringol Esp. 2010;61:48–53. doi: 10.1016/j.otorri.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Koc Z, Koc F, Yerdelen D, Ozdogu H. Rhino-orbital-cerebral mucormycosis with different cerebral involvements: Infarct, hemorrhage, and ophthalmoplegia. Int J Neurosci. 2007;117:1677–90. doi: 10.1080/00207450601050238. [DOI] [PubMed] [Google Scholar]
- 3.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, et al. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670–4. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SG, Greenberg MS. Rhinomaxillary mucormycosis in a kidney transplant patient. Oral Surg Oral Med Oral Pathol. 1980;50:33–8. doi: 10.1016/0030-4220(80)90328-x. [DOI] [PubMed] [Google Scholar]
- 5.Davis RL, Robertson DM. 2nd ed. Baltimore: Williams & Wilkins; 1991. Textbook of Neuropathology; pp. 761–3. [Google Scholar]
- 6.Lamia A, Kilani B, Tiouiri H, et al. Mucormycose: Apropos de quatre observations. Tunis Med. 2008;86:165–8. [Google Scholar]
- 7.Hosseini SM, Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur Arch Otorhinolaryngol. 2005;262:932–8. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am. 2000;33:349–65. doi: 10.1016/s0030-6665(00)80010-9. [DOI] [PubMed] [Google Scholar]
- 9.Pecket P, Schattner A. Concurrent Bell's palsy and diabetes mellitus: A diabetic mononeuropathy? J Neurol Neurosurg Psychiatry. 1982;45:652–5. doi: 10.1136/jnnp.45.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickerts V, Just-Nübling G, Konrad F, Kern J, Lambrecht E, Böhme A, et al. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur J Clin Microbiol Infect Dis. 2006;25:8–13. doi: 10.1007/s10096-005-0078-7. [DOI] [PubMed] [Google Scholar]
- 11.Ferry AP, Abedi S. Diagnosis and management of rhino-orbitocerebral mucormycosis (phycomycosis). A report of 16 personally observed cases. Ophthalmology. 1983;90:1096–104. doi: 10.1016/s0161-6420(83)80052-9. [DOI] [PubMed] [Google Scholar]
- 12.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 13.Yadav SP, Goel AK. Rhino-orbital mucormycosis – A case report. Int J Pediatr Otolaryngol Extra. 2010;5:9–12. [Google Scholar]
- 14.Reed C, Bryant R, Ibrahim AS, Edwards J, Jr, Filler SG, Goldberg R, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–71. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun QN, Najvar LK, Bocanegra R, Loebenberg D, Graybill JR. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob Agents Chemother. 2002;46:2310–2. doi: 10.1128/AAC.46.7.2310-2312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


