Abstract
γδ T cells are a subset of T lymphocytes that have been implicated in immunosurveillance against infections and tumours. In the peripheral blood of humans the γδ T cell pool is made up predominantly of Vδ2 cells, which can detect both foreign and self-metabolites of the isoprenoid biosynthesis pathway. This unique axis of antigen recognition enables Vδ2 cells to respond to a range of pathogenic infections as well as perturbations in endogenous isoprenoid biosynthesis that can occur during cell stress and malignant transformation. There has been growing interest in Vδ2 cells as a potential avenue for cancer immunotherapy, and a number of strategies have been utilized in an attempt to boost the anti-tumour response of Vδ2 cells in patients. In this review we discuss critically the evidence that Vδ2 cells contribute to the cytotoxic response against tumours and evaluate current immunotherapeutic approaches that target these cells in cancer patients, with specific focus on their shortcomings and how they may be improved.
Keywords: cancer immunotherapy, γδ T cells, tumour immunology
Introduction
γδ T cells are a unique subset of lymphocytes that express T cell receptors (TCRs) composed of γ and δ chains. In humans, two subsets of γδ T cell predominate, defined by the variable domain of their δ chain. Vδ2+ γδ T cells are the most abundant subset found in peripheral blood, and are often regarded as sentinels against infection, whereas Vδ1+ γδ T cells are the most abundant subset in mucosal epithelia, forming part of the protective barrier against invading pathogens 1. In addition, both subsets have been implicated in immunosurveillance against tumours 2–4, and thus manipulation of γδ T cells in order to enhance their anti-tumour properties is a potential approach to cancer immunotherapy. To date, research has focused on peripheral blood Vδ2 cells because this subset has potent reactivity against tumours and is readily accessible. In human peripheral blood, Vδ2 cells typically constitute 1–5% of the total T cell population 5, and all these cells have the potential to target tumour. This is a large pool of tumour-reactive cytotoxic cells compared with their peptide-specific αβ T cell counterparts, and thus a promising cell population to exploit in cancer immunotherapy. In this review we will discuss the current evidence that Vδ2 cells function as anti-tumour immune cells, paying particular attention to the limitations encountered thus far. We will then assess the therapeutic approaches that have been employed to effectively bolster their anti-tumour activity, and discuss how these treatment strategies may be enhanced.
Vδ2 cell tumour recognition
Within the peripheral blood population of Vδ2 cells in humans there are three functionally distinct subsets: naive (CD45RA+CD27+), central memory (CD45RA–CD27+) and effector memory (CD45RA–CD27–) 6. In response to antigenic stimulation, naive and central memory Vδ2 cells proliferate, with central memory cells displaying a higher magnitude of response compared to naive cells. In contrast, effector memory Vδ2 cells display low levels of proliferation but are potent producers of cytokines such as interferon (IFN)-γ and tumour necrosis factor (TNF)-α. At sites of inflammation, Dieli et al. identified a fourth terminally differentiated subset (CD45RA+CD27–), which displays potent cytotoxicity against target cells. The majority of peripheral blood Vδ2 cells express the inflammatory homing chemokine receptor CCR5/CD195 and lack expression of the lymph node homing chemokine receptor CCR7/CD197, and thus home to sites of inflammation that are expressing the CCR5/CD195 ligands CCL3, CCL4 and CCL5 7,8. These characteristics suggest that Vδ2 cells are patrolling the periphery, awaiting the appropriate migratory signals that will direct them to sites of inflammation where they rapidly proliferate and produce cytokines, and subsequently differentiate into cytotoxic cells that kill infected and/or malignant cells.
Underpinning the immune responses of human Vδ2 cells is a unique system of TCR-mediated antigen recognition that is seemingly unique to primates. The Vδ2 cell TCR, which is composed typically of Vδ2 chains paired with Vγ9 chains, recognizes small phosphate-rich metabolites of the isoprenoid biosynthesis pathway, namely (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) of the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway and isopentenyl pyrophosphate (IPP) of the mevalonate pathway 9. Purified forms of these phosphoantigens have been shown to activate Vδ2 cells in a TCR-dependent manner, with HMBPP displaying a 30 000-fold higher potency than IPP 10. Of particular interest to this review is that malignant cells can have a dysfunctional mevalonate pathway, which can cause them to over-express IPP at levels capable of activating Vδ2 cells 11.
It is important to note that not all malignant cells are susceptible to Vδ2 cell killing via IPP recognition, as suggested by the TCR-independent killing observed in certain cell lines by Wrobel et al. 12. There are, however, synthetic drugs, the nitrogen-containing bisphosphonates (NBP), that have been shown to disrupt isoprenoid biosynthesis, and thus increase tumour susceptibility to Vδ2 cell killing 13. Specifically, NBPs block a particular enzyme of the mevalonate pathway called farnesyl pyrophosphate synthase, which converts dimethylallyl and geranyl pyrophosphate into downstream metabolites 14. Blocking the activity of this enzyme causes intracellular accumulation of IPP, and in-vitro studies have demonstrated that tumour cells pre-exposed to NBPs become more susceptible to Vδ2 cell killing 15–17. It is important to note that this effect of NBPs is not common to all tumour cell lines, possibly because of reduced cellular uptake and low mevalonate activity in these cells 18. Moreover, tumour cells are not the only cell type affected by NBPs. It has been shown that peripheral blood mononuclear cells (PBMCs) treated with zoledronic acid (ZA) contain activated Vδ2 cells, an effect that appears to be mediated by the up-regulation of phosphoantigens in peripheral blood monocytes 19.
Although phosphoantigens such as IPP and HMBPP are known to activate Vδ2 cells in a TCR-dependent manner, the underlying mechanism is poorly understood. Early studies demonstrated that recognition of purified phosphoantigen is dependent upon antigen-presenting cells (APCs) of primate origin, but independent of previously identified antigen-presenting molecules such as human leucocyte antigen (HLA) class I, HLA class II and CD1 20. Recent advances have been made that implicate a critical role of butyrophilin (BTN) 3/CD277 in the phosphoantigen-mediated activation of human Vδ2 cells 21. CD277 is a member of the immunoglobulin-supergene family of transmembrane proteins whose extracellular domains share sequence homology to the B7 family 22. In 2012, Harly et al. discovered that an agonist antibody specific for CD277 was able to activate Vδ2 cells in a similar fashion to phosphoantigens and render non-susceptible tumour cells cytolytic targets for Vδ2 cells in a TCR-dependent manner 23. The authors also found that cytotoxicity against susceptible tumour cells was blocked by antagonist antibodies against CD277, and that the 3A1 isoform of CD277 was a critical mediator of phosphoantigen-induced γδ T cell activation. Wang et al. confirmed these observations by showing that APCs treated with the same agonistic antibody were stimulatory for Vδ2 cells 24. In their studies, the antibody did not alter IPP levels in APCs and its ability to render APCs stimulatory for Vδ2 cells was unaffected by statins, which are 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors that block cholesterol synthesis upstream of IPP. The authors went on to show that silencing BTN3A1 in APCs abrogated the activity of this antibody, as did substituting the intracellular region of BTN3A1 for that of BTN3A3. In a later study by Sandstrom et al., phosphoantigens were shown to bind to an intracellular domain of the BTN3A1 molecule and subsequently confer Vδ2 cell reactivity 25. Taken together, this research has led to a new wave of hypotheses that phosphoantigens bind the intracellular domains of BTN3A1 and induce conformational changes in the extracellular domains that are then detected by the Vδ2 cell TCR. Harly et al. discussed that they were unable to demonstrate direct binding of recombinant Vδ2 TCRs to CD277, and they proposed that BTN3A1 may be an accessory molecule for an as-yet undiscovered antigen for Vδ2 cells 23. However, in 2013 Vavassori et al. demonstrated that soluble Vδ2 cell TCRs interact with phosphoantigen-bound BTN3A1 complexes 26, and subsequently proposed an alternative hypothesis that BTN3A1 is a presenting molecule for phosphoantigens.
Tumour immunosurveillance by Vδ2 cells
Due to the lack of appropriate animal models, there is no direct evidence to suggest that human Vδ2 cells eradicate or reduce tumour burden in vivo; however, a number of studies imply that Vδ2 cells may contribute to anti-tumour immunity, and are thus a promising target for cancer immunotherapy.
In-vitro experiments, although limited in their extrapolation into a physiological system, have demonstrated that Vδ2 cells are capable of recognizing tumour cells and killing them through multiple pathways, including granule exocytosis, Fas/Fas-ligand (CD95/CD178)-induced apoptosis, antibody-dependent cell-mediated cytotoxicity and TNF-related apoptosis inducing ligand 15,27–29. Human Vδ2 cells were found to kill a broad range of tumour cell lines derived from haematological and solid malignancies in both allogeneic and autologous settings 12,30,31. Mechanistically, the use of γδTCR- and natural killer group 2, member D (NKG2D)-specific antibodies in Vδ2 cell cytotoxicity assays demonstrated that tumour recognition can be TCR- and/or NKG2D-dependent 12. However, experimental data for whether or not this effect takes place in situ are understandably lacking. Immunocompromised mice bearing human tumours have been utilized in the attempt to model a physiological system, and results have shown that human Vδ2 cells exert some degree of protection against tumour growth in these systems 18,32–35; however, there is a marked degree of uncertainty as to whether the activity of human Vδ2 cells in a xenograft model is the same as in their syngeneic host.
In patients, both positive and negative correlations have been made between clinical responses and tumour-infiltrating Vδ2 cells. For instance, Cordova et al. found that γδ T cells, consisting of both Vδ1+ and Vδ2+ cells, were the predominant tumour-infiltrating lymphocytes in melanoma lesions, and that low numbers of tumour-infiltrating γδ T cells correlated with advanced disease 36. In a cohort of ovarian cancer patients, Raspollini et al. reported a positive correlation between the number of tumour-infiltrating γδ T cells and a brief disease-free interval 37. In contrast, Inman et al. found relatively low percentages of tumour-infiltrating γδ T cells in renal cancer patients 38, and Ma et al. reported a positive correlation between disease progression and the number of tumour-infiltrating γδ T cells in a cohort of breast cancer patients 39. Interestingly, despite no correlation between numbers of tumour-infiltrating Vδ2 cells and clinical responses in a cohort of renal cancer patients, Viey et al. found that peripheral blood Vδ2 cells were inefficient at migrating towards renal tumour cell lines in vitro compared with tumour-infiltrating Vδ2 cells 40. This observation has important ramifications regarding the utility of peripheral blood Vδ2 cells in the absence of appropriate priming. With such a limited number of studies it remains unclear as to whether peripheral blood Vδ2 cells infiltrate tumours, and whether or not their presence in the tumour microenvironment has any bearing on disease prognosis. Moreover, correlations between the numbers of infiltrating γδ T cells and clinical responses do not address whether the Vδ2 cells detected within the tumour mass are activated and exerting cytotoxic activity against the tumour cells. Indeed, we have already mentioned that not all tumour cells are susceptible to Vδ2 cell killing. More studies that critically assess the phenotype and function of immune cells that infiltrate the tumour microenvironment are required, and further efforts to conduct such studies should be made.
If it is hypothesized that Vδ2 cells indeed play a role in immunosurveillance against malignant transformations why, then, do tumours develop? If this hypothesis were true then one would expect people with low numbers of peripheral blood Vδ2 cells to be more susceptible to cancer and/or the activity of Vδ2 cells in patients to be somehow impaired. Indeed, the activity of γδ T cells from cancer patients has been compared with that of healthy controls, and in melanoma, glioblastoma and nasopharyngeal carcinoma, reduced numbers of peripheral blood γδ T cells and/or impaired functional responses have been noted 41–44. However, characterization of immune cells in the periphery is not necessarily an indication of what is happening within the tumour microenvironment. One particular study by Yi et al. reported that tumour-infiltrating γδ T cells in hepatocellular carcinoma exhibited impaired degranulation and IFN-γ responses compared with γδ T cells isolated from peritumoural tissue, suggesting that the tumour microenvironment may be hampering γδ T cell function 45. In this study, tumour-infiltrating T regulatory cells and their production of immunosuppressive cytokines such as transforming growth factor (TGF)-β and interleukin (IL)-10 seemed to coincide with γδ T cell suppression. It is thought that tumours may have evolved immune escape mechanisms that enable them to hamper the activity of infiltrating cytotoxic cells 46. For instance, tumours and tumour-associated macrophages have been reported to express the inhibitory programmed cell death ligand (PDL)-1/CD274 47, and γδ T cells have been shown to up-regulate the receptor for this ligand (PD-1/CD279) following antigenic stimulation 48. Accordingly, in-vitro studies have demonstrated that Vδ2 cells display reduced cytokine and cytotoxic responses in the presence of PDL-1/CD274+ tumour cells compared with PDL-1/CD274– tumour cells 48. Interestingly, when PDL-1/CD274+ tumour cells were treated with ZA, the inhibitory effect on PD-1/CD279+ Vδ2 cell cytotoxicity was reduced. An additional hypothesis is that suboptimal Vδ2 cell function in cancer patients is linked to genetic mutations; for example, Gaafar et al. found that a granzyme B polymorphism was associated with breast cancer, and that this polymorphism coincided with decreased cytotoxic function in peripheral blood Vδ2 cells 49. Although evidence suggests that Vδ2 cells in cancer patients may be impaired they are not inert, and can respond to antigenic stimulation in vitro in the appropriate manner 30,50,51. Importantly, this suggests that tumour immunosuppression can be overcome, and therapies that can do this will provide useful tools for Vδ2 cell-based cancer immunotherapy. For example, antibodies that block cytotoxic T lymphocyte-associated protein (CTLA)-4/CD152 and PD-1/CD279 are available as therapeutic drugs for cancer, and may have potential as combinatorial partners for Vδ2 cell-based cancer immunotherapies 52,53.
Vδ2 cells in cancer immunotherapy
Bolstering the activity of peripheral blood Vδ2 cells in cancer patients in order to enhance their capacity to infiltrate and kill tumour has captured growing interest in the field of cancer immunotherapy. To date, two approaches have been employed: the first involves targeting Vδ2 cells in situ via intravenous (i.v.) administration of NBPs; the second involves adoptive transfer of in-vitro-primed Vδ2 cells. Although both these methodologies have yielded promising results, there are a number of limitations associated with each that, once overcome, may help to further improve their efficacy.
In vitro, NBPs have been shown to rapidly expand peripheral blood Vδ2 cell populations when used in combination with IL-2, as well as increase the sensitivity of tumour cells to Vδ2 cell killing 54,55. Based on these observations, it was hypothesized that NBPs, when administered i.v. in combination with IL-2 [given either subcutaneously (s.c.) or i.v.], will expand the pool of tumour-reactive Vδ2 cells in the peripheral blood of cancer patients while concomitantly increasing the sensitivity of the tumour to Vδ2 cell killing. Administration of NBPs in combination with IL-2 has been conducted in early-phase clinical trials in patients with acute myeloid leukaemia (AML), breast cancer, malignant melanoma, multiple myeloma (MM), non-Hodgkin's lymphoma (NHL), prostate cancer and renal cell carcinoma (RCC). In a clinical trial by Wilhelm et al., patients with either NHL or MM received i.v. pamidronic acid combined with i.v. IL-2, and significant levels of in-vivo Vδ2 cell activation and proliferation were observed in five of nine patients, three of whom showed clinical responses 56. In a cohort of patients with prostate cancer, Dieli et al. reported that i.v. ZA combined with s.c. IL-2 increased the numbers of peripheral blood effector memory Vδ2 cells, an affect which correlated with clinical responses and a decline in prostate serum antigen 57. Similarly, Meraviglia et al. reported that sustained numbers of peripheral blood Vδ2 cells correlated with clinical responses and a decline in serum cancer antigen in breast cancer patients 58. In contrast, Lang et al. reported that i.v. ZA used in combination with s.c. IL-2 in RCC resulted in no clinical responses; however, the authors observed only partial induction of responses in peripheral blood Vδ2 cells, and reported that repeated cycles of ZA and IL-2 reduced overall percentages of peripheral blood Vδ2 cells as well as their capacity to proliferate in response to in-vitro restimulation 59. Kunzmann et al. conducted a trial of i.v. ZA and s.c. IL-2 on small cohorts of patients with RCC, malignant melanoma and AML and found that, although expansion and activation of peripheral blood Vδ2 cells was observed in all patients, clinical responses were observed only in AML, the only cancer type of blood/bone origin 60. Interestingly, in the study by Kunzmann et al., elevated vascular endothelial growth factor levels were found to correlate negatively with clinical response.
An underlying hypothesis for the i.v. administration of NBPs in combination with IL-2 therapy is that peripheral blood Vδ2 cells become activated and subsequently undergo expansion, thus resulting in increased numbers of tumour-reactive Vδ2 cells in the circulation. For these cytotoxic cells to offer optimal protection against tumour they must migrate from the circulation to the tumour mass; however, this has yet to be demonstrated in vivo. Peripheral blood Vδ2 cells are predominantly CCR5/CD195+ and CCR7/CD197–, suggesting that they will home to sites of inflammation such as the tumour microenvironment 7. Indeed, the production of inflammatory chemokines such as CCL3, CCL4 and CCL5 by tumour cells, stromal cells and/or infiltrating immune cells within the tumour microenvironment has been noted, which may potentially draw in CCR5/CD195+ Vδ2 cells from the peripheral blood 61–64. However, we have shown recently that Vδ2 cells within ZA-treated PBMCs have down-regulated expression of CCR5/CD195 and reduced migration towards CCL5, suggesting that, following i.v. ZA, Vδ2 cells may have reduced homing towards inflammatory sites and possibly tumours 65. Others have also shown that γδ T cells undergo marked changes in their inflammatory homing programme following antigenic stimulation. In a study by Brandes et al., TCR-activated γδ T cells were reported to have down-regulated CCR5/CD195 and up-regulated CCR7/CD197 expression, which coincided with reduced migration towards CCR5/CD195 ligands and increased migration towards CCR7/CD197 ligands in Transwell assays 7. This suggests that TCR-mediated activation of peripheral blood γδ T cells results in reduced inflammatory homing and increased lymph node homing, and further supports the notion of inhibited migration towards tumour following ZA treatment. Although the loss of CCR5 was only transient in our studies, in-vivo models have shown that an increase in tumour susceptibility to Vδ2 cell killing following i.v. NBPs may also be transient 33. Taken together, these studies suggest that at the time of optimal tumour susceptibility to Vδ2 cell killing following i.v NBPs, peripheral blood Vδ2 cells have down-regulated expression of the inflammatory chemokine receptors that may be involved in tumour migration. Furthermore, our data suggest that ZA renders peripheral blood monocytes targets for Vδ2 cells, an effect which may result in exhaustion of Vδ2 cells before they reach the tumour 65. Indeed, Sugie et al. and Lang et al. have shown that repeated administration of ZA in breast cancer patients can decrease the responsiveness of Vδ2 cells to in-vitro restimulation 59,66.
Even if it is assumed that Vδ2 cells are migratory towards a tumour mass following i.v. administration of NBPs, there remains the question of how susceptible is the tumour to Vδ2 cell killing? NBPs are capable of increasing the susceptibility of a broad range of tumour cell lines to Vδ2 cell cytotoxicity in vitro, and Santolaria et al. have shown that in immunocompromised mice bearing human tumours, i.v. injections of pamidronic acid enhanced the capacity of cells isolated from the tumour to stimulate human Vδ2 cells in vitro 33. There is little evidence, however, to suggest that a patient's tumour mass becomes more susceptible to Vδ2 cell killing following i.v. infusion of NBPs. This is compounded by the fact that the hydroxyl group of ZA and other NBPs confer high affinity for calcium, and thus bone 67. Indeed, pharmacokinetic studies have shown that within hours of i.v. infusion, ZA is either deposited on bone surfaces or found in the kidneys, where it is then excreted in the urine 67. It is therefore questionable as to whether ZA administered by i.v. infusion reaches tumours that are not associated with either the blood or bone. Accordingly, in the clinical trial conducted by Kunzmann et al., in which ZA and IL-2 was administered to patients with either solid or haematological malignancies, objective clinical responses were observed only in the latter 60. Therefore, rigorous investigations need to be made in order to assess the effect of NBPs on tumour following i.v. infusion in patients.
In light of these discussions, it would be interesting to investigate ways of improving the exposure of solid tumours not associated with blood or bone to NBPs. Indeed, nanoparticle-based drug delivery systems have been utilized in the attempt to reduce NBP binding to bone and thus increase its extraskeletal bioavailability. This technology also has the potential to target NBPs specifically to tumours and immunosuppressive cell types, such as tumour-associated macrophages 68, and increased efficacy of ZA has been reported in preclinical models 69,70. Alternative routes of administration such as intratumoural injection may also prove successful, and the efficacy of such an approach could be tested in easily accessible lesions such as those associated with melanoma. This approach could be combined with other therapeutic strategies capable of priming anti-tumour responses in peripheral blood Vδ2 cells. For instance, the attenuated preparation of Mycobacterium bovis bacillus Calmette–Guérin (BCG) can induce clinical responses when administered intravesicularly in bladder cancer, and may have potential when given intradermally in other cancers such as melanoma 71. We have shown recently that BCG can boost anti-tumour responses in peripheral blood Vδ2 cells 72,73. Specifically, BCG activated IFN-γ production by Vδ2 cells and enhanced their cytotoxicity against susceptible tumour target cells in a manner that is dependent upon myeloid dendritic cells and memory CD4+ αβ T cells. In support of our in-vitro observations, Cairo et al. have reported that intradermal injections of BCG in Macaca fascicularis induced Vδ2 cell proliferation in vivo, and enhanced PBMC IFN-γ production and Vδ2 cell proliferation in response to in-vitro restimulation with IPP 74. Data suggest that BCG may also down-regulate chemokine receptors on Vδ2 cells; for example, Glatzel et al. have demonstrated that human PBMCs treated with heat-killed M. tuberculosis extract contain Vδ2 cells with down-regulated CCR5/CD195 expression, an effect that is dependent upon CCR5/CD195 ligands 8. However, combination therapy would enable clinicians to optimize the timing of intradermal BCG and intratumoural injections of ZA so that tumour susceptibility and peripheral blood Vδ2 cell activity are co-ordinated effectively.
An alternative approach to Vδ2-based cancer immunotherapy has involved the adoptive transfer of in-vitro-expanded populations of Vδ2 cells. This approach has been tested in early-phase clinical trials in breast cancer, lung cancer, MM and RCC patients. In these trials, PBMCs were isolated from patients and treated in vitro with either ZA or synthetic phosphoantigens combined with IL-2 for 2 weeks. The resulting cell population is enriched with Vδ2+CD3+ cells, exhibits marked cytotoxicity against tumour target cells and expresses high levels of CCR5/CD195 and CXCR3/CD183 31,75. In addition to testing the safety and feasibility of this technique, some of these trials demonstrated clinical responses. In 2007, Kobayashi et al. reported reduced tumour growth in three of five RCC patients receiving adoptively transferred γδ T cells 76. The authors then repeated this trial in 2011 using adoptively transferred γδ T cells in combination with ZA and IL-2 therapy, and found reduced tumour growth in 11 of 11 RCC patients 77. Similarly, in 2011 Nicol et al. tested adoptive transfer of γδ T cells in combination with ZA and IL-2 therapy in patients with solid malignancies, and reported clinical responses in those patients who were concomitantly receiving other therapies 78. Furthermore, Abe et al. observed stable levels of serum M-protein in four of six MM patients receiving injections of in-vitro-stimulated γδ T cells, and reported that soluble MHC class I chain-related protein A was detected only in the sera from the two non-responding patients 79, which may represent a potential tumour escape mechanism 80,81. However, in trials conducted by Bennouna et al., Nakajima et al. and Sakamoto et al., no clinical responses were reported in cohorts of patients with RCC or non-small cell lung cancer receiving adoptive transfer of in-vitro-stimulated γδ T cells 82–84.
Adoptive cell transfer is an appealing approach for cancer immunotherapy. It has the potential to generate consistently large pools of tumour-reactive cells because the cells are stimulated outside the immunosuppressive environment of their tumour-bearing host. Furthermore, the cells can be harvested and used at the point at which they display optimal effector functions and migratory function. However, the success of adoptive transfer of Vδ2 cells is dependent upon how susceptible the tumour is to Vδ2 cell killing. Interestingly, of the two clinical trials conducted by Kobayashi et al., one using adoptively transferred Vδ2 cells alone and the other in combination with i.v. ZA, the combination treatment regimen generated a higher proportion of clinical responses 76,77. Although these two treatment strategies were not tested in parallel, one could speculate that the combination therapy was more effective because i.v. ZA renders the tumour mass more susceptible to the adoptively transferred Vδ2 cells. Moreover, it remains unclear as to whether adoptively transferred Vδ2 cells migrate to sites of tumour. In one study by Nicol et al., in vitro-stimulated γδ T cells were labelled with indium (III)-oxine and injected i.v. into three patients (two with melanoma and one with colorectal cancer), and the trafficking of these cells monitored in situ. These cells were shown to express high levels of tissue homing CCR5/CD195 and CXCR3/CD183, and traffic to the lungs, liver and spleen, as well as metastatic lesions 78. However, although it seems possible to generate large numbers of tumour-homing Vδ2 cells for adoptive transfer they may still come under the influence of the immunosuppressive microenvironment of the tumour, and therefore continued research into the effect of the tumour microenvironment on the function of infiltrating Vδ2 cells is necessary. Lastly, with bespoke adoptive cell transfer therapies there are considerable limitations in terms of the associated costs, the time taken to generate sufficient clinically usable material and whether such treatments, if proved to be successful, will be widely available.
Conclusions
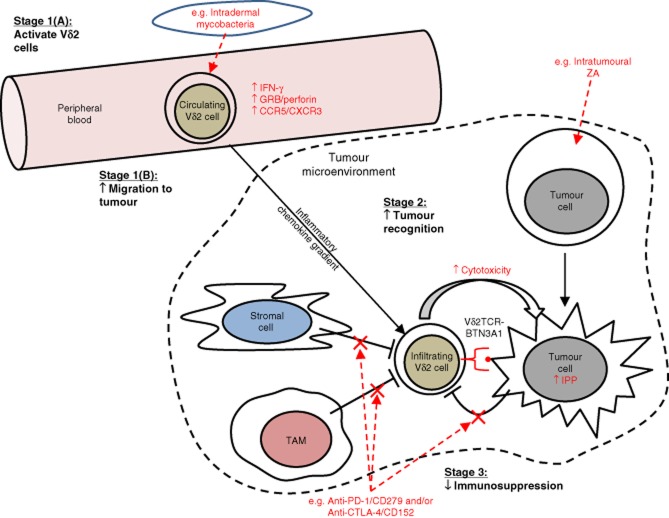
There is a growing body of in-vitro data that supports the notion that Vδ2 cells can exert potent responses against tumour. Moreover, initial clinical evaluations of therapies aimed specifically at boosting Vδ2 cell anti-tumour immunity have supported this approach to cancer immunotherapy. In this review we have highlighted three areas within the field of Vδ2 cell-based cancer immunotherapy that need further investigation: (1) Vδ2 cell migration, (2) tumour susceptibility to Vδ2 cell killing and (3) tumour immunosuppression of Vδ2 cell responses. Of foremost importance in Vδ2 cell-based cancer immunotherapy is ensuring that Vδ2 cells can migrate to the site of a tumour, and therefore the features that enable peripheral blood Vδ2 cells to infiltrate tumours need to be defined more clearly, and therapeutic regimens that manipulate Vδ2 cell migratory behaviour appropriately need to be identified. Once Vδ2 cells have infiltrated a tumour mass, the susceptibility of the tumour to killing, and how this can be increased, is a critical parameter to investigate in order to fully gauge the effectiveness of this approach. Furthermore, a better understanding of the immunosuppressive nature of the tumour microenvironment on the function of infiltrating Vδ2 cells will help us to identify potential combinatorial partners that can be used to counteract this, thus allowing Vδ2 cells to optimally carry out their effector response. Further research into these areas will enhance the progression of Vδ2 cell immunotherapy and enable us to effectively prime the migratory and cytotoxic capacity of peripheral blood Vδ2 cells, while simultaneously increasing tumour susceptibility to Vδ2 killing. A schematic overview of the topics discussed in this review is given in Fig. 1.
Figure 1.
Schematic overview of potential strategies to harness the power of Vδ2 cells in cancer immunotherapy. In this schematic, we have depicted and compartmentalized into key stages the hypothetical processes involved in achieving optimal Vδ2 cell targeting of tumour and the potential ways in which clinical intervention may be used to manipulate the activity of Vδ2 cells in patients. ‘Stage 1(A): activate Vδ2 cells': peripheral blood Vδ2 cells must be primed in order to enhance their anti-tumour response. Intradermal administration of mycobacterial preparations such as bacillus Calmette–Guérin (BCG) may achieve this by increasing Vδ2 cell expression of cytolytic effector molecules [e.g. granzyme B (GRB) and perforin] and production of proinflammatory cytokines [e.g. interferon (IFN)-γ]. ‘Stage 1(B): increase migration to tumour’: primed Vδ2 cells must then migrate from the peripheral blood to the site of tumour. This may involve inflammatory chemokines, and thus require optimal expression of inflammatory chemokine receptors on Vδ2 cells following the priming stage. ‘Stage 2: increase tumour recognition’: Once Vδ2 cells have successfully infiltrated the tumour mass, tumour cells must be susceptible to Vδ2 cell cytotoxicity. Vδ2 cell recognition of tumour can be manipulated in certain cancers by delivering nitrogen-containing bisphosphonates (NBPs) such as zoledronic acid (ZA) to the site of the tumour. ‘Stage 3: decrease immunosuppression’: immunosuppressive elements within the tumour microenvironment must be counteracted in order to ensure an uninhibited Vδ2 cell response against tumour. Examples of potential immunosuppressive pathways include programmed death (PD)-1/CD279 and cytotoxic T lymphocyte antigen-4 (CTLA-4)/CD152, both of which can be blocked using monoclonal antibodies.
Acknowledgments
The authors thank the Cancer Vaccine Institute for funding this project.
Disclosure
The authors declare no competing interests.
References
- Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D. Epithelial defence by gamma delta T cells. Int Arch Allergy Immunol. 2005;137:73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- Zocchi MR, Poggi A. Role of gammadelta T lymphocytes in tumor defense. Front Biosci. 2004;9:2588–2604. doi: 10.2741/1419. [DOI] [PubMed] [Google Scholar]
- Hayday AC. Gammadelta T cells and the lymphoid stress–surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Lang AB, et al. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D. Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J Immunol. 2002;168:4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Puan KJ, Jin C, Wang H, et al. Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int Immunol. 2007;19:657–673. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel P, Shojaei H, Schittek B, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–1297. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- Todaro M, D'Asaro M, Caccamo N, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- D'Asaro M, La Mendola C, Di Liberto D, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–3268. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- Nishio N, Fujita M, Tanaka Y, et al. Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human gammadelta T cells. J Immunother. 2012;35:598–606. doi: 10.1097/CJI.0b013e31826a745a. [DOI] [PubMed] [Google Scholar]
- Benzaid I, Monkkonen H, Stresing V, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011;71:4562–4572. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita CT, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Willcox CR, Mohammed F, Willcox BE. Resolving the mystery of pyrophosphate antigen presentation. Nat Immunol. 2013;14:886–887. doi: 10.1038/ni.2689. [DOI] [PubMed] [Google Scholar]
- Afrache H, Gouret P, Ainouche S, Pontarotti P, Olive D. The butyrophilin (BTN) gene family: from milk fat to the regulation of the immune response. Immunogenetics. 2012;64:781–794. doi: 10.1007/s00251-012-0619-z. [DOI] [PubMed] [Google Scholar]
- Harly C, Guillaume Y, Nedellec S, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Henry O, Distefano MD, et al. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J Immunol. 2013;191:1029–1042. doi: 10.4049/jimmunol.1300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom A, Peigne CM, Leger A, et al. The intracellular B30 2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S, Kumar A, Wan GS, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol. 2013;14:908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu Q, Peng H, Cheng R, Sun Z, Ye Z. IFN-gamma enhances HOS and U2OS cell lines susceptibility to gammadelta T cell-mediated killing through the Fas/Fas ligand pathway. Int Immunopharmacol. 2011;11:496–503. doi: 10.1016/j.intimp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Tokuyama H, Hagi T, Mattarollo SR, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs – rituximab and trastuzumab. Int J Cancer. 2008;122:2526–2534. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- Dokouhaki P, Schuh NW, Joe B, et al. NKG2D regulates production of soluble TRAIL by ex vivo expanded human gammadelta T cells. Eur J Immunol. 2013;43:3175–3182. doi: 10.1002/eji.201243150. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Narita M, Watanabe N, et al. Anti-tumor cytotoxicity of gammadelta T cells expanded from peripheral blood cells of patients with myeloma and lymphoma. Med Oncol. 2008;25:137–147. doi: 10.1007/s12032-007-9004-4. [DOI] [PubMed] [Google Scholar]
- Bouet-Toussaint F, Cabillic F, Toutirais O, et al. Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother. 2008;57:531–539. doi: 10.1007/s00262-007-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Pitters E, Zoller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–6776. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- Santolaria T, Robard M, Leger A, Catros V, Bonneville M, Scotet E. Repeated systemic administrations of both aminobisphosphonates and human Vgamma9Vdelta2 T cells efficiently control tumor development in vivo. J Immunol. 2013;191:1993–2000. doi: 10.4049/jimmunol.1300255. [DOI] [PubMed] [Google Scholar]
- Deniger DC, Maiti S, Mi T, et al. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin Cancer Res. 2014;20:5708–5719. doi: 10.1158/1078-0432.CCR-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo E, Bocca P, Emionite L, et al. Mechanisms of the antitumor activity of human Vgamma9Vdelta2 T cells in combination with zoledronic acid in a preclinical model of neuroblastoma. Mol Ther. 2013;21:1034–1043. doi: 10.1038/mt.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Toia F, La Mendola C, et al. Characterization of human gammadelta T lymphocytes infiltrating primary malignant melanomas. PLOS ONE. 2012;7:e49878. doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspollini MR, Castiglione F, Rossi Degl'innocenti D, et al. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann Oncol. 2005;16:590–596. doi: 10.1093/annonc/mdi112. [DOI] [PubMed] [Google Scholar]
- Inman BA, Frigola X, Harris KJ, et al. Questionable relevance of gamma delta T lymphocytes in renal cell carcinoma. J Immunol. 2008;180:3578–3584. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Zhang Q, Ye J, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viey E, Lucas C, Romagne F, Escudier B, Chouaib S, Caignard A. Chemokine receptors expression and migration potential of tumor-infiltrating and peripheral-expanded Vgamma9Vdelta2 T cells from renal cell carcinoma patients. J Immunother. 2008;31:313–323. doi: 10.1097/CJI.0b013e3181609988. [DOI] [PubMed] [Google Scholar]
- Argentati K, Re F, Serresi S, et al. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120:829–834. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- Petrini I, Pacini S, Galimberti S, Taddei MR, Romanini A, Petrini M. Impaired function of gamma-delta lymphocytes in melanoma patients. Eur J Clin Invest. 2011;41:1186–1194. doi: 10.1111/j.1365-2362.2011.02524.x. [DOI] [PubMed] [Google Scholar]
- Puan KJ, Low JS, Tan TW, et al. Phenotypic and functional alterations of Vgamma2Vdelta2 T cell subsets in patients with active nasopharyngeal carcinoma. Cancer Immunol Immunother. 2009;58:1095–1107. doi: 10.1007/s00262-008-0629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NL, Suarez-Cuervo C, Gillespie GY, et al. Characterization and immunotherapeutic potential of gammadelta T-cells in patients with glioblastoma. Neuro Oncol. 2009;11:357–367. doi: 10.1215/15228517-2008-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, He HW, Wang JX, et al. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J Hepatol. 2013;58:977–983. doi: 10.1016/j.jhep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Tanaka Y, Kobayashi H, et al. Expression and function of PD-1 in human gammadelta T cells that recognize phosphoantigens. Eur J Immunol. 2011;41:345–355. doi: 10.1002/eji.201040959. [DOI] [PubMed] [Google Scholar]
- Gaafar A, Aljurf MD, Al-Sulaiman A, et al. Defective gammadelta T-cell function and granzyme B gene polymorphism in a cohort of newly diagnosed breast cancer patients. Exp Hematol. 2009;37:838–848. doi: 10.1016/j.exphem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Chargui J, Combaret V, Scaglione V, et al. Bromohydrin pyrophosphate-stimulated Vgamma9delta2 T cells expanded ex vivo from patients with poor-prognosis neuroblastoma lyse autologous primary tumor cells. J Immunother. 2010;33:591–598. doi: 10.1097/CJI.0b013e3181dda207. [DOI] [PubMed] [Google Scholar]
- Murayama M, Tanaka Y, Yagi J, Uchiyama T, Ogawa K. Antitumor activity and some immunological properties of gammadelta T-cells from patients with gastrointestinal carcinomas. Anticancer Res. 2008;28:2921–2931. [PubMed] [Google Scholar]
- Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. 2014;27:3–10. doi: 10.1093/intimm/dxu076. [DOI] [PubMed] [Google Scholar]
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153:145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Kondo M, Izumi T, Fujieda N, et al. Expansion of human peripheral blood gammadelta T cells using zoledronate. J Vis Exp. 2011;55:pii: 3182. doi: 10.3791/3182. doi: 10.3791/3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten A, Lilienfeld-Toal M, Buchler MW, Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J Immunother. 2007;30:370–377. doi: 10.1097/CJI.0b013e31802bff16. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraviglia S, Eberl M, Vermijlen D, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JM, Kaikobad MR, Wallace M, et al. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–1460. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann V, Smetak M, Kimmel B, et al. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- Oldham KA, Parsonage G, Bhatt RI, et al. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol. 2012;61:385–394. doi: 10.1016/j.eururo.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Berghuis D, Santos SJ, Baelde HJ, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223:347–357. doi: 10.1002/path.2819. [DOI] [PubMed] [Google Scholar]
- Hong M, Puaux AL, Huang C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427–438. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD. Zoledronic acid causes gammadelta T-cells to target monocytes and downmodulate inflammatory homing. Immunology. 2014;143:539–549. doi: 10.1111/imm.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie T, Murata-Hirai K, Iwasaki M, et al. Zoledronic acid-induced expansion of gammadelta T cells from early-stage breast cancer patients: effect of IL-18 on helper NK cells. Cancer Immunol Immunother. 2013;62:677–687. doi: 10.1007/s00262-012-1368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022–1033. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- Agrati C, Marianecci C, Sennato S, et al. Multicompartment vectors as novel drug delivery systems: selective activation of Tgammadelta lymphocytes after zoledronic acid delivery. Nanomedicine. 2011;7:153–161. doi: 10.1016/j.nano.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Salzano G, Marra M, Porru M, et al. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int J Pharm. 2011;403:292–297. doi: 10.1016/j.ijpharm.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Marra M, Salzano G, Leonetti C, et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv. 2012;30:302–309. doi: 10.1016/j.biotechadv.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Stewart JH, 4th, Levine EA. Role of bacillus Calmette–Guerin in the treatment of advanced melanoma. Expert Rev Anticancer Ther. 2011;11:1671–1676. doi: 10.1586/era.11.163. [DOI] [PubMed] [Google Scholar]
- Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD. Mycobacteria activate gammadelta T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother. 2012;61:535–547. doi: 10.1007/s00262-011-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD. Tripartite immune cell co-operation in the bacillus Calmette–Guerin-induced activation of gammadelta T cells. Immunol Cell Biol. 2013;91:461–468. doi: 10.1038/icb.2013.30. [DOI] [PubMed] [Google Scholar]
- Cairo C, Hebbeler AM, Propp N, Bryant JL, Colizzi V, Pauza CD. Innate-like gammadelta T cell responses to mycobacterium bacille Calmette–Guerin using the public V gamma 2 repertoire in Macaca fascicularis. Tuberculosis (Edinb) 2007;87:373–383. doi: 10.1016/j.tube.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Sakuta K, Noguchi A, et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Yagi J, et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol AJ, Tokuyama H, Mattarollo SR, et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y, Muto M, Nieda M, et al. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- Marten A, von Lilienfeld-Toal M, Buchler MW, Schmidt J. Soluble MIC is elevated in the serum of patients with pancreatic carcinoma diminishing gammadelta T cell cytotoxicity. Int J Cancer. 2006;119:2359–2365. doi: 10.1002/ijc.22186. [DOI] [PubMed] [Google Scholar]
- Bennouna J, Bompas E, Neidhardt EM, et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Nakajima J, Murakawa T, et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded gammadeltaTcells: a phase I clinical study. J Immunother. 2011;34:202–211. doi: 10.1097/CJI.0b013e318207ecfb. [DOI] [PubMed] [Google Scholar]
- Nakajima J, Murakawa T, Fukami T, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg. 2010;37:1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]