Abstract
Background:
Liver abscess is a burning problem in tropical nations, with often lethal consequences and diagnostic/therapeutic challenges. We have determined etiopathology, clinical, radiological, and bacteriological characteristics of this condition and review its management strategies.
Materials and Methods:
During the period of the month from May 2007 to September 2009, a prospective study was performed involving 125 patients admitted to the in-patient ward of the Department of General Surgery of N.R.S Medical College their diagnosis was made on the basis of clinical features (such as right upper abdomen pain, and fever), laboratory investigations and radiological evidence of liver abscess.
Results:
Amoebic liver abscess was the most common (88%) type of liver abscess among the study groups. There was a strong correlation with the occurrence of liver abscesses and addiction to alcohol, history of diabetes mellitus and low socioeconomic status. The most common etiology of pyogenic liver abscess was Escherichia coli. Ultrasonography (USG) of the abdomen was accurate and cost-effective in diagnosis of liver abscesses. Percutaneous catheter drainage was the most effective method of treatment (with a 100% success rate).
Conclusion:
Most patients in our study had liver abscess of amoebic origin and had temporal relationship with diabetes, alcoholism, and staggering socioeconomic status. We suggest early recognition of clinical features and prompt abdominal USG as cost-effective means for treatment initiation and reducing complications.
Keywords: Amoebic liver abscess, pyogenic liver abscess, ultrasonography
INTRODUCTION
Liver abscess are associated with mortality of up to 20%[1] and are categorized into various types based on etiology, of which amoebic (ALA) and pyogenic (PLA) liver abscess are major types. Interestingly, ALA is more common in the developing nations.[1] PLA constitutes the bulk of hepatic abscesses in developed nations. PLA result from ascending biliary tract infection, hematogenous spread through portal venous system, septicemia with involvement of liver by way of hepatic arterial circulation and secondary spread from intraperitoneal infection. Escherichia coli, Klebsiella, and Streptococcus are the most common etiology of PLA.
Although no distinct clinical criteria exist for distinguishing ALA and PLA, the differential diagnosis can be made based on the following criteria- younger age, resident, or recent travel to areas of endemic amoebiasis, diarrhea, and marked abdominal pain raise clinical suspicion of ALA. The diagnosis is confirmed by ultrasonography (USG), serological tests such as indirect hemagglutination test, reddish brown (anchovy paste like material) aspirate from the abscess, negative gram stain, rapid resolution after metronidazole treatment. The diagnosis of PLA is based on picket fence configuration of temperature chart, nausea, vomiting, anorexia, hematological analysis of leukocytosis, anemia, and positive blood or aspirate culture for bacterial etiology. The treatment of liver abscesses has evolved remarkably with minimal invasive drainage taking the center stage. Radiological imaging has improved diagnostic competence and has altered therapeutic strategy by allowing the possibility of percutaneous approach using needle aspiration or catheter drainage. While open surgery should be reserved for management of complicated cases. We designed a prospective study to analyze the relationship of occurrence of liver abscesses to patient particulars such as age, sex, religion, and socioeconomic status, source of drinking water, addiction to alcohol and history of diabetes mellitus. We also aimed to identify a fast, accurate and cost-effective diagnosis of liver abscess and evaluate the most effective treatment for liver abscesses.
MATERIALS AND METHODS
This was a prospective study carried over a period of 1½ years (May 2007-September 2009). All patients included in the study were admitted to the inpatient ward of General Surgery Department of N.R.S. Medical College. The diagnosis of liver abscess was made based on history, clinical features, laboratory investigations, radiology, serological investigations, blood culture, and culture from the aspirate. Patients were treated with medical treatment with or without one of the following-percutaneous needle aspiration, percutaneous catheter drainage or open surgical drainage.
Following parameters were recorded:
From history-age, sex, religion, socioeconomic status, drinking water source, addiction to alcohol, and medical history of diabetes mellitus.
Clinical features- symptoms (abdominal pain, fever, jaundice, weight loss, diarrhea, anorexia, cough, and others). Signs (right upper quadrant pain, intercostal tenderness, hepatomegaly, jaundice, chest infections, and others).
Laboratory findings (leukocytosis, eosinophilia, raised erythrocyte sedimentation rate (ESR), Hb% (<10 mg%), bilirubin (>1 mg/dl), raised alkaline phosphatase, raised serum glutamic oxaloacetic transaminase, raised serum glutamic pyruvic transaminase, abnormal prothrombin time, and hypoalbuminemia.
Radiology- chest X-ray, abdominal X-ray, ultrasound abdomen (nature of the abscess- single or multiple, the lobe involved and size of the abscess)
Culture from the aspirate
Blood culture
Response to type of treatment-all patients were examined daily for clinical improvement. Improvement in pain, fever, anorexia, and hepatomegaly, improved liver function tests, ultrasonographic evidence of decrease in size of abscess cavity were considered criteria for successful treatment.
Total stay in hospital in days
Follow up-on discharge each patient was followed up weekly for 1 month and then every 2 months for 6 months.
During each visit patient's body weight was recorded, any new clinical symptom was noted; USG of the upper abdomen was performed. The data collected was analyzed. Raw data were entered into a MicroSoft Excel spreadsheet(Microsoft corporation 2007, Washington, US) and analyzed using standard statistical software SPSS® statistical package version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the total 125 patients screened, 110 (88%) had ALA and 15 (12%) had PLA. The major epidemiological findings and clinical features recorded were as follows:
Radiology
Amoebic liver abscess-chest X-ray was abnormal in 50% patients. Ultrasonogram of the abdomen showed right lobe involvement in 80% cases and left lobe involvement in 10% and in rest (10%) both lobes were involved. In 80% patients, the abscess was single and in 20% it was multiple. About 87% patients had abscess volume >300cc and 13% had volume <300cc.
Pyogenic-chest X-ray was abnormal in 40% patients. Ultrasonogram whole abdomen showed right lobe involvement in 60% cases, left lobe in 20% cases, and both lobes in 20%. The abscess was single in 60% and multiple in 40% cases. 80% patients with PLA had abscess volume >300cc.
Blood and aspirate culture
Blood culture was positive in one case of PLA showing presence of E. coli. Culture from aspirate was positive in three cases of PLA showing the presence of E. coli.
Treatment outcome
Of 110 patients with ALA 15 patients were treated with medical or conservative treatment with a success rate of 70%, 37 patients was treated with percutaneous needle aspiration with a success rate of 67%, 37 patients were treated with percutaneous catheter drainage and the success rate of this procedure was 100%, and 21 patients presented with features of peritonitis, were treated with surgical exploration and drainage. The success rate in this group was 65%. There were seven deaths in patients treated surgically.
Of the 15 patients, 3 were treated conservatively, 6 were treated with percutaneous needle aspiration with the success rate of 50%, and 6 patients were treated with percutaneous catheter drainage with the success rate of 100%.
Totally 103 patients with ALA (93.63%) were treated successfully. There were seven deaths. These patients were treated with surgical exploration and drainage. The main cause of death was diffuse peritonitis due to rupture of the abscess. These patients had presented late and had poor general condition. Interestingly, all patients with PLA were treated successfully and subsequently discharged.
DISCUSSION
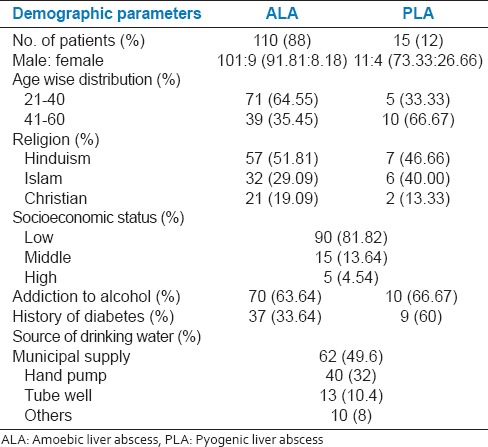
In our study, the maximum age incidence for ALA was 21-40 years, with male:female ratio of 101:9,[Table-1] which is consistent with previous reports.[2,3,4,5] Among PLA patient the maximum age incidence was 41-60 years, which although contradicts the report by Alvarez et al.,[6] is consistent with other report.[7] We observed a higher incidence ratio of PLA in males (11:4). However, in their study Gyorffy et al.[8] they found slightly higher incidence in females (male: Female-13:20), which contradicts our and other studies.[9,10] Nevertheless, males tend to have a poorer prognosis from PLA.
Table 1.
Comparison of demographic data between the two study groups
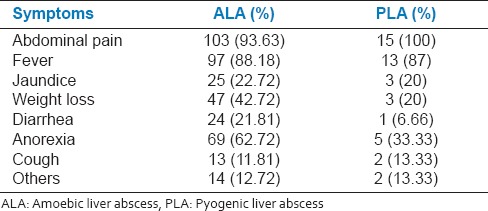
In our study, 21.81% patients with ALA had a recent history of diarrhea, but not dysentery, while 6.66% PLA patients had a history of diarrhea [Table-2]. A previous study has reported lower incidence of diarrhea among ALA patients[4] and hepatic complications are reported in individuals who never had amoebic dysentery.
Table 2.
Comparison of symptoms between two groups
In our study, 33.64% patients with ALA and 60% patients with PLA were diabetic, [Table 1] which is consistent with previous reports.[5,11] The higher incidence of liver abscesses in diabetics may be due to lower immunity in this patient population.
Consistent to previous studies[3,5] we observed 63.64% and 66.67% patients with ALA and PLA had a history of addiction to alcohol, respectively[Table 1]. The higher incidence of ALA in chronic alcoholics is due to higher content of iron deposition in their liver. 81.82% patients in our study were of lower socioeconomic status suggesting that liver abscesses are more common in people of lower socioeconomic status [Table 1]. The main reason for this was poor living conditions such as crowded home, poor hygiene, and drinking contaminated water.
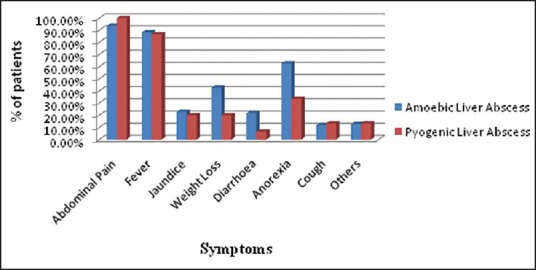
The clinical features observed by us in patients with ALA were abdominal pain (93%), fever (88%), anorexia (62%), jaundice (22%), intercostals tenderness (91%), hepatomegaly (72%), [Table 3, Figure 1] and are consistent with previous reports.[3,5,12]
Table 3.
Comparison of signs between two groups
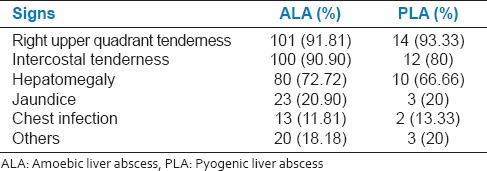
Figure 1.
Comparison of symptoms between two groups
The main clinical features observed in PLA were abdominal pain (100%), fever (87%), jaundice (20%), intercostals tenderness (80%), and hepatomegaly (66%). Several reports have suggested fever[9,10,13] and abdominal pain[7] being the main presenting feature [Table 3, Figure 1] in PLA.
The laboratory revealed leukocytosis (75%), raised ESR (82%), and anemia (52%). The most marked LFT abnormality was raised alkaline phosphatase (62% cases). Abnormal prothrombin time was observed in 43% cases. Most marked LFT abnormality in patients with PLA was abnormal alkaline phosphatase (93%) and 26% patients had abnormal prothrombin time. Eosinophilia was a feature observed in PLA (41% vs. 8% in ALA). About 40% patients in our study had hypoproteinemia [Table 4, Figure 2]. Abnormally, high alkaline phosphatase levels (seen in 60-80% cases) is the most reliable and consistent biochemical marker of ALA.[5]
Table 4.
Comparison of laboratory investigations between two groups
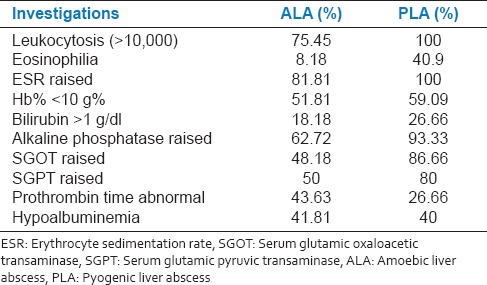
Figure 2.
Comparison of laboratory investigations between two groups
The most important and accurate[14] diagnostic tool in our study was USG, which had accuracy of 96%. CECT abdomen was performed in two cases due to diagnostic confusion and right lobe[3,12] (80% patients) was most commonly affected. 87% patients had abscess cavity size >300cc. In cases PLA the right lobe was involved in 60% cases and 60% patients had single abscess. USG had accuracy of 100% in this patient group. Serology based anti-amoebic antibody estimation using ELISA was not routinely done in our study due to endemic nature of amoebiasis.
The main etiology of PLA in our study was E. coli (60% cases)[6] as opposed to Klebsiella pneumonia reported by other studies.[7,9,15]
15 ALA patients with abscess size <300cc and without complications were treated with conservative management (using metronidazole and chloroquine). The patients who failed to respond to conservative therapy were treated using percutaneous catheter drainage. While other have used USG guided aspiration.[4] Fifteen patients with abscess size >300cc were treated with percutaneous needle aspiration with a success rate of 67%. This result was comparatively less successful in our hands compared to other studies[16] reporting 96.5% success rate. However, most practitioners do not recommend surgical drainage of ALA.[4,11,12,17] In our study, 21 patients presented with features of peritonitis and were treated by surgical exploration and drainage. The success rate in this group was 65%. Unfortunately, there were seven deaths due to late presentation with features of generalized peritonitis and shock. The overall mortality in ALA patients was similar to other reports.[18] Other authors have reported a mortality rate of 12.3%[14] and 17-20%.[1]
PLA should be managed by interventions like needle aspiration or catheter drainage.[19] One patient with small abscess was treated with intravenous antibiotics. Six patients were treated with percutaneous needle aspiration. There was one failure in this group who was managed by catheter drainage. Thus, success rate of needle aspiration and catheter drainage was 50% and 100%, respectively and is consistent with previous report.[20,21] Nevertheless, needle aspiration[7,22] has the advantage over catheter drainage in better maneuverability within abscess cavity, possibly less likely of secondary infection, and reduced equipment cost. As the sample size of PLA in our study was small (n = 15), our findings do not give a strong support in favor of catheter drainage in management of PLA over needle aspiration despite 100% success rate by catheter drainage. There was no mortality in patients with PLA.
CONCLUSION
Liver abscess is a fatal disease if early diagnosis and proper treatment is not initiated. ALA is the main type of liver abscess among patients attending tertiary care institute. Males are more commonly affected and there is strong relationship in occurrence of liver abscesses with diabetes mellitus, addiction to alcohol and lower socioeconomic status; although people of mid- and high-socioeconomic status are also affected. This may be due to drinking contaminated water. Early recognition of clinical features and proper investigation including abdominal USG (which is relatively cheap and very sensitive) is very important. E. coli was the most common causative organism of PLA in our region. For small abscesses conservative or medical management is effective. However, for larger abscesses (>300cc) and left lobe abscesses medical management plus intervention such as catheter drainage (compared to needle aspiration) results in high cure rates with surgical option reserved for complications such as peritonitis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Perez JY., Jr Amoebic liver abscess: Revisited. Philip J Gastroenterol. 2006;2:11–3. [Google Scholar]
- 2.Pillai DR, Keystone JS, Sheppard DC, MacLean JD, MacPherson DW, Kain KC. Entamoeba histolytica and Entamoeba dispar: Epidemiology and comparison of diagnostic methods in a setting of nonendemicity. Clin Infect Dis. 1999;29:1315–8. doi: 10.1086/313433. [DOI] [PubMed] [Google Scholar]
- 3.Makkar RP, Sachdev GK, Malhotra V. Alcohol consumption, hepatic iron load and the risk of amoebic liver abscess: A case-control study. Intern Med. 2003;42:644–9. doi: 10.2169/internalmedicine.42.644. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay M, Saha AK, Sarkar A, Mukherjee S. Amoebic liver abscess: Presentation and complications. Indian J Surg. 2010;72:37–41. doi: 10.1007/s12262-010-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathur S, Gehlot RS, Mohta A, Bhargava N. Clinical profile of amoebic liver abscess. J Indian Acad Clin Med. 2002;3:367–73. [Google Scholar]
- 6.Alvarez JA, González JJ, Baldonedo RF, Sanz L, Junco A, Rodrfíguez JL, et al. Pyogenic liver abscesses: A comparison of older and younger patients. HPB (Oxford) 2001;3:201–6. doi: 10.1080/136518201753242217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugti QA, Baloch MA, Wadood AU, Mulghani AH, Azeem B, Ahmed J. Pyogenic liver abscess: Demographical, clinical, radiological and bacteriological characteristics and management strategies. Gomal J Med Sci. 2005;3:10–4. [Google Scholar]
- 8.Gyorffy EJ, Frey CF, Silva J, Jr, McGahan J. Pyogenic liver abscess. Diagnostic and therapeutic strategies. Ann Surg. 1987;206:699–705. doi: 10.1097/00000658-198712000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew KV, Lau TC, Ho CH, Cheng TK, Ong YS, Chia SC, et al. Pyogenic liver abscess — A tropical centre's experience in management with review of current literature. Singapore Med J. 2000;41:489–92. [PubMed] [Google Scholar]
- 10.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: Recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654–9. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 11.Krige JE, Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system. BMJ. 2001;322:537–40. doi: 10.1136/bmj.322.7285.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kebede A, Kassa E, Ashenafi S, Woldemichael T, Polderman AM, Petros B. Amoebic liver abscess: A 20 year retrospective analysis at Tikur Anbessa Hospital, Ethiopia. Ethiop J Health Dev. 2004;18:199–202. [Google Scholar]
- 13.Chan KS, Chen CM, Cheng KC, Hou CC, Lin HJ, Yu WL. Pyogenic liver abscess: A retrospective analysis of 107 patients during a 3-year period. Jpn J Infect Dis. 2005;58:366–8. [PubMed] [Google Scholar]
- 14.Mohsen AH, Green ST, Read RC, McKendrick MW. Liver abscess in adults: Ten years experience in a UK centre. QJM. 2002;95:797–802. doi: 10.1093/qjmed/95.12.797. [DOI] [PubMed] [Google Scholar]
- 15.Seo TJ, Park CH, Lee SH, Park JH, Lee WS, Joo YE, et al. A clinical study on liver abscess for recent 15 years in Gwangju-Chonnam Province. Korean J Med. 2005;68:26–38. [Google Scholar]
- 16.Zafar A, Ahmed S. Amoebic liver abscess: A comparative study of needle aspiration versus conservative treatment. J Ayub Med Coll Abbottabad. 2002;14:10–2. [PubMed] [Google Scholar]
- 17.Sharma MP, Ahuja V. Amoebic liver abscess. J Indian Acad Clin Med. 2003;4:107–11. [Google Scholar]
- 18.Boonyapisit S, Chinapak O, Plengvanit U. Amoebic liver abscess in Thailand, clinical analysis of 418 cases. J Med Assoc Thai. 1993;76:243–6. [PubMed] [Google Scholar]
- 19.D’Angelica M, Fong Y. The liver. In: Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston Text Book of Surgery. 19th ed. Ch. 54. Philadelphia: Elsevier Saunders; 2012. pp. 1411–75. [Google Scholar]
- 20.Rajak CL, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: Needle aspiration versus catheter drainage. AJR Am J Roentgenol. 1998;170:1035–9. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 21.Cheng DL, Liu YC, Yen MY, Liu CY, Shi FW, Wang LS. Pyogenic liver abscess: Clinical manifestations and value of percutaneous catheter drainage treatment. J Formos Med Assoc. 1990;89:571–6. [PubMed] [Google Scholar]
- 22.Yu SC, Ho SS, Lau WY, Yeung DT, Yuen EH, Lee PS, et al. Treatment of pyogenic liver abscess: Prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932–8. doi: 10.1002/hep.20133. [DOI] [PubMed] [Google Scholar]


