Abstract
Background:
We investigated anti-diabetic, hypolipedimic and antioxidant activity of hydroalcoholic extract from leaves and fruit peel of Punica granatum.
Materials and Methods:
Streptozotocin induced diabetic Wister rats were used in this study consisting of seven groups of six animals each. Groups (1) normal control, (2) diabetic control, (3) leaves extract 100 mg/kg b.w. of P. granatum, (4) leaves extract 200 mg/kg b.w. of P. granatum, (5) fruit peel extract 100 mg/kg b.w. of P. granatum, (6) peel extract 200 mg/kg b.w. of P. granatum and (7) glibenclamide respectively. Fasting blood sugar was recorded on 1st, 7th, 14th, 21st and 28th day. At the end of the experiment Lipid profile and levels of antioxidants were determined. Safety profile of both extracts was evaluated using acute and chronic toxicity studies.
Results:
Higher dose of fruit peel extract of P. granatum (PEPG) and glibenclamide significantly lowered blood glucose level from 7th day onwards however glibenclamide was found to be more effective. Leaves extract at higher dose and fruit extract at lower dose also significantly lowered blood glucose level from 14th day onwards. Leaves extract at lower dose also significantly lowered blood glucose level from 21st day onwards. Glibenclamide and higher dose of fruit PEPG extract significantly reduced the total cholesterol, triglyceride levels and significantly increased the high density lipoprotein cholesterol level. Glibenclamide followed by higher dose was found more effective in reducing plasma thiobarbituric acid reactive substances and increasing levels of antioxidant enzymes (superoxide dismutase and catalase). No toxicity was observed even when both extracts were administered at 10 times of higher dose used in this study and no significant changes were seen when it were used chronically.
Conclusion:
Leaves and fruit PEPG possesses significant anti-diabetic, hypolipedimic and antioxidant properties. This study supports the traditional use of P. granatum in diabetes. Fruit peel which is normally thrown by many while eating pomegranate fruit is having anti-diabetic, hypolipedimic and Antioxidant activity. Furthermore high therapeutic index is safe for chronic use.
Keywords: Anti-diabetic, antioxidant, glibenclamide, hypolipedimic, Punica granatum, streptozotocin
INTRODUCTION
Diabetes is a chronic endocrine disorder associated with several secondary complications.[1,2,3] Despite the currently available anti-diabetic drugs, there is need for alternatives which are economical and safe.[4] Many Indian medicinal plants recommended for the treatment of diabetes mellitus lack rigorous scientific justification. Punica granatum, commonly known as pomegranate is one of the plants that have long been used in traditional herbal medicine against diabetes and other disorders.[5,6,7,8]
Many scientific studies have reported anti-diabetic activity of flower and juice of the pomegranate seeds.[9,10,11] However, only few studies have evaluated the anti-diabetic property of fruit peel and leaves of P. granatum. Hence, the present study was designed to scientifically validate and compare the anti-diabetic as well as the antioxidant effect of fruit peel and leaves of P. granatum.
MATERIALS AND METHODS
Collection of the plant materials
The leaves of P. granatum were collected in the month of October from Nagpur, Maharashtra, India and were then authenticated by Mr. Hivarkar botanist of local Science College. The fruits of P. granatum were purchased from local fruit shop and peel was removed from it after authenticated by Botanist.
Preparation of extract of leaves of P. granatum
Fresh leaves of P. granatum were carefully cleaned, shade dried, powdered and stored in airtight containers until it was used for further studies. Hydroalcoholic extract was prepared.[12] A total of 40 g of dried powder was packed in the timble of soxhlet apparatus and was extracted using 95% ethanol refluxing at 50-70°C which yielded a dark brown extract. The stock extract was preserved in airtight glass container and stored at 4°C.
Preparation of extract of fruit peel of P. granatum
Fruit peel of P. granatum was carefully removed, cleaned, shade dried, powdered and stored in airtight containers until it was used for further studies. Hydroalcoholic extract was prepared as above.
Chemicals
Streptozotocin (STZ) was obtained from Sigma Chemicals, Bangalore, India. Glibenclamide of Cipla Company was procured from local medical store. The solvents and chemicals of analytical grade were used and obtained from Swastik Chemicals Nagpur. Kits to estimate total cholesterol (TC), triglycerides (TGs) and high density lipoprotein cholesterol (HDL-C) kit were purchased from Merck, Mumbai India.
Equipment
The glucometer manufactured by Prestige Company (Prestige IQ) was used for estimation of blood glucose for study.
Animals
A 70-80-day-old healthy adult Wistar male albino rats 1 (60 ± 10 g) were used. The animals were maintained under standard laboratory conditions (light period of 12 h/day and temperature 27°C ± 2°C) with access to water ad libitum. The animals were used in groups of six for all the studies.
Ethical clearance
Ethical clearance was taken from Institutional Animal Ethics Committee of the institute before commencement of the study where the research was conducted (MG/IAEC/3/2010).
Acute toxicity study and dose selection
Healthy adult male albino Wistar rats were used for this study. Pilot study was performed using three doses 500 mg/kg body weight, 1000 mg/kg body weight and 2000 mg/kg body weight of the leaves and fruit peel extract of P. granatum (PEPG). The doses selected were 5, 10 and 20 times more than the earlier study.[13] The animals were observed continuously for 4 h and then occasionally for further 4 h and finally overnight. Animals were observed for tremors, clonic convulsions, tonic extensions, catatonia, spasticity, opisthotonus, ataxia, sedation, ptosis, respiration. After a period of 24 and 72 h they were observed for any lethality or death.
Induction of diabetes mellitus
The anti-diabetic activity of P. granatum was assessed using Steptozotocin (STZ) induced diabetes in rats. Diabetes mellitus was induced in overnight fasted male Wister albino rats weighing 160 ± 10 g by single intraperitoneal injection of freshly prepared STZ at dose of 40 mg/kg body weight in 0.1 M citrate buffer (pH = 4.5). After 7 days of STZ administration, blood was collected from tail vein and blood glucose level was determined. Rats with blood glucose level above 200 mg/dl were considered diabetic and included in the study.
Experimental design
In the experiment a total of 42 rats (6 normal; 36 STZ diabetic surviving rats) were used. Only those animals with fasting blood sugar (FBS) between 200 and 300 mg/dl were selected for the study. Rats were divided into seven groups of six animals each as follows (1) normal control, (2) diabetic control, (3) diabetic + leaves extract of P. granatum 100 (LEPG @ 100/mg/kg), (4) diabetic + LEPG @ 200/mg/kg, (5) diabetic + fruit PEPG @ 100/mg/kg, (6) diabetic + PEPG @ 200/mg/kg and (7) diabetic + standard control glibenclamide (600 μg/kg) respectively. Normal control and diabetic control rats were given normal lab diet ad libitum up to 28 days. All extracts/drugs dissolved in distilled water were administered once orally/day in the morning between 9 and 10 am for 28 days. In all groups fasting blood glucose (FBG) levels were recorded on 1st (First time recording FBS levels), 7th, 14th, 21st and 28th day. FBS levels were taken after overnight fasting. Body weights of animals were also recorded on 1st, 14th and 28th day in all groups.
Estimation of lipid profile and antioxidant levels
At the end of the experiment, rats were euthanized by cervical decapitation and blood was collected by direct cardiac puncture. Serum was separated by centrifugation at 3000 rpm for 10 min. It was then kept frozen at −20°C until analysis. TC level was calculated by enzymatic method and expressed in mg/dl. HDL-C level was calculated using polyanion precipitation and expressed as mg/dl. Low density lipoprotein cholesterol level was calculated using Friedewald's equation and expressed in mg/dl. TG in serum was converted to glycerol and then estimated using glycerol kinase enzyme based kinetic method and expressed in mg/dl.
Liver was removed immediately and washed with ice-cold physiological saline and then stored at −20°C until analysis. The antioxidant action of the leaves and fruit PEPG was assessed by measuring thiobarbituric acid reactive substances (TBARS) in tissues.[14] Superoxide dismutase (SOD) activity[15] and catalase activity.[16]
Chronic toxicity studies
Animals were divided into three groups of six animals each. First group was control and received laboratory food ad libitum, second group was given LEPG 200 and third group was given PEPG 200. Plant extracts were dissolved in distilled water and given twice daily orally for 3 months. The various parameters such as food intake, any gross change in behavior and motor activity were observed on 1st, 30th and 60th day.
Statistical analysis
Data was analyzed using SPSS statistical software version 17.0 produced by SPSS Inc. Results are expressed as mean ± standard deviation statistical analysis was performed using analysis of variance followed by post-hoc test (Bonferroni). P < 0.05 was considered as statistically significant.
RESULTS
Acute toxicity study of leaves and fruit PEPG in rats
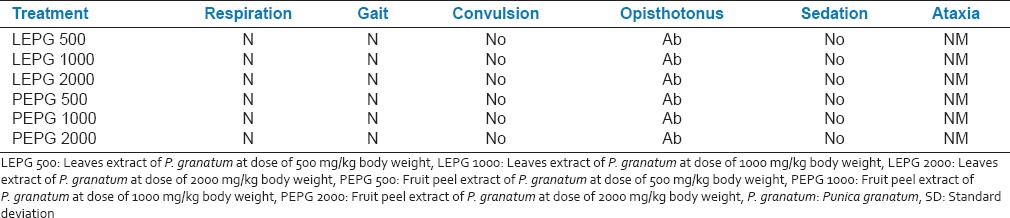
Acute toxicity studies showed the non-toxic nature of the leaves as well as fruit PEPG up to dose of 2000 mg/kg body weight. There was no mortality of any animals when observed for 72 h. There was no lethality or any toxic reactions found at any of the doses selected until the end of the study period [Table 1].
Table 1.
Signs in different doses of leaves and fruit peel extract of P. granatum (n = 6)
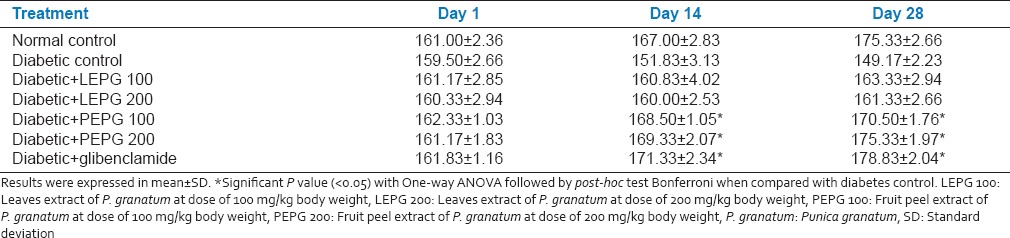
Effect of leaves and fruit PEPG on steptozotocin induced diabetes
There was sustained increase in the mean blood glucose level until 28th day after induction of diabetes by streptozotocin in diabetic group. In diabetic control group mean blood glucose level was 261.50 ± 6 mg/dl on day 1 and was 264.83 ± 3.189 mg/dl on 28th day.
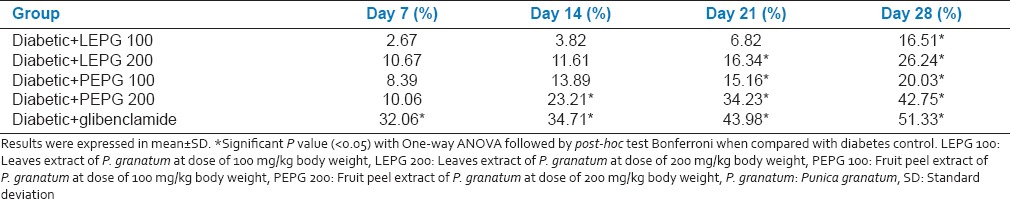
LEPG 100 group showed a significant drop in the mean blood glucose level from 21st day onwards. On 1st day mean blood glucose level was 266.00 ± 4.05 mg/dl, which significantly dropped to 222.00 ± 4.51 mg/dl on 28th day. LEPG 200 group was found better than LEPG 100 and showed a significant drop in the mean blood glucose level from 14th day onwards. On 1st day mean blood glucose level was 266.00 ± 7.40 mg/dl, which significantly dropped to 196.17 ± 8.95 mg/dl on 28th day. PEPG 100 group showed a significant drop in the mean blood glucose level from 14th day onwards (264.17 ± 6.70 mg/dl at day 1-211.17 ± 3.06 mg/dl on 28th day). PEPG 200 group showed a significant drop in the mean blood glucose level from 7th day onwards (266.17 ± 3.86 mg/dl at day 1-152.33 ± 5.57 mg/dl on 28th day). Glibenclamide group showed a sustained drop in the mean blood glucose levels when compared between 1 and 28th days (268.00 ± 6.06 mg/dl on day 1-130.33 ± 7.42 mg/dl on 28th day) and was found better than all extracts of P. granatum in lowering the blood glucose levels [Table 2]. The reduction in mean blood glucose levels was 51.33%, 16.51%, 26.24%, 20.03% and 42.75% in glibenclamide, LEPG 100, LEPG 200, PEPG 100 and PEPG 200 groups respectively [Table 3].
Table 2.
Effect of oral administration of leaves and fruit peel extract of P. granatum on steptozotocin induced diabetic rats (n = 6)
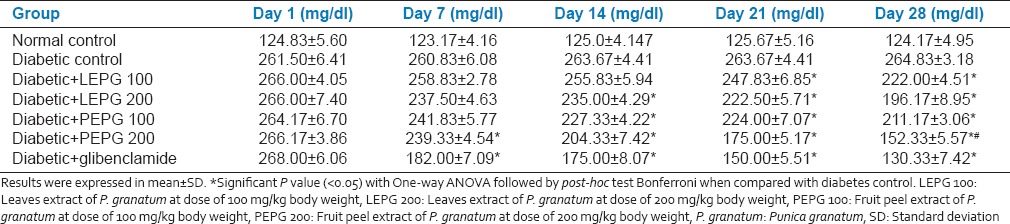
Table 3.
Effect of oral administration of leaves and fruit peel extract of P. granatum on percentage reduction of mean blood glucose levels (n = 6)
Effect of leaves and fruit PEPG on serum lipid profile
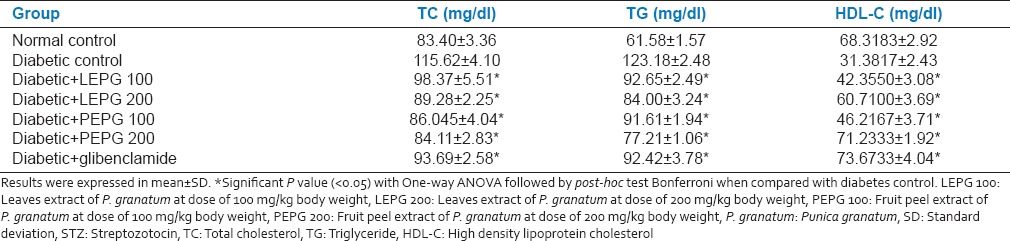
There was increased level of TC, TGs and decreased level of HDL-C in diabetic rats compared with the normal control. Administration of leaves and fruit PEPG extract at all doses for 28 days significantly reduced the TC, TG levels and significantly increased the HDL-C level when compared with diabetic rats. PEPG 200 was significantly better than its lower dose and leaves extract at both the doses. Standard drug glibenclamide was found better than all the extracts of P. granatum [Table 4].
Table 4.
Effect of oral administration of leaves and fruit peel extract of P. granatum on lipid profile in STZ induced diabetic rats after 28 days (n = 6)
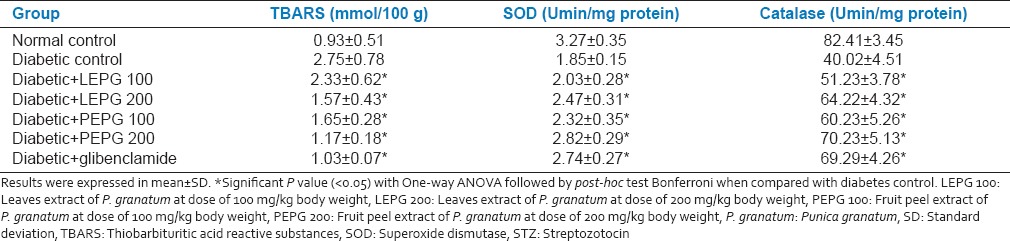
Effect of leaves and fruit PEPG on TBARS and antioxidant levels
In diabetic control group a marked increase in the TBARS levels and a concomitant decrease in the antioxidant levels were observed. However, administration of LEPG at dose of 200 mg/kg and fruit PEPG at both doses significantly reduced the TBARS. PEPG 200 was significantly better compared to other extracts. The activities of SOD and CAT in liver were significantly lower in diabetic rats compared to control rats. Treatment with LEPG 200 and fruit PEPG at both doses showed a significant increase in SOD and CAT activity in the diabetic rats but less compared to glibenclamide group [Table 5].
Table 5.
Effect of oral administration of leaves and fruit peel extract of P. granatum on levels of TBARS, SOD and catalase in STZ induced diabetic rats after 28 days (n = 6)
Effect of Leaves and fruit PEPG on body weight
Significant decrease in body weight was observed in diabetic control group. Although, significant increase in body weight was observed in fruit PEPG treated groups in both doses and glibenclamide group when compared with diabetic control rats but no change in body weight was observed in both doses of LEPG treated groups [Table 6].
Table 6.
Effect of oral administration of leaves and fruit peel extract of P. granatum on body weight in STZ induced diabetic rats (n = 6)
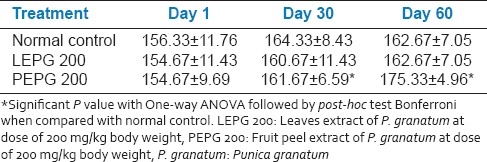
Chronic toxicity of leaves and fruit PEPG on rats
The photoactometer readings in control group were 156.33 ± 11.76, 164.33 ± 8.43 and 162.67 ± 7.005 on 1st, 30th and 60th day respectively. LEPG 200 group didn’t show any significant change in photoactometer readings on 30th and 60th day, whereas the PEPG 200 group showed significant change in photoactometer readings at the end of 30th and 60th day. There was no change in gross behavior of animals but food intake was less in group which received LEPG [Table 7].
Table 7.
Effect of oral administration of leaves and fruit peel extract of P. granatum on spontaneous motor activity in rats (n = 6)
DISCUSSION
The study showed the anti-diabetic activity of leaves as well as fruit PEPG in STZ induced diabetic rats.[17,18,19,20,21] Leaves and fruit peel extracts of P. granatum decreased the elevated blood sugar levels. The possible mechanism for this anti-diabetic action of leaves and fruit peel extracts of P. granatum may be improving glycemic control and insulin secretion from pancreatic beta cells in diabetic rats. The study done by Das and Sarma also showed the anti-diabetic activity of fruit PEPG.[22] Diabetes induced by STZ leads to loss of body weight due to the increased muscle wasting.[23] Fruit PEPG extract for 28 days significantly increased the body weight when compared with diabetic control in dose dependent manner. The increase in weight could be due to control of hyperglycemia by fruit PEPG. However, the animals received LEPG at both doses showed no increase in body weight. As shown by Lei et al. this effect appears to be partly mediated by inhibiting the pancreatic lipase activity and suppressing energy intake.[24]
The level of serum lipids are usually raised in diabetes and such an elevation represents a risk factor for coronary heart disease.[25,26] We have noted a significant increase in TC, TG and significant decrease in HDL-C levels in steptozotocin induced diabetic rats. Fruit peel and LEPG extract significantly decreased TGs and significantly increased HDL-C levels in dose dependent manner. Higher dose of peel extract was found better than both leaves extract and peel extract at lower dose. Since lipid abnormalities accompanied with premature atherosclerosis is the major cause of cardiovascular diseases in diabetic patients, therefore ideal treatment for diabetes, in addition to glycemic control, should have a favorable effect on lipid profile.[27,28,29,30] Free reactive oxygen species generated due to sustained hyperglycemia causes lipid peroxidation.
Treatment with fruit peel and LEPG extract for 28 days significantly decreased TBARS levels in dose dependent manner. The increase in the levels of lipid peroxidation might be indicative of a decrease in the enzymatic antioxidant defense mechanism.[31,32,33,34] In the present study significant increase in SOD and catalase activity was observed following treatment with the extracts, which is in concurrence with previous reports on hypoglycemic, antioxidant and hypolipedimic effect of P. granatum flower extract.[35] The hypoglycemic, antioxidant and hypolipedimic effect of leaves and fruit peel extract could be due to the presence of phytochemicals such as alkaloids, flavonoids, saponins and tannins. As per the previous study done by Elfalleh et al. fruit peel of P. granatum has the most antioxidant content followed by flower, leaves and seed.[36] This could be the reason for better activity of fruit peel extract than the LEPG.
CONCLUSION
Fruit peel and LEPG has shown anti-diabetic and hypoglycemic activity. Fruit PEPG has better anti-diabetic activity than leaves extract. Further elaborative work is necessary for the better understanding of the mechanism of their anti-diabetic activity. Detailed clinical studies in this direction are required to potentiate this claim in human beings.
Footnotes
Source of Support: Mahatma Gandhi Institute of Medical Sciences, Sewagram.
Conflict of Interest: None declared.
REFERENCES
- 1.Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Org; 1999. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Mohan V, Seedat YK, Pradeepa R. The rising burden of diabetes and hypertension in Southeast Asian and African regions: Need for effective strategies for prevention and control in primary health care settings. Int J Hypertens 2013. 2013 doi: 10.1155/2013/409083. 409083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor LD. Florida, Boca Raton: CRC Press; 1990. Handbook of Ayurvedic Medicinal Plants. [Google Scholar]
- 5.Boukef K, Souissi HR, Balansard G. Contribution to the study of plants used in traditional medicine in Tunisia. Plant Med Phytother. 1982;16:260–79. [Google Scholar]
- 6.Nagaraju N, Rao KN. A survey of plant crude drugs of Rayalaseema, Andhra Pradesh, India. J Ethnopharmacol. 1990;29:137–58. doi: 10.1016/0378-8741(90)90051-t. [DOI] [PubMed] [Google Scholar]
- 7.Gujral ML, Varma DR, Sareen KN. Oral contraceptives. Part I. Preliminary observations on the antifertility effect of some indigenous drugs. Indian J Med Res. 1960;48:46–51. [PubMed] [Google Scholar]
- 8.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005;135:2096–102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang TH, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, et al. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: Role of lowering circulating lipids. Br J Pharmacol. 2005;145:767–74. doi: 10.1038/sj.bjp.0706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz SR, Newman RA, Lansky EP. Punica granatum: Heuristic treatment for diabetes mellitus. J Med Food. 2007;10:213–7. doi: 10.1089/jmf.2006.290. [DOI] [PubMed] [Google Scholar]
- 11.Parmar HS, Kar A. Antidiabetic potential of Citrus sinensis and Punica granatum peel extracts in alloxan treated male mice. Biofactors. 2007;31:17–24. doi: 10.1002/biof.5520310102. [DOI] [PubMed] [Google Scholar]
- 12.Mahanta M, Mukherjee AK. Neutralisation of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts. J Ethnopharmacol. 2001;75:55–60. doi: 10.1016/s0378-8741(00)00373-1. [DOI] [PubMed] [Google Scholar]
- 13.Amjad L, Shafighi M. Antioxidant activity of leaf different extracts in Punica granatum. Int J Biol Med Res. 2012;3:2065–7. [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 16.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Dunn JS, McLetchie NG. Experimental alloxan induced diabetes in the rat. Lancet. 1943;11:384–7. [Google Scholar]
- 18.Lenzen S, Panten U. Alloxan: History and mechanism of action. Diabetologia. 1988;31:337–42. doi: 10.1007/BF02341500. [DOI] [PubMed] [Google Scholar]
- 19.Rakieten N, Rakieten ML, Nadkarni MV. Studies on the diabetogenic action of streptozotocin (NSC-37917) Cancer Chemother Rep. 1963;29:91–8. [PubMed] [Google Scholar]
- 20.Mahimainathan L, Das F, Venkatesan B, Choudhury GG. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes. 2006;55:2115–25. doi: 10.2337/db05-1326. [DOI] [PubMed] [Google Scholar]
- 21.Rao NK, Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2006;6:17. doi: 10.1186/1472-6882-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Sarma G. Antidiabetic action of ethanolic extracts of Punica granatum Linn. in Alloxan-induced diabetic albino rats. S J Pharm Sci. 2009;2:14–21. [Google Scholar]
- 23.Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol. 2006;107:285–90. doi: 10.1016/j.jep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, et al. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond) 2007;31:1023–9. doi: 10.1038/sj.ijo.0803502. [DOI] [PubMed] [Google Scholar]
- 25.Sharma RD, Sarkar A, Hazra DK, Misra B, Singh IB. Hypolidemic effect of fenugreek seeds. Phytother Res. 1996;10:332–4. [Google Scholar]
- 26.al-Shamaony L, al-Khazraji SM, Twaij HA. Hypoglycaemic effect of Artemisia herba alba. II. Effect of a valuable extract on some blood parameters in diabetic animals. J Ethnopharmacol. 1994;43:167–71. doi: 10.1016/0378-8741(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 27.Kesari AN, Kesari S, Singh SK, Gupta RK, Watal G. Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J Ethnopharmacol. 2007;112:305–11. doi: 10.1016/j.jep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Kumar V, Prakash O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4:347–52. doi: 10.1016/S1995-7645(11)60101-6. [DOI] [PubMed] [Google Scholar]
- 29.Patil R, Patil R, Ahirwar B, Ahirwar D. Isolation and characterization of anti-diabetic component (bioactivity-guided fractionation) from Ocimum sanctum L. (Lamiaceae) aerial part. Asian Pac J Trop Med. 2011;4:278–82. doi: 10.1016/S1995-7645(11)60086-2. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Kumar V, Prakash O. Antidiabetic and anti-lipemic effects of Cassia siamea leaves extract in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2010;3:871–3. [Google Scholar]
- 31.Nizamutdinova IT, Jin YC, Chung JI, Shin SC, Lee SJ, Seo HG, et al. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res. 2009;53:1419–29. doi: 10.1002/mnfr.200800526. [DOI] [PubMed] [Google Scholar]
- 32.Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 2009;64:235–44. doi: 10.1590/S1807-59322009000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cemek M, Kaða S, Simþek N, Büyükokuroðlu ME, Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med. 2008;62:284–93. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 34.Adewole SO, Ojewole JA. Protective effects of Annona muricata Linn.(Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2008;6:30–41. doi: 10.4314/ajtcam.v6i1.57071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhaskar A, Kumar A. Antihyperglycemic, antioxidant and hypolipidemic effect of Punica granatum L flower extract in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(3 Supplement):S1764–9. [Google Scholar]
- 36.Elfalleh W, Hannachi H, Tlili N, Yahia Y, Nasri N, Ferchichi A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J Med Plants Res. 2012;6:4724–30. [Google Scholar]


